Key Points
The negative impact on thrombin generation of zymogen FVII competing with rFVIIa for TF is counteracted by FVII (auto)activation.
Correction of hemophilia A occurs in a rFVIIa concentration range where detectable effects of FVII competition are minimal or absent.
Abstract
Successful competition of activated factor VII (FVIIa) with zymogen factor VII (FVII) for tissue factor (TF) and loading of the platelet surface with FVIIa are plausible driving forces behind the pharmacological effect of recombinant FVIIa (rFVIIa) in hemophilia patients. Thrombin generation measurements in platelet-rich hemophilia A plasma revealed competition for TF, which potentially could reduce the effective (r)FVIIa:TF complex concentration and thereby attenuate factor Xa production. However, (auto)activation of FVII apparently counteracted the negative effect of zymogen binding; a small impact was observed at endogenous concentrations of FVII and FVIIa but was virtually absent at pharmacological amounts of rFVIIa. Moreover, corrections of the propagation phase in hemophilia A required rFVIIa concentrations above the range where a physiological level of FVII was capable to downregulate thrombin generation. These data strongly suggest that rFVIIa acts independently of TF in hemophilia therapy and that FVII displacement by rFVIIa is a negligible mechanistic component.
Introduction
Recombinant factor VIIa (rFVIIa; NovoSeven) is approved as a bypassing agent to treat hemophilia patients with inhibitory antibodies against factor VIII (FVIII) or IX.1 It is debated whether the pharmacologic effect of rFVIIa, taking place after (r)FVIIa has functioned in the initiation phase that serves to prime the coagulation process with a nonhemostatic amount of thrombin, primarily results from its occupation of tissue factor (TF) by displacing zymogen factor VII (FVII) or from TF-independent factor X activation on its localization to activated platelets.2 In vivo, FVII is present in large excess over FVIIa. Even though blood clotting on physiologic TF exposure is initiated by as little as picomolar concentrations of FVIIa, along the TF-dependent avenue, it has been suggested that a high dose of rFVIIa (typically 90 μg/kg, plasma concentration ∼25 nM) would be needed to efficiently compete with 10 nM endogenous FVII for TF and ensure hemostasis.3,4 As an alternative, the TF-independent mechanism postulates that a similar dose would provide an adequate amount of rFVIIa bound to activated platelets to mediate a sufficient rate of factor X activation.5,6 The most recent mechanistic studies also arrive at conflicting conclusions: one suggesting that a TF-dependent component dominates7 and the other demonstrating that rFVIIa acts in a TF-independent manner.8
We used thrombin generation measurements in hemophilia A plasma to demonstrate zymogen FVII competition with FVIIa for TF and a counteractive effect of FVII (auto)activation and assessed the net outcome of these 2 events at physiological and pharmacological levels of (r)FVIIa. We then monitored the dose-dependent effect of rFVIIa and could assign the major contribution to a TF-independent mode of action.
Study design
Materials
FVIII- and FVII-deficient plasmas were from George King Bio-Medical (Overland Park, KS), and normal plasma was from Precision BioLogic (Dartmouth, NS, Canada). Double-deficient plasma was FVII-deficient plasma treated with 0.1 mg/mL polyclonal sheep anti-human FVIII antibody (Haematologic Technologies, Essex Junction, VT). rFVIIa (NovoSeven), FVII, and FVIIR152A were produced in-house.9 The FVIIa content in the FVII preparation was 0.3% as measured by the amidolytic activity of 300 nM FVII in the presence of 1 µM soluble TF using a FVIIa standard curve. N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)/Tyrodes buffer consisted of 10 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 1.7 mM MgCl2, 5 mM d-glucose, and 0.4 mM NaH2PO4, pH 6.5 or 7.4. Prostaglandin E1 was from Sigma-Aldrich (St. Louis, MO), relipidated TF (Dade-Innovin) was from Siemens Healthcare (Erlangen, Germany), SFLLRN agonist peptide was from Bachem (Bubendorf, Switzerland), convulxin was from Santa Cruz Biotechnology (Dallas, TX), and reagents for the thrombin generation assay were from Thrombinoscope (Maastricht, The Netherlands).
Isolation of platelets
Human blood was obtained from healthy donors, members of the Danish National Corps of Voluntary Blood Donors, as approved by the Danish National Committee on Biomedical Research Ethics (H-D-2007-0055). Platelets were isolated from citrate-stabilized plasma by centrifugation.10 Briefly, the platelet pellet was washed in HEPES/Tyrodes buffer, pH 6.5, containing prostaglandin E1 (5 μg/mL), and resuspended in HEPES/Tyrodes buffer, pH 7.4.
Thrombin generation assay
Thrombin generation was performed according to the calibrated automated thrombogram method.11 Citrate-stabilized, platelet-rich human plasma (80 μL, 150 000 platelets/μL) was placed at 37°C for 5 minutes, followed by the addition of 10 μL FVIIa (0.1-300 nM) and buffer with or without added FVII or FVIIR152A (10 μL, 10 or 100 nM). Thrombin generation was initiated with 20 μL FluCa reagent supplemented with either TF (1 pM) or convulxin (100 ng/mL) plus SFLLRN (30 μM). Final concentrations are noted in parentheses.
Results and discussion
Competition between FVII and FVIIa for TF
Zymogen FVII and FVIIa bind TF with equal affinity,12 and no or slow (auto)activation would lead to inhibition of TF-dependent rFVIIa activity by FVII (reduced fraction of (r)FVIIa:TF complexes). FVII can potentially be activated by FVIIa (TF-dependent autoactivation) or by the other products FVIIa generates, FXa or FIXa13-17 (feedback activation), and the term (auto)activation as used herein is intended to cover FVII activation mediated by any of these activators because they are not distinguishable in our plasma system. Using FVII-deficient platelet-rich human plasma where FVIII had been neutralized to mimic hemophilia A, we investigated whether the presence of FVII influenced FVIIa-mediated, TF-induced thrombin generation. FVII competition with rFVIIa for TF became evident at high FVII/rFVIIa ratios. FVII (100 nM) did not affect thrombin generation at 25 nM rFVIIa, but the peak was reduced by 40% at 6 nM rFVIIa and by >50% when rFVIIa dropped to <1 nM (Figure 1A). As expected, the reduction was more modest at 10 nM FVII, reaching 25% at the physiological FVIIa level. However, the degree of inhibition did not reflect the molar FVII/FVIIa ratio. By using 100 nM FVIIR152A (zymogen incapable of conversion to FVIIa) and comparing the thrombin generation to that obtained in the presence of the same amount of FVII, we could show that the actual competition was more pronounced (Figure 1B-C). This strongly suggested that (auto)activation of FVII to a large extent neutralized the inhibitory effect of competing with FVIIa for TF. The lowering of the effective TF concentration by 100 nM FVIIR152A in the presence of 25 nM rFVIIa is equivalent to reducing TF to one-fifth in the presence of rFVIIa alone. Although the former condition reduced the functional TF:FVIIa density on the TF-containing vesicles, the latter reduced the number of vesicles (with unaltered TF density), but both gave similar effective rFVIIa:TF concentrations and parallel reductions of the thrombin peak (Figure 1B; also at 6 nM FVIIa, Figure 1C). Thus, FVII (auto)activation has a remarkable ability to counteract the competitive effect of FVII. Although competition between FVII and rFVIIa is an inevitable phenomenon, detectable under certain conditions, it has limited or no effect at physiological (0.1 nM) or clinically relevant concentrations (>5 nM) of FVIIa and a physiological concentration of FVII (10 nM; Figure 1A,D).
Impact of competition between FVII and FVIIa for TF. Thrombin generation was measured in FVII-deficient, FVIII-neutralized plasma supplemented with rFVIIa in the absence or presence of FVII or FVIIR152A and initiated with 1 pM TF (Innovin) unless otherwise stated. (A) The effect of the presence of 10 or 100 nM FVII with 0.1 to 25 nM rFVIIa. The degree of inhibition of thrombin generation was calculated at each [rFVIIa] as the decrease in thrombin peak level compared with a sample without added FVII. The FVIIa present in FVII (0.3%) was taken into account when 100 nM FVII was used at a total FVIIa concentration <6 nM. Data are mean of repeated experiments (n = 2 or 3). (B) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 25 nM rFVIIa. The curves represent the thrombin generation obtained in the presence of rFVIIa alone initiated with either 1 pM TF (thick solid line) or 1/5 pM TF (dotted line) or initiated with 1 pM TF in the presence of either 100 nM FVII (thin solid line) or 100 nM FVIIR152A (dashed line). (C) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 6 nM FVIIa. Same setup as in B, but initiation was with 1/15 pM TF in the low-TF sample (dotted line). (D) The effect of endogenous level (10 nM) of FVII on the thrombin generation profile at therapeutic concentrations of rFVIIa. Thin solid and dotted lines depict the results with 6 nM rFVIIa, and thick solid and dashed lines are the corresponding results with 25 nM rFVIIa, in the absence and presence of 10 nM FVII, respectively. The presence of 100 nM FVIIR152A together with 6 or 25 nM FVIIa reduced the functional TF density as measured by a significant decrease in the thrombin generation, but still no inhibitory effect of 10 nM zymogen FVII could be revealed (data not shown). In B-D, the thrombin generation profiles were normalized for easier comparison, and a peak level of 1 was assigned to the samples initiated with 1 pM TF without added zymogen FVII. Data are mean of repeated experiments (n = 3).
Impact of competition between FVII and FVIIa for TF. Thrombin generation was measured in FVII-deficient, FVIII-neutralized plasma supplemented with rFVIIa in the absence or presence of FVII or FVIIR152A and initiated with 1 pM TF (Innovin) unless otherwise stated. (A) The effect of the presence of 10 or 100 nM FVII with 0.1 to 25 nM rFVIIa. The degree of inhibition of thrombin generation was calculated at each [rFVIIa] as the decrease in thrombin peak level compared with a sample without added FVII. The FVIIa present in FVII (0.3%) was taken into account when 100 nM FVII was used at a total FVIIa concentration <6 nM. Data are mean of repeated experiments (n = 2 or 3). (B) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 25 nM rFVIIa. The curves represent the thrombin generation obtained in the presence of rFVIIa alone initiated with either 1 pM TF (thick solid line) or 1/5 pM TF (dotted line) or initiated with 1 pM TF in the presence of either 100 nM FVII (thin solid line) or 100 nM FVIIR152A (dashed line). (C) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 6 nM FVIIa. Same setup as in B, but initiation was with 1/15 pM TF in the low-TF sample (dotted line). (D) The effect of endogenous level (10 nM) of FVII on the thrombin generation profile at therapeutic concentrations of rFVIIa. Thin solid and dotted lines depict the results with 6 nM rFVIIa, and thick solid and dashed lines are the corresponding results with 25 nM rFVIIa, in the absence and presence of 10 nM FVII, respectively. The presence of 100 nM FVIIR152A together with 6 or 25 nM FVIIa reduced the functional TF density as measured by a significant decrease in the thrombin generation, but still no inhibitory effect of 10 nM zymogen FVII could be revealed (data not shown). In B-D, the thrombin generation profiles were normalized for easier comparison, and a peak level of 1 was assigned to the samples initiated with 1 pM TF without added zymogen FVII. Data are mean of repeated experiments (n = 3).
Mode of action of rFVIIa in FVIII deficiency
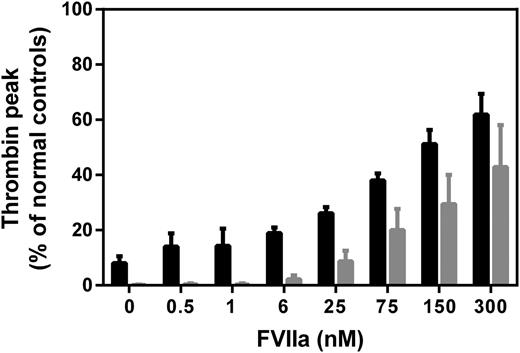
To assess the contributions of TF-dependent and -independent events, rFVIIa titrations were made in platelet-rich hemophilia plasma, and coagulation was initiated with either TF or direct platelet activators (convulxin and SFLLRN). When triggered with TF, addition of rFVIIa up to 6 nM had hardly any effect on the thrombin generation profile, followed by a successively increasing thrombin peak at higher rFVIIa concentrations (Figure 2, black bars). The effect of rFVIIa on thrombin generation in plasma triggered with direct platelet activators was qualitatively very similar, with an increase in thrombin peak becoming evident and measurable at 6 nM rFVIIa (Figure 2, gray bars). This indicated that the TF-independent (platelet surface) factor Xa generation by rFVIIa is manifested at ≥6 nM, where the zymogen competition effect is no longer observed (Figure 1B).
Assessment of rFVIIa mode of action under FVIII-deficient conditions. rFVIIa was added to FVIII-deficient plasma, and thrombin generation was initiated with 1 pM TF (Innovin, black bars) or direct platelet activators (100 ng/mL convulxin and 30 μM SFLLRN, gray bars). The thrombin peak level is plotted against increasing rFVIIa concentration (0-300 nM). The presence of polyclonal goat-anti-human TF antibodies did not significantly influence the thrombin peak after initiation with the platelet activators (data not shown). Thrombin peak levels are expressed in percentage relative to the thrombin peak in normal human plasma. Data are mean with standard deviation of repeated experiments (n = 3).
Assessment of rFVIIa mode of action under FVIII-deficient conditions. rFVIIa was added to FVIII-deficient plasma, and thrombin generation was initiated with 1 pM TF (Innovin, black bars) or direct platelet activators (100 ng/mL convulxin and 30 μM SFLLRN, gray bars). The thrombin peak level is plotted against increasing rFVIIa concentration (0-300 nM). The presence of polyclonal goat-anti-human TF antibodies did not significantly influence the thrombin peak after initiation with the platelet activators (data not shown). Thrombin peak levels are expressed in percentage relative to the thrombin peak in normal human plasma. Data are mean with standard deviation of repeated experiments (n = 3).
The fact that endogenous FVII competition with rFVIIa for TF ceases to have an effect on the thrombin generation at the concentration of rFVIIa (∼6 nM) needed for detectable restoration of thrombin generation in hemophilia suggests that the dose-effect relationships of rFVIIa reflect a TF-independent mode of action (Figure 2). In other words, competition between FVII and rFVIIa for TF cannot explain the increased hemostatic potential, according to the thrombin generation measurements, of ≥6 nM concentrations (especially ≥25 nM) of rFVIIa. Nevertheless, TF doubtlessly plays a crucial role, not for the hemostatic effect of rFVIIa, but by being instrumental in generating the initial thrombin needed especially for platelet activation. It is important to keep in mind that the initiation of the blood coagulation cascade, presumably also facilitated by FVII (auto)activation, is effectively mediated by a subnanomolar concentration of FVIIa, which makes it unlikely that pharmacologic amounts of rFVIIa dramatically improve hemostasis through a TF-dependent mechanism. Our overall conclusion that rFVIIa in hemophilia treatment works primarily through a TF-independent mechanism, most likely (primarily) on platelets, is also in agreement with the hemostatic equipotency of murine FVIIa and a variant thereof unable to bind TF in hemophilia B mice.8
Presented in abstract form at the 24th congress of the International Society on Thrombosis and Haemostasis, Amsterdam, The Netherlands, June 29-July 3, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr Mirella Ezban, Novo Nordisk A/S, for contributions to the study initiation and valuable discussions.
Authorship
Contribution: C.A. designed and performed experiments, analyzed data, and wrote the manuscript; and E.P. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: C.A. and E.P. are employees of Novo Nordisk A/S.
Correspondence: Egon Persson, Novo Nordisk A/S, Novo Nordisk Park, DK-2760 Måløv, Denmark; e-mail: egpe@novonordisk.com.

![Figure 1. Impact of competition between FVII and FVIIa for TF. Thrombin generation was measured in FVII-deficient, FVIII-neutralized plasma supplemented with rFVIIa in the absence or presence of FVII or FVIIR152A and initiated with 1 pM TF (Innovin) unless otherwise stated. (A) The effect of the presence of 10 or 100 nM FVII with 0.1 to 25 nM rFVIIa. The degree of inhibition of thrombin generation was calculated at each [rFVIIa] as the decrease in thrombin peak level compared with a sample without added FVII. The FVIIa present in FVII (0.3%) was taken into account when 100 nM FVII was used at a total FVIIa concentration <6 nM. Data are mean of repeated experiments (n = 2 or 3). (B) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 25 nM rFVIIa. The curves represent the thrombin generation obtained in the presence of rFVIIa alone initiated with either 1 pM TF (thick solid line) or 1/5 pM TF (dotted line) or initiated with 1 pM TF in the presence of either 100 nM FVII (thin solid line) or 100 nM FVIIR152A (dashed line). (C) The effect of FVII, FVIIR152A, and reduced TF level on the thrombin generation profile at 6 nM FVIIa. Same setup as in B, but initiation was with 1/15 pM TF in the low-TF sample (dotted line). (D) The effect of endogenous level (10 nM) of FVII on the thrombin generation profile at therapeutic concentrations of rFVIIa. Thin solid and dotted lines depict the results with 6 nM rFVIIa, and thick solid and dashed lines are the corresponding results with 25 nM rFVIIa, in the absence and presence of 10 nM FVII, respectively. The presence of 100 nM FVIIR152A together with 6 or 25 nM FVIIa reduced the functional TF density as measured by a significant decrease in the thrombin generation, but still no inhibitory effect of 10 nM zymogen FVII could be revealed (data not shown). In B-D, the thrombin generation profiles were normalized for easier comparison, and a peak level of 1 was assigned to the samples initiated with 1 pM TF without added zymogen FVII. Data are mean of repeated experiments (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/20/10.1182_blood-2014-05-576892/4/m_3172f1.jpeg?Expires=1769180226&Signature=10fRbB5~0gSAfAaAwAluedGr9VWPo-ElRD6XiHeDEohIXfr8yhCHdijsYrxUOrBwg~YDFaT2fgXOOBHqfYE-FP8PjobdeQh2Lx8u4yQ2qrSVTRl4VM8nkXTvXxtb17XV3HRT-Dse4O9fjs5vQg8rxsnvQzOw3QOT-bMolx7r-6Q164Xuaq-cGtmx8yEb7hFfVVjQarV2NaDxjN1PT1jesKltUnYZtczznjN8DOQM0R0mfz4bvxrVKXtVqDmsPCiJ2~Zmx8SizwgBrz5fkwOUa321Qh1ViYe-S5AWtyUNAHyaTHb1TZ37DzNvSAUHKTOadxsgguU0oqCfa6kYSbumZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)