Abstract
Background
Erlotinib, a reversible TKI, is currently approved for the treatment of patients with locally advanced or metastatic NSCLC after failure of at least 1 prior chemotherapy. In addition to its EGFR inhibitory activity, erlotinib is a potent Syk inhibitor. Syk is frequently activated in AML and anecdotal reports have suggested that erlotinib can induce complete remission in patients with AML being treated for a concomitant non-small cell lung cancer.
We thus assess the efficacy of Erlotinib in patients with relapsed / refractory AML.
Patients and Methods
This was a phase II, single-arm, open-label study. We enrolled 30 patients with refractory or relapsed AML between May 2013 and July 2014. Patients were >18yr, had ECOG PS £2, and adequate liver (total bilirubin £2x ULN, ALT ≤2.5x ULN) and renal (creatinine £2x ULN) function. They received erlotinib at a dose of 150 mg orally, once daily in 28-day cycles until clinically significant progression of the disease or unacceptable toxicity.
Evaluation during treatment consisted of physical exam and assessment of adverse events; complete blood counts once weekly for the first 3 months, then every 2-4 weeks until 6 months of therapy. BM aspiration was performed on day 28 (± 7 days), then every 2-3 months for 1 year. Responders are patients who obtain a complete remission (CR), CR with incomplete hematologic recovery (CRi), or partial remission (PR), with or without cytogenetic response, hematologic improvement (HI), and morphologic leukemia free state (MLF).
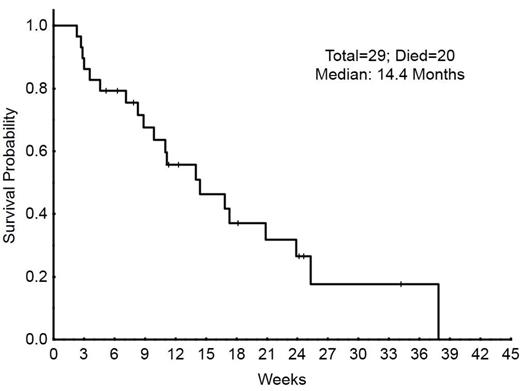
Results: Out of 30 enrolled patients, 29 (97%) were found eligible for evaluation on response. The median age was 67 yrs (range, 20-83) and patients had received a median of 2 prior chemotherapy regimens (range 1-6). Table 1 demonstrates patient’s characteristics. 1 patient (3%) achieved CR, and 2 patients (7%) had blast response (B; >50% reduction in blasts). Baseline blasts in BM decreased from a median of 58% (range 6-94) to a median of 37% (range 1-84) among patients who received 1 or more cycle of Erlotinib. Three (10%) patients had a decrease in bone marrow blasts of 50% or more (from 91% to 23%, 62% to 9% and 6% to 1%). Patients received therapy for a median of 29 days (range 12 to 142). The most common reason for treatment discontinuation was disease progression in 26 patients (90%). Treatment discontinuation before day 28 occurred in 12 patients (41%), in all cases due to disease progression. The most common treatment emergent adverse events were fatigue in 10 patients (32%), diarrhea in 10 (32%), nausea in 8 (26%), and rash in 7 (23%). Thirteen patients (42%) had neutropenic fever, all grade 3. Fourteen (45%) were hospitalized due to pneumonia, 5 of them requiring ICU care. One patient (6%) developed a subdural hematoma not related to study drug. The median Event-free survival (EFS) was 5 weeks (4.2-5.7) and Overall Survival (OS) 14 weeks (9.1-18.8) (Figure 1). 20 patients have died, 19 from progressive disease, and 1 from a cascade of events due to C. Diff infection.
Conclusion Erlotinib has limited clinical efficacy in patients with AML. The occasional responses require further investigation (ongoing) to determine potential biomarkers that might identify patients who could benefit from use of erlotinib as part of their treatment.
| PARAMETER . | . | Median(Range) . |
|---|---|---|
| Age years, median (range) | 67 (20-83) | |
| Sex Male/Female, n(%) | 17/12 (58.6/41.3) | |
| MEDIAN | Range | |
| White Blood Cell Count (x 109/L) Median(Range) | 2.20 | (0.5-36) |
| Hemoglobin (g/dL) | 9.40 | (8.2-12.4) |
| ANC | 0.25 | (0.0-6.4) |
| Platelets (x 109/L) | 27.00 | (8-138) |
| Bone marrow blasts (%) | 53.50 | (6-94) |
| Prior chemo regimens | 2.00 | (1-6) |
| CYTOGENETICS | Characteristic | n(%) |
| Diploid | 8 (28) | |
| Complex | 21 (72) | |
| Molecular | Mutation | n(%) |
| FLT3 | 5 (17) | |
| IDH1 | 4 (13) | |
| IDH2 | 6 (19) | |
| NPM1 | 6 (19) | |
| KIT | 2 (7) | |
| RESPONSE TO ERLOTINIB | n | % |
| CR | 1 | 3 |
| PR | 0 | 0 |
| HI | 2 | 7 |
| Died on therapy | 0 | 0 |
| PD | 19 | 61 |
| PARAMETER . | . | Median(Range) . |
|---|---|---|
| Age years, median (range) | 67 (20-83) | |
| Sex Male/Female, n(%) | 17/12 (58.6/41.3) | |
| MEDIAN | Range | |
| White Blood Cell Count (x 109/L) Median(Range) | 2.20 | (0.5-36) |
| Hemoglobin (g/dL) | 9.40 | (8.2-12.4) |
| ANC | 0.25 | (0.0-6.4) |
| Platelets (x 109/L) | 27.00 | (8-138) |
| Bone marrow blasts (%) | 53.50 | (6-94) |
| Prior chemo regimens | 2.00 | (1-6) |
| CYTOGENETICS | Characteristic | n(%) |
| Diploid | 8 (28) | |
| Complex | 21 (72) | |
| Molecular | Mutation | n(%) |
| FLT3 | 5 (17) | |
| IDH1 | 4 (13) | |
| IDH2 | 6 (19) | |
| NPM1 | 6 (19) | |
| KIT | 2 (7) | |
| RESPONSE TO ERLOTINIB | n | % |
| CR | 1 | 3 |
| PR | 0 | 0 |
| HI | 2 | 7 |
| Died on therapy | 0 | 0 |
| PD | 19 | 61 |
Off Label Use: Erlotinib as a treatment for Relapsed / Refractory AML. Cortes:OSI, Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal