Key Points
A long-term acquired hemophilia A model expressing spontaneous joint bleeds and other bleeds was newly established in nonhuman primates.
Weekly SC dose of the anti-FIXa/X bispecific antibody ACE910 prevented joint bleeds and other bleeds in the primate hemophilia A model.
Abstract
ACE910 is a humanized anti-factor IXa/X bispecific antibody mimicking the function of factor VIII (FVIII). We previously demonstrated in nonhuman primates that a single IV dose of ACE910 exerted hemostatic activity against hemophilic bleeds artificially induced in muscles and subcutis, and that a subcutaneous (SC) dose of ACE910 showed a 3-week half-life and nearly 100% bioavailability, offering support for effective prophylaxis for hemophilia A by user-friendly SC dosing. However, there was no direct evidence that such SC dosing of ACE910 would prevent spontaneous bleeds occurring in daily life. In this study, we newly established a long-term primate model of acquired hemophilia A by multiple IV injections of an anti-primate FVIII neutralizing antibody engineered in mouse-monkey chimeric form to reduce its antigenicity. The monkeys in the control group exhibited various spontaneous bleeding symptoms as well as continuous prolongation of activated partial thromboplastin time; notably, all exhibited joint bleeds, which are a hallmark of hemophilia. Weekly SC doses of ACE910 (initial 3.97 mg/kg followed by 1 mg/kg) significantly prevented these bleeding symptoms; notably, no joint bleeding symptoms were observed. ACE910 is expected to prevent spontaneous bleeds and joint damage in hemophilia A patients even with weekly SC dosing, although appropriate clinical investigation is required.
Introduction
Patients with severe hemophilia A (<1% of normal factor VIII [FVIII] level) typically suffer from recurrent bleeding episodes, primarily in the musculoskeletal system.1,2 Approximately 85% of the bleeding episodes are into joints,3 and repeated joint bleeding from early childhood results in a chronic degenerative arthritis. Although traditional on-demand treatment by a FVIII agent cannot prevent hemophilic arthropathy, routine prophylaxis with FVIII to maintain ≥1% FVIII:C is beneficial in preventing it.4,5 However, the need for frequent IV injections of FVIII negatively affects patients’ quality of life and their adherence to the routine prophylactic regimen, which is particularly problematic when treating pediatric patients at home.2,6
Furthermore, ∼30% of severe patients develop alloantibodies against FVIII (FVIII inhibitors),2,7 which largely restrict treatment with FVIII. FVIII inhibitors make hemorrhage more difficult to be controlled because alternative bypassing agents have shorter half-lives and are not always effective.7,8 Attempts to induce immune tolerance to FVIII inhibitors with high doses of FVIII are very expensive and do not always work.9
Therefore, a novel drug is needed: one that is long-lasting, subcutaneously injectable, effective regardless of FVIII inhibitors, and does not induce FVIII inhibitors.10-13 To achieve this desirable profile, we produced a series of humanized immunoglobulin G (IgG) antibodies bispecific to factors IXa and X (anti-FIXa/X antibodies) that mimic the FVIII cofactor function by binding and placing FIXa and FX into spatially appropriate positions (supplemental Figure 1, see supplemental Data available on the Blood Web site),14 and identified a clinical investigational drug termed ACE910.15 In a short-term primate model of acquired hemophilia A, ACE910 at a single IV dose of 1 or 3 mg/kg exerted hemostatic activity against artificial ongoing bleeds in muscles and subcutis to the same extent as recombinant porcine FVIII (rpoFVIII) at twice-daily IV doses of 10 U/kg.16 Furthermore, a multiple-dosing simulation calculated from the pharmacokinetic (PK) parameters of ACE910 in cynomolgus monkeys suggested that the plasma ACE910 concentration capable of stopping even ongoing bleeds would be maintained by once-weekly subcutaneous (SC) administration of 0.64 to 1.5 mg/kg ACE910.16
Prevention of joint bleeding is of major importance in the care of hemophilia A patients.3 However, it remained unproven whether repeated SC dosing of ACE910 could actually prevent spontaneous bleeding episodes, including the joint bleeds that are a pathologic hallmark of hemophilia A. To address this question nonclinically, we required a primate model because ACE910 is highly species-specific in its FVIII-mimetic cofactor activity.16
In this study, we aimed first to establish a long-term acquired hemophilia A model expressing spontaneous bleeding episodes, including joint bleeds, in nonhuman primates, and second to evaluate the preventive effect of once-weekly SC dosing of ACE910 in this model for investigating the potential of a prophylactic treatment in hemophilia A patients.
Materials and methods
ACE910
ACE910 was expressed in HEK293 or CHO cells cotransfected with a mixture of plasmids encoding the anti-FIXa heavy chain, anti-FX heavy chain, and common light chain.15 ACE910 was purified by protein A and ion-exchange chromatography from the culture supernatants.
Anti-primate FVIII neutralizing antibodies
A mouse monoclonal anti-primate FVIII neutralizing antibody, termed VIII-2236, was prepared from hybridoma culture supernatants.14,16 A chimeric mouse-monkey anti-primate FVIII neutralizing antibody, termed cyVIII-2236, was constructed comprising the mouse variable region from VIII-2236 and the monkey constant region of IgG, which we originally cloned from cynomolgus monkey thymus. The cyVIII-2236 antibody was produced in HEK293 cells and isolated by protein A and gel permeation chromatography from the culture supernatants.
Comparison of cyVIII-2236 with VIII-2236 in an APTT assay
First, to compare the FVIII-neutralizing activity between cyVIII-2236 and VIII-2236, each was added to citrated plasma pooled from 3 normal male cynomolgus monkeys. Then, activated partial thromboplastin time (APTT) was measured with a standard method using Thrombocheck APTT-SLA (Sysmex) and coagulation analyzer KC4 Delta (Stago). Second, to compare the effect of cyVIII-2236 and VIII-2236 on the APTT-shortening activity of ACE910, each was added to FVIII-deficient human plasma (George King) at the final plasma concentration of 300 µg/mL together with various concentrations of ACE910, then APTT was measured.
Animals
Ten male cynomolgus monkeys (2.35-4.25 kg, aged 3-4 years) were purchased from Hamri. All animal studies were approved by the institutional animal care and use committee of Chugai Pharmaceutical, and were conducted in accordance with the approved protocols and the Guidelines for the Care and Use of Laboratory Animals at the company. Chugai Pharmaceutical is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Long-term primate model of acquired hemophilia A
In the first part of this study, 2 cynomolgus monkeys received weekly IV injections (3 or 10 mg/kg each) of VIII-2236. Citrated blood was collected over time. Blood hemoglobin (Hgb) concentration was measured by hematology analyzer SF-3000 (Sysmex) to monitor hemorrhagic anemia. APTT was assessed with a standard method using Data-Fi APTT (Siemens) and coagulation analyzer AMAX CS-190 (Trinity). In addition, bleeding symptoms (bruises [dark-red areas on the body surface], hematuria, limping, and general condition including joint swelling) were monitored on 21 working days from day 0 until day 28. To observe bruises easily, the body hair was sheared on day 0, and the area of bruises and the number of days when bruises had been detected were assessed.
In the second part of this study, another 8 cynomolgus monkeys received weekly IV injections (10 mg/kg) of cyVIII-2236. Two groups (n = 4 each) were established: a control group (the vehicle group) treated with vehicle and a test group (the ACE910 group) treated with ACE910. ACE910 was administered as an initial 3.97 mg/kg SC dose 2 hours after cyVIII-2236 injection on day 0, and thereafter as weekly 1 mg/kg SC doses. The same dosing regimen of vehicle (histidine buffer containing a surfactant) was applied to the vehicle group. Citrated blood was collected over time. Blood Hgb concentration was measured; the change in blood Hgb level was expressed as a percentage of that on day 0 (2 hours after cyVIII-2236 injection). APTT was measured with Thrombocheck APTT-SLA and KC4 Delta. Bleeding symptoms were monitored on 41 working days until day 56. Necropsy was performed on day 56; organs and tissues from the whole body were macroscopically examined, and hemorrhagic findings in joints (shoulder, elbow, wrist, hip, knee, and ankle on both sides) were scored. Then, they were histopathologically examined.
We assessed cyVIII-2236 concentration, FVIII-neutralizing titer of cyVIII-2236, ACE910 concentration, and development of anti-ACE910 alloantibodies. The methods are described in detail in the supplemental Methods. Briefly, cyVIII-2236 concentration was determined with a sandwich enzyme-linked immunosorbent assay (ELISA) using recombinant human FVIII and an anti-human IgG antibody. FVIII-neutralizing titers of cyVIII-2236 on days 0 (just after cyVIII-2236 injection) and 56 were assessed with a modified Bethesda assay. ACE910 concentration was determined with a sandwich ELISA to quantify human IgG.16 Anti-ACE910 alloantibodies present at necropsy were examined with an electrochemiluminescent bridging immunoassay using labeled ACE910.16
In both parts, the monkeys were carefully monitored under the supervision of the attending veterinarian to determine the necessity of pain relief or other treatment. Consequently, no monkeys received an analgesic drug.
Statistical analysis and multiple-dosing simulation
The data are presented as individual values or as mean ± standard deviation (SD). In the in vivo efficacy study, some were analyzed by 2-tailed Student t test (SAS Preclinical Package, version 5.00) between the vehicle and the ACE910 groups. A P value of .05 or less was considered statistically significant. Multiple doses of ACE910 were simulated on the basis of the PK parameters previously determined16 with SAAM II, version 1.2 software (SAAM Institute).
Results
Failure of long-term neutralization of endogenous FVIII by VIII-2236 in cynomolgus monkeys
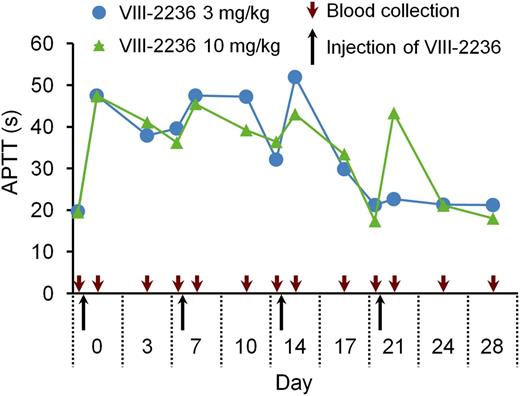
First, we attempted to establish a long-term acquired hemophilia A model by weekly IV injection of VIII-2236 in cynomolgus monkeys. APTT prolongation was observed for the first 2 weeks (Figure 1), but gradually shortened after the third injection, and finally disappeared by the end of the fourth week, suggesting the development of alloantibodies to VIII-2236. VIII-2236 is a mouse IgG, and therefore entirely foreign to primates. In addition, no sign of joint bleeds was observed, although some bruises and a transient decrease in blood Hgb were detected (data not shown). Consequently, we sought an alternative way of establishing a longer-term primate model of acquired hemophilia A.
Change in APTT after weekly IV injection of the mouse anti-primate FVIII neutralizing antibody VIII-2236 in cynomolgus monkeys. VIII-2236 was injected at 3 or 10 mg/kg IV doses to cynomolgus monkeys on days 0, 7, 14, and 21. Citrated blood was collected on days 0, 3, 7, 10, 14, 17, 21, 24, and 28 (before and 2 hours after VIII-2236 injection on days 0, 7, 14, and 21). The time course of APTT is shown for each monkey.
Change in APTT after weekly IV injection of the mouse anti-primate FVIII neutralizing antibody VIII-2236 in cynomolgus monkeys. VIII-2236 was injected at 3 or 10 mg/kg IV doses to cynomolgus monkeys on days 0, 7, 14, and 21. Citrated blood was collected on days 0, 3, 7, 10, 14, 17, 21, 24, and 28 (before and 2 hours after VIII-2236 injection on days 0, 7, 14, and 21). The time course of APTT is shown for each monkey.
Chimerization of VIII-2236 to cyVIII-2236
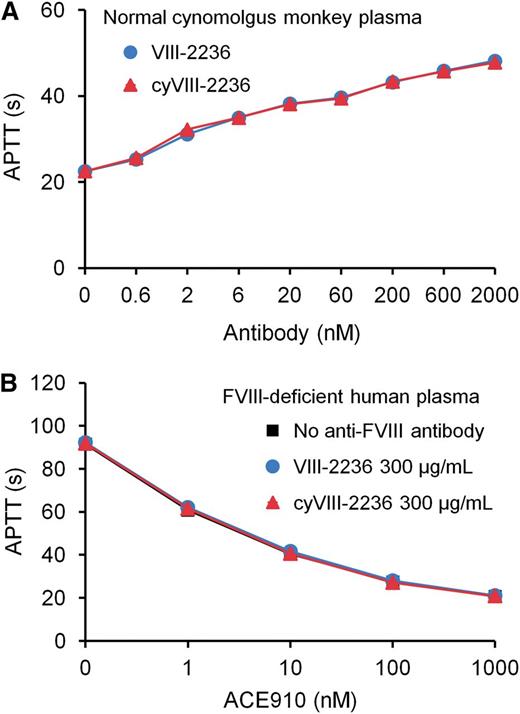
As an attempt to avoid anti-VIII-2236 alloantibody development, we chimerized VIII-2236 (mouse IgG) by replacing its constant region with that of cynomolgus monkey IgG. The chimerized VIII-2236 (cyVIII-2236) successfully prolonged APTT in cynomolgus monkey plasma in vitro with a similar concentration dependency to that of original VIII-2236 (Figure 2A). Additionally, cyVIII-2236 did not interfere with the APTT-shortening activity of ACE910, the same results were also found with VIII-2236 (Figure 2B). These results indicate that VIII-2236 was successfully chimerized to cyVIII-2236 while retaining the original characteristics.
Comparison of the mouse-monkey chimeric anti-primate FVIII neutralizing antibody cyVIII-2236 with the original mouse antibody VIII-2236 in an APTT assay. (A) Effects of cyVIII-2236 and VIII-2236 on APTT in normal cynomolgus monkey plasma. (B) Influence of 300 µg/mL cyVIII-2236 and VIII-2236 on APTT-shortening activity of ACE910 in FVIII-deficient human plasma. Data are expressed as means ± SD (n = 3). The bars depicting SD are shorter than the height of the symbols. The symbols for the group without anti-FVIII antibody are hidden behind the symbols for the other groups in panel B.
Comparison of the mouse-monkey chimeric anti-primate FVIII neutralizing antibody cyVIII-2236 with the original mouse antibody VIII-2236 in an APTT assay. (A) Effects of cyVIII-2236 and VIII-2236 on APTT in normal cynomolgus monkey plasma. (B) Influence of 300 µg/mL cyVIII-2236 and VIII-2236 on APTT-shortening activity of ACE910 in FVIII-deficient human plasma. Data are expressed as means ± SD (n = 3). The bars depicting SD are shorter than the height of the symbols. The symbols for the group without anti-FVIII antibody are hidden behind the symbols for the other groups in panel B.
Establishment of a long-term acquired hemophilia A model expressing spontaneous joint bleeds in cynomolgus monkeys
Using cyVIII-2236, we retried to establish long-term neutralization of endogenous FVIII, expecting reproducible development of spontaneous joint bleeds. The experimental protocol is illustrated in Figure 3A. In all the monkeys given vehicle (n = 4), APTT was prolonged to approximately twice the normal baseline for 8 weeks (Figure 3B). Plasma cyVIII-2236 concentration fluctuated, but remained over 58.6 µg/mL (nearly 400 nM) (supplemental Figure 2). As over 400 nM cyVIII-2236 prolonged APTT by twofold in vitro (Figure 2A), these observations seemed consistent. Thus, an acquired hemophilia A state was successfully maintained by using cyVIII-2236.
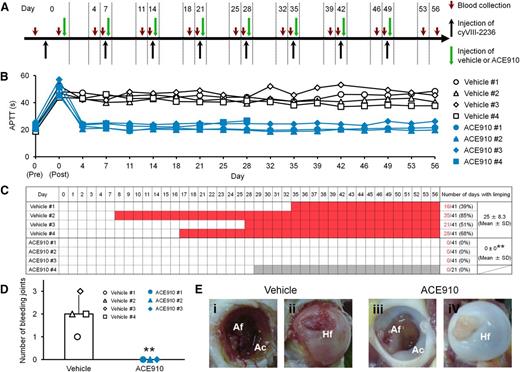
Preventive effects of ACE910 on spontaneous joint bleeds in a long-term primate model of acquired hemophilia A. (A) Experimental protocol used for evaluating preventive effects of ACE910 in a long-term hemophilia A model induced by the weekly IV doses of 10 mg/kg cyVIII-2236. The 8 cynomolgus monkeys received weekly IV injections of cyVIII-2236 on days 0, 7, 14, 21, 28, 35, 42, and 49. ACE910 was administered as an initial 3.97 mg/kg SC dose 2 hours after cyVIII-2236 injection on day 0, and thereafter as a weekly 1 mg/kg SC dose on days 7, 14, 21, 28, 35, 42, and 49. Citrated blood was collected on days 0, 4, 7, 11, 14, 18, 21, 25, 28, 32, 35, 39, 42, 46, 49, 53, and 56 (before and 2 hours after cyVIII-2236 injection on day 0; just before cyVIII-2236, vehicle, and ACE910 injections on days 7, 14, 21, 28, 35, 42, and 49). (B) The time courses of APTT are shown as individual values for respective cynomolgus monkeys (#1-4) of the vehicle and ACE910 groups. The APTT of the vehicle #3 monkey on day 46 was not determined due to a handling failure. (C) The days in which limping was observed are shown in red for the individual cynomolgus monkeys. The gray boxes indicate that no data are available because the ACE910 #4 monkey was killed for humane reasons on day 28 (See “Results”). The number of days with limping (C) and the number of bleeding joints at necropsy (D) are shown as individual values (#1-4) and as means ± SD in the vehicle group (n = 4) and the ACE910 group (n = 3). **P < .01 indicates significant differences from the vehicle group (2-tailed Student t test). (E) Representative macroscopic findings of the joints at necropsy. Left hip joint with limping in the vehicle #1 monkey; dark-red area in the Af (i) and Hf (ii) is detected. Left hip joint without limping in the ACE910 #3 monkey; no abnormalities are noted in the Af (iii) and Hf (iv). Ac, acetabulum; Af, acetabular fossa; Hf, head of femur.
Preventive effects of ACE910 on spontaneous joint bleeds in a long-term primate model of acquired hemophilia A. (A) Experimental protocol used for evaluating preventive effects of ACE910 in a long-term hemophilia A model induced by the weekly IV doses of 10 mg/kg cyVIII-2236. The 8 cynomolgus monkeys received weekly IV injections of cyVIII-2236 on days 0, 7, 14, 21, 28, 35, 42, and 49. ACE910 was administered as an initial 3.97 mg/kg SC dose 2 hours after cyVIII-2236 injection on day 0, and thereafter as a weekly 1 mg/kg SC dose on days 7, 14, 21, 28, 35, 42, and 49. Citrated blood was collected on days 0, 4, 7, 11, 14, 18, 21, 25, 28, 32, 35, 39, 42, 46, 49, 53, and 56 (before and 2 hours after cyVIII-2236 injection on day 0; just before cyVIII-2236, vehicle, and ACE910 injections on days 7, 14, 21, 28, 35, 42, and 49). (B) The time courses of APTT are shown as individual values for respective cynomolgus monkeys (#1-4) of the vehicle and ACE910 groups. The APTT of the vehicle #3 monkey on day 46 was not determined due to a handling failure. (C) The days in which limping was observed are shown in red for the individual cynomolgus monkeys. The gray boxes indicate that no data are available because the ACE910 #4 monkey was killed for humane reasons on day 28 (See “Results”). The number of days with limping (C) and the number of bleeding joints at necropsy (D) are shown as individual values (#1-4) and as means ± SD in the vehicle group (n = 4) and the ACE910 group (n = 3). **P < .01 indicates significant differences from the vehicle group (2-tailed Student t test). (E) Representative macroscopic findings of the joints at necropsy. Left hip joint with limping in the vehicle #1 monkey; dark-red area in the Af (i) and Hf (ii) is detected. Left hip joint without limping in the ACE910 #3 monkey; no abnormalities are noted in the Af (iii) and Hf (iv). Ac, acetabulum; Af, acetabular fossa; Hf, head of femur.
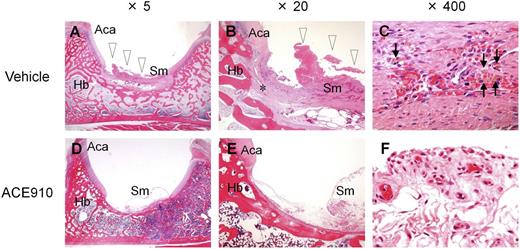
In this long-lasting acquired hemophilia A state, all the control monkeys given vehicle developed abnormal leg motion known as limping: a behavior to avoid using the affected leg (Figure 3C). The limping continued until the experiments ended, with days when limping was detected reaching 25.0 ± 8.3 days (61% ± 20%) of the 41 observation days. Two control monkeys (#3 and #4) developed joint swelling at the ankle in the limping limb from days 28 to 42 and from days 18 to 42, respectively. Macroscopic observation at necropsy on day 56 revealed intraarticular dark red areas (consistent with hemorrhagic findings in the histopathological examination) in joints (elbow, hip, or ankle) of all the control monkeys; the number of bleeding joints was 2.0 ± 0.8 per head (Figure 3D, Table 1). Bleeding was detected in the hip or ankle joint of the side that limped. Representative macroscopic findings of the hip joints are shown in Figures 3Ei-ii. Hemorrhagic findings or reactive findings associated with hemorrhage were histopathologically confirmed in the joints that limped: hemorrhage/hemosiderin deposition, mononuclear cell infiltration, and vascular proliferation in the synovial membranes; synovial hyperplasia; granulation tissue; and destruction of articular cartilage or underlying bone (Figure 4A-C). Thus, a long-term acquired hemophilia A model expressing spontaneous joint bleeds was successfully established in cynomolgus monkeys.
Macroscopic hemorrhagic findings at necropsy on day 56 in a long-term primate model of acquired hemophilia A
| Organ/tissue . | Site . | No. of animals with findings . | ||
|---|---|---|---|---|
| Vehicle, n = 4 . | ACE910, n = 3 . | |||
| Joint | Elbow | Left | 1 | 0 |
| Right | 1 | 0 | ||
| Hip | Left | 4 | 0 | |
| Right | 1 | 0 | ||
| Ankle | Left | 1 | 0 | |
| Skeletal muscle | Lower abdomen | 2 | 0 | |
| Femur | 1 | 0 | ||
| Urinary bladder | Wall | 2 | 0 | |
| Seminal vesicle | Serosa | 1 | 0 | |
| Rectum | Serosa | 1 | 0 | |
| Skin | Back | Subcutis | 1 | 0 |
| Organ/tissue . | Site . | No. of animals with findings . | ||
|---|---|---|---|---|
| Vehicle, n = 4 . | ACE910, n = 3 . | |||
| Joint | Elbow | Left | 1 | 0 |
| Right | 1 | 0 | ||
| Hip | Left | 4 | 0 | |
| Right | 1 | 0 | ||
| Ankle | Left | 1 | 0 | |
| Skeletal muscle | Lower abdomen | 2 | 0 | |
| Femur | 1 | 0 | ||
| Urinary bladder | Wall | 2 | 0 | |
| Seminal vesicle | Serosa | 1 | 0 | |
| Rectum | Serosa | 1 | 0 | |
| Skin | Back | Subcutis | 1 | 0 |
Histopathological findings in a representative joint in a long-term primate model of acquired hemophilia A. Left hip joint with limping in the vehicle #1 monkey (A-C) and left hip joint without limping in the ACE910 #3 monkey (D-F) are shown at original magnification ×5 (A, D), ×20 (B, E), and ×400 (C, F). Hemorrhagic changes (arrowheads) including hemosiderin deposition (arrows) and destruction of articular cartilage/underlying bone (*) are detected in the joint with limping (A-C). No hemorrhagic changes are noted in the joint without limping (D-F). Hematoxylin and eosin stain. Aca, articular cartilage of acetabulum; Hb, hip bone; Sm, synovial membrane.
Histopathological findings in a representative joint in a long-term primate model of acquired hemophilia A. Left hip joint with limping in the vehicle #1 monkey (A-C) and left hip joint without limping in the ACE910 #3 monkey (D-F) are shown at original magnification ×5 (A, D), ×20 (B, E), and ×400 (C, F). Hemorrhagic changes (arrowheads) including hemosiderin deposition (arrows) and destruction of articular cartilage/underlying bone (*) are detected in the joint with limping (A-C). No hemorrhagic changes are noted in the joint without limping (D-F). Hematoxylin and eosin stain. Aca, articular cartilage of acetabulum; Hb, hip bone; Sm, synovial membrane.
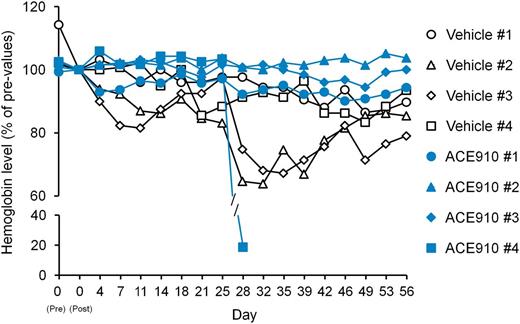
Other spontaneous bleeding symptoms, that is, bruises and hematuria, were detected by daily observation in the vehicle group (data not shown). By macroscopic observation at necropsy, hemorrhagic findings were also observed in skeletal muscle (lower abdomen and femoral region), urinary bladder, seminal vesicle, rectum, and subcutis (back) in 1 or 2 of the 4 control monkeys (Table 1). Additionally, blood Hgb temporarily decreased during the observation period with the lowest values reaching 64% to 87% (Figure 5), suggesting substantial hemorrhagic blood loss.
Relative blood Hgb concentrations in a long-term primate model of acquired hemophilia A. The time courses of blood Hgb concentration relative to concentration on day 0 (2 hours after cyVIII-2236 injection) are shown as individual values in respective cynomolgus monkeys (#1-4) of the vehicle and ACE910 groups.
Relative blood Hgb concentrations in a long-term primate model of acquired hemophilia A. The time courses of blood Hgb concentration relative to concentration on day 0 (2 hours after cyVIII-2236 injection) are shown as individual values in respective cynomolgus monkeys (#1-4) of the vehicle and ACE910 groups.
Bleeding preventive effect of ACE910 in the long-term acquired hemophilia A model
The preventive effect of a weekly SC dose of ACE910 on spontaneous joint bleeds was evaluated in the model described in “Establishment of a long-term acquired hemophilia A model expressing spontaneous joint bleeds in cynomolgus monkeys.” The dosing regimen was simulated to maintain the plasma concentration trough at >30 µg/mL from the first week after administration using the PK parameters of ACE910 in cynomolgus monkeys previously obtained because around 26 µg/mL of plasma ACE910 would show hemostatic activity comparable to that of 10 U/kg (twice-daily) rpoFVIII in a short-term primate model of acquired hemophilia A.16
In all the monkeys treated with ACE910 (n = 4), APTT was initially prolonged to approximately twice the normal baseline after the first IV injection of cyVIII-2236, as in the group treated with vehicle. Then, repeated ACE910 administration shortened the prolonged APTT to the baseline level over the entire dosing period (Figure 3B), although careful observation indicates that the APTT-shortening effect was slightly reversed in 1 monkey (ACE910 #4) on days 25 and 28. It should be noted that the APTT of FVIII-neutralized cynomolgus monkey plasma was normalized even at around 30 μg/mL (nearly 200 nM) of ACE910, which also indicated rpoFVIII relative activity of <10% on the basis of peak height in the thrombin generation assay.16 This is assumedly because ACE910 does not require an activation process to exert its cofactor activity, whereas FVIII needs additional time to be activated by thrombin or FXa in the APTT assay. Therefore, the effect of ACE910 on APTT should be carefully interpreted in relation to the hemostatic efficacy. Plasma cyVIII-2236 in the ACE910 group remained over 96.1 μg/mL, which exceeded the minimal cyVIII-2236 level (58.6 μg/mL) in the vehicle group (supplemental Figure 2). Furthermore, in all monkeys other than the ACE910 #4 monkey, the FVIII-neutralizing titer measured by the modified Bethesda assay was maintained on day 56 (data not shown). These results suggest that endogenous FVIII was also successfully neutralized in the ACE910 group.
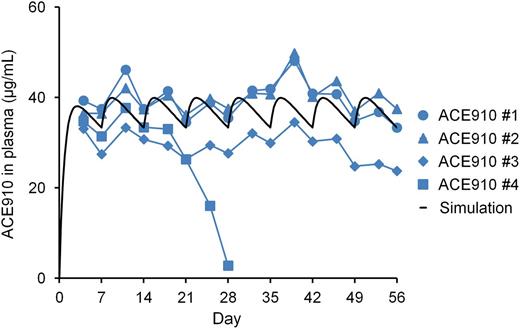
The ACE910 #4 monkey showed a rapid and significant decrease in plasma ACE910 concentration from day 21 to day 28, on which it was only 2.8 µg/mL (Figure 6), and anti-ACE910 alloantibodies were detected in the plasma (data not shown). Furthermore, this monkey accidentally experienced a fracture of the mandibular cuspid on day 28, although no other monkeys experienced such a rare accident. Owing to the consequent massive bleeding in the oral cavity, the blood Hgb concentration decreased to 2.2 g/dL (19% of day 0 values, Figure 5). Therefore, in consideration of animal ethics and the impossibility of maintaining an effective ACE910 level, this monkey was killed humanely on day 28 and macroscopic observation of its organs and tissues was carried out at necropsy.
Plasma ACE910 concentrations in a long-term primate model of acquired hemophilia A. ACE910 was administered at an initial SC dose of 3.97 mg/kg on day 0 followed by weekly SC doses of 1 mg/kg on days 7, 14, 21, 28, 35, 42, and 49. The time courses of actual measured and simulated plasma concentrations of ACE910 are shown. The actual measured concentrations are presented as individual values for cynomolgus monkeys (#1-4) of the ACE910 group.
Plasma ACE910 concentrations in a long-term primate model of acquired hemophilia A. ACE910 was administered at an initial SC dose of 3.97 mg/kg on day 0 followed by weekly SC doses of 1 mg/kg on days 7, 14, 21, 28, 35, 42, and 49. The time courses of actual measured and simulated plasma concentrations of ACE910 are shown. The actual measured concentrations are presented as individual values for cynomolgus monkeys (#1-4) of the ACE910 group.
In the other 3 monkeys, plasma ACE910 level remained around the target concentration (30 µg/mL) from day 4 to day 56 (Figure 6), demonstrating that the multiple-dosing simulation worked very well. Anti-ACE910 alloantibodies were examined in plasma collected at necropsy (day 28 for the #4 monkey; day 56 for the other 3 monkeys). In addition to the #4 monkey, 1 other monkey (ACE910 #3) had anti-ACE910 alloantibodies. In this monkey, however, the plasma ACE910 level slightly decreased only after day 49, and remained around 26 µg/mL, a level which is expected to show hemostatic activity.16
Regarding the joint bleeding symptoms, no limping and no macroscopic bleeding joints at necropsy were observed in any of the ACE910-treated monkeys, including monkey #4. In the ACE910 group (n = 3; excluding #4 statistically), the number of limping days (Figure 3C) and the number of bleeding joints at necropsy (Figure 3D, Table 1) significantly decreased (P < .01, 2-tailed Student t test), compared with those of the vehicle group (n = 4). In the ACE910 group, synovial hyperplasia and vascular proliferation in the synovial membranes of joints were histopathologically noted (Figure 4D-F). However, these findings were less severe and less frequent in the ACE910 group than those in the joints with neither macroscopic bleeding nor limping in the vehicle group.
Regarding the other spontaneous bleeding symptoms, bruises (the maximum value of areas and the number of days detected during the observation period) were ameliorated, and no hematuria and no organ bleeds at necropsy were found except for the oral cavity bleeds of the ACE910 #4 monkey (Table 1). Furthermore, monkeys #1 to 3 in the ACE910 group exhibited blood Hgb of 90% or above for 8 weeks (Figure 5). The minimum value of blood Hgb during the observation period was 94.9% ± 4.9% in the ACE910 group (n = 3, excluding monkey #4), which was significantly higher than 75.2% ± 11.3% in the vehicle group (n = 4) (P < .05, 2-tailed Student t test).
Discussion
Joint damage is one of the most problematic bleeding-related complications in hemophilia A patients. Manco-Johnson et al demonstrated in a prospective clinical study that the progression of joint damage could be decreased by routine alternate-day doses of FVIII from infancy.4 Although this dosing regimen could render a severe disease (<1% FVIII:C) into a moderate one (1%-5%), its preventive effect on joint bleeds was not perfect,4,17 and an analysis by den Uijl et al determined that the threshold FVIII:C level required to be free from joint bleeds would be 12%.18 Our previous study in a primate model of acquired hemophilia A predicted that ≥26 µg/mL ACE910 would exhibit hemostatic activity within the range of the mild phenotype (>5%) and that such a hemostatic level would be maintained by weekly SC doses of 0.64 mg/kg ACE910.16 Therefore, in the present study, we investigated weekly SC doses of 1 mg/kg ACE910 and demonstrated that such a regimen significantly prevented spontaneous joint bleeding, which also confirmed that the simulation of plasma ACE910 concentrations worked well. Because PK data of therapeutic antibodies from cynomolgus monkeys can be scaled to project human PK profiles,19,20 a similar PK profile and efficacy are expected for ACE910 in a clinical setting.
In this study, a long-term acquired hemophilia A model was newly established. The FVIII:C in this model was speculated to keep <4% and <3% in the vehicle and ACE910 groups, respectively, according to plasma cyVIII-2236 level and the cyVIII-2236 concentration vs FVIII:C correlation in the pooled cynomolgus monkey plasma (supplemental Figures 2 and 3). In the case of congenital hemophilia A, a small percentage of FVIII:C is considered to render the severity of bleeding; however, in acquired hemophilia A, measured FVIII:C does not necessarily correlate with the severity.21 Although cyVIII-2236 would not have necessarily decreased FVIII:C in the 1-stage clotting assay to <1% in the monkeys, the control monkeys actually presented severe bleeding symptoms. Thus, we considered that this model was a severe acquired hemophilia A one. In an attempt to further validate this model by looking at reactivity to injected FVIII, we examined the effects of rpoFVIII, which is not neutralized by cyVIII-2236 (data not shown). Aiming to evaluate the marginal level of rpoFVIII efficacy, we administered rpoFVIII for 8 weeks with a twice-weekly 20 U/kg IV dosing regimen, which was set to keep ≥1% rpoFVIII:C for 6 days a week (supplemental Figures 4 and 5A).22 The prolonged APTT was shortened immediately after the initial rpoFVIII injection (supplemental Figure 5B); however, in 3 of 4 monkeys, the APTT-shortening effect gradually disappeared in the middle of the dosing period, suggesting that anti-rpoFVIII alloantibodies had developed. In terms of bleeding symptoms, the number of days with limping was lower in the rpoFVIII group than in the vehicle group (supplemental Figure 5C); furthermore, the course of blood Hgb tended to worsen when anti-rpoFVIII alloantibodies presumably emerged (supplemental Figure 5D). In the monkey in which rpoFVIII maintained the APTT-shortening effect, limping and decrease in blood Hgb were not observed; nevertheless, 2 bleeding joints were noted by macroscopic observation at necropsy. Although it was difficult to fully evaluate the effect of rpoFVIII because anti-rpoFVIII alloantibodies developed, these results suggest that increasing FVIII activity could ameliorate the bleeding tendency in this model; however, the twice-weekly 20 U/kg IV dosing regimen was not sufficient to fully prevent joint bleeding.
ACE910 and rpoFVIII are proteins foreign to cynomolgus monkeys; therefore, the development of alloantibodies to these is theoretically inevitable.23 Incidence rates of anti-humanized antibody alloantibodies in cynomolgus monkeys are reported to vary (0%-100%), and antigenicity in cynomolgus monkeys cannot predict that in humans.19 Although the antigenicity risk score of ACE910 in an in silico T-cell epitope prediction system was comparable to that of trastuzumab and palivizumab, which are nonimmunogenic in a clinical setting,15 the rate of anti-ACE910 alloantibody development must be evaluated in the actual clinical setting.
In this long-term acquired hemophilia A model, joint damage involving abnormal motion and histopathological features associated with intraarticular hemorrhage were similar to those of hemophilia A patients.3,24,25 Although a number of animal models have been reported for hemophilia A, it has been difficult to develop reproducible spontaneous joint bleeds.26-28 The FVIII-deficient mouse model of hemophilia A needs an artificial injuring procedure to induce joint bleeding.27,29 Although congenital hemophilia A models in dogs, sheep, rats, and pigs have been reported to develop joint bleeds, it seems difficult to express reproducible joint bleeds in individuals and to efficiently evaluate drug efficacy in a practicable experimental period.28-34 Our model stably developed leg-joint damage within 8 weeks, which was possibly produced by the severe acquired hemophilic state and by bodyweight loading due to the bipedal motion of the monkeys. Thus, this model may be particularly useful in testing the efficacy of therapeutic agents from the orthopedic aspect of hemophilia A. In addition, this primate model should be useful for the many therapeutic antibodies that have poor interspecies cross-reactivity. Moreover, along with our model of hemophilia A, the strategy of using a mouse-host animal chimeric antibody may be beneficial for establishing long-term acquired animal models of other protein-deficiency diseases in the hematology research field.
Although limping and macroscopic intraarticular hemorrhage at necropsy were observed in all 4 control monkeys, joint swelling was noted in only 2 monkeys. Therefore, it was not so reproducible and may be inappropriate for quantitative assessment. The joint swelling was linked to limping in both monkeys. However, in one of the monkeys (vehicle #4), the ankle joint that had swollen did not present macroscopic intraarticular hemorrhage. We assume that the intraarticular hemorrhage may have been absorbed during 2 weeks after the remission of joint swelling.
In this study, although ACE910 completely prevented the macroscopic joint impairment, subclinical histopathological changes in the joint synovium were found, despite the presence of ACE910. We assume that the subclinical or small joint bleeds would occur in this model, and ACE910 prevented them from becoming larger and from leading to clinical symptoms, but did not provide a histopathological complete recovery at around 30 µg/mL of plasma ACE910 concentration. An appropriate clinical investigation will be required to elucidate whether such an action of ACE910 can completely prevent macroscopic and clinical joint damage in the long run.
In conclusion, a long-term acquired hemophilia A model expressing reproducible spontaneous joint bleeds and other bleeds was newly established in cynomolgus monkeys, and weekly SC doses of ACE910 significantly prevented these bleeding symptoms. The difficulty in venous access negatively affects the adoption of and adherence to the routine prophylaxis regimen in home settings.35 ACE910 is expected to provide hemophilia A patients, regardless of FVIII inhibitors, with a more effective and user-friendly way of bleeding prophylaxis. Clinical investigation of ACE910 is currently ongoing.
Presented in abstract form at the World Federation of Hemophilia 2014 World Congress, Melbourne, Australia, May 11-15, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their colleagues at Chugai Pharmaceutical Co, Ltd: T. Matsuura, M. Hiranuma, R. Takemoto, T. Koike, H. Kitamura, and T. Houjo for the in vivo pharmacologic experiments; T. Kojima, T. Wakabayashi, E. Tanaka, K. Esaki, Y. Kikuchi, M. Wada, M. Goto, H. Tsunoda, T. Suzuki, Y. Okuyama-Nishida, A. Harada, M. Funaki, S. Suzuki, T. Toyoda, and M. Ijiri for the preparation of test items and the in vitro experiments; Y. Sakamoto, T. Tachibana, and M. Nanami for the PK studies; and H. Saito for providing advice for the various experiments. The work was carried out at the Research Division, Chugai Pharmaceutical Co, Ltd.
This work was supported by research funding from Chugai Pharmaceutical Co, Ltd.
Authorship
Contribution: A.M., K.Y., M.T., T. Kitazawa, T.S., and Y.K. designed, performed, and analyzed the pharmacologic studies; T.I., Z.S., T. Kuramochi, and A.S. prepared the test items; K. Haraya conducted the PK studies; K.A. conducted the histopathological studies; K.N. and M.S. interpreted data and provided advice from the viewpoints of their medical expertise in hemophilia; K. Hattori provided direction and organized the program; and A.M. and T. Kitazawa wrote this manuscript.
Conflict-of-interest disclosure: A.M., K.Y., T. Kitazawa., T.S., T.I., Z.S., K. Hattori, M.T., T. Kuramochi, A.S., K. Haraya, K.A., and Y.K. are employees of Chugai Pharmaceutical, and the former 7 authors are inventors of the patents relating to anti-FIXa/X bispecific antibodies, of which all rights have been assigned to the company. K.N. and M.S. receive research support paid to their institution from Chugai Pharmaceutical, and have received consulting honoraria from Chugai Pharmaceutical and F. Hoffmann-La Roche.
Correspondence: Atsushi Muto, Research Division, Chugai Pharmaceutical Co, Ltd, 1-135 Komakado, Gotemba, Shizuoka 412-8513, Japan; e-mail: mutoats@chugai-pharm.co.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal