Key Points
Smac mimetic and GCs synergize to induce apoptosis in ALL cells in vitro and in vivo.
Smac mimetic and GCs cooperate to deplete IAP proteins and to trigger formation of a RIP1/FADD/caspase-8 complex (ripoptosome).
Abstract
Apoptosis resistance contributes to poor outcome in pediatric acute lymphoblastic leukemia (ALL). Here, we identify a novel synergistic combination of Smac mimetic BV6 and glucocorticoids (GCs) (ie, dexamethasone, prednisolone) to trigger apoptosis in ALL cells. BV6 and GCs similarly cooperate to induce apoptosis in patient-derived leukemia samples, underlining the clinical relevance. Importantly, BV6/dexamethasone cotreatment is significantly more effective than monotherapy to delay leukemia growth in a patient-derived xenograft model of pediatric ALL without causing additional side effects. In contrast, BV6 does not increase cytotoxicity of dexamethasone against nonmalignant peripheral blood lymphocytes, mesenchymal stromal cells, and CD34-positive hematopoietic cells. We identify a novel mechanism by showing that BV6 and dexamethasone cooperate to deplete cIAP1, cIAP2, and XIAP, thereby promoting assembly of the ripoptosome, a RIP1/FADD/caspase-8-containing complex. This complex is critical and is required for BV6/dexamethasone-induced cell death, because RIP1 knockdown reduces caspase activation, reactive oxygen species production, and cell death. Ripoptosome formation occurs independently of autocrine/paracrine loops of death receptor ligands, because blocking antibodies for TNFα, tumor necrosis factor-related apoptosis-inducing ligand, or CD95 ligand or knockdown of death receptors fail to rescue BV6/dexamethasone-induced cell death. This is the first report showing that BV6 sensitizes for GC-triggered cell death by promoting ripoptosome formation with important implications for apoptosis-targeted therapies of ALL.
Introduction
ALL represents the number 1 malignancy in childhood.1 Despite major achievements, the prognosis for very high-risk or relapsed patients remains poor.1,2 For decades, GCs have represented a central element of treatment protocols for pediatric ALL because of their ability to kill leukemic blasts efficiently.3 However, GC resistance occurs in a fraction of children with ALL and remains a key unsolved obstacle, because it is associated with poor prognosis and outcome.3 Because the susceptibility of childhood ALL to steroids has been linked to GC-triggered apoptosis,4 novel treatment approaches to overcome GC resistance may have to include strategies to reactivate apoptosis.
Apoptosis, one of the best characterized forms of programmed cell death,5 plays an important role in the lymphohematopoietic system.6,7 Two key apoptosis-signaling pathways exist, that is, the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway.8 Second mitochondria-derived activator of caspases (Smac) is released from the mitochondria into the cytosol,9 and it promotes caspase-3 activation by binding to and antagonizing the X-linked inhibitor of apoptosis protein (XIAP).10 Evasion from cell death is one of the hallmarks of human cancers and a major cause of treatment resistance.11 For example, antiapoptotic proteins such as “inhibitor of apoptosis” (IAP) proteins are expressed at aberrantly high levels in cancers, which has been associated with poor outcome and treatment resistance.10 In childhood ALL, elevated protein levels of XIAP have been linked to poor prednisone response,12 and high cellular inhibitor of apoptosis protein-1 (cIAP1) expression was detected in pediatric acute leukemia samples.13 XIAP inhibits caspase activation by binding caspase-9 and -3/-7 via their baculovirus IAP repeat (BIR) domains,14 whereas cIAP1 and cIAP2 are predominately involved in regulating NF-κB signaling via their really interesting new gene (RING) domain that exhibits E3 ubiquitin ligase activity and mediates (auto)ubiquitination.10 Whereas ubiquitinated receptor-interacting protein (RIP)1 mediates TNFα-stimulated NF-κB activation, deubiquitinated RIP1 promotes TNFα-induced cell death by enhancing the formation of complex II that contains RIP1/Fas-associated protein with death domain (FADD)/caspase-8.15 In addition, a cytosolic RIP1/FADD/caspase-8-containing cell death platform can assemble independently of death receptors on depletion of IAP proteins and is then termed “ripoptosome.”16-18
Because of their key role in the regulation of cell death and survival signaling, IAP proteins have been at the center of interest for the design of molecular targeted cancer therapeutics. To this end, small-molecule inhibitors of IAP proteins including Smac mimetics have been developed that trigger autoubiquitination and proteasomal degradation of IAP proteins with a RING domain.19-21 Data from preclinical cancer models suggest that the therapeutic potential of Smac mimetics may particularly reside in rational combination regimens.10 Several Smac mimetics are currently undergoing evaluation in early clinical trials for the treatment of cancer alone and in combination with other anticancer agents.22 We previously reported that small-molecule IAP inhibitors enhance death receptor- or chemotherapy-induced apoptosis in childhood leukemia.23-25 However, the question of whether or not targeting IAP proteins represents a suitable strategy to bypass GC resistance in ALL has not yet been answered. Therefore, in the present study we investigated the question of whether or not neutralization of XIAP, cIAP1, and cIAP2 by the small-molecule Smac mimetic BV619 can enhance the antileukemic activity of GC against childhood ALL.
Methods
Cell culture
The local Ethical Committee approved the study and informed consent was obtained in accordance with the Declaration of Helsinki. Leukemia cells, human mesenchymal cells (MSCs), human G-CSF mobilized CD34+ (immature) hematopoietic cells, monocytes, and dendritic cells were cultured as described previously23,25 or as described in supplemental Methods (available on the Blood Web site; see the supplemental Materials link at the top of the online article). Leukemia blasts were derived from children with ALL at initial diagnosis before the onset of therapy at the University Children's Hospital Frankfurt, isolated using Ficoll Isopaque (Amersham Bioscience, Freiburg, Germany), and stimulated in X-VIVO Medium (Lonza, Walkersville, MD) supplemented with 10% FCS and 0.5% penicillin/streptomycin. Gene silencing was performed as described26 using pGIPZ-shRNAmir vectors (Thermo Fisher Scientific, Dreieich, Germany) or small interfering RNA (siRNA) (Invitrogen, Karlsruhe, Germany) following the manufacturer's instructions. Chemicals, antibodies, shRNAs, and siRNAs are listed in supplemental Methods.
Cell death, mitochondrial perturbations, western blotting, and immunoprecipitation
Apoptosis was determined by flow cytometric analysis (FACSCanto II, BD Biosciences) of DNA fragmentation of propidium iodide (PI)-stained nuclei or by forward/side scatter analysis as described.27 Mitochondrial membrane potential and Bax/Bak activation were examined as described,28 reactive oxygen species (ROS) production by flow cytometry using 1 µM CellROX or 5 µM MitoSox (Invitrogen). Western blotting27 and immunoprecipitation25 were done as described.
Patient-derived xenograft model of pediatric ALL
To establish an in vivo mouse model of childhood ALL, patient-derived leukemic blasts were subcutaneously injected into the flank of male nonobese diabetic/severe combined immunodeficient (NOD/SCID) IL2Rγ−/− (NSG) mice.29 For the present experiment, one of these xenograft models, the ALL-SCID 4, was selected.29 All animal experiments were approved by local responsible authorities (LaGeSo) and were performed according to guidelines for the welfare and use of animals in cancer research.30
Statistical analysis
Statistical significance was assessed by Student's t test. Drug interactions were analyzed by the combination index (CI) method based on that described by Chou31 using CalcuSyn software (Biosoft, Cambridge, United Kingdom). CI < 0.9 indicates synergism, 0.9-1.1 additivity, >1.1 antagonism.
Results
Smac mimetic and dexamethasone cooperate to induce cell death in ALL cell lines and primary ALL blasts
To investigate the potential of Smac mimetic BV6 that neutralizes XIAP, cIAP1, and cIAP219 to augment GC-induced cell death in ALL cells, we used T-cell (Jurkat, Molt-4, Molt-8, CEM C1-15) and B-cell precursor (Reh, MHH-cALL4, Tanoue) ALL cell lines. Importantly, BV6 significantly enhanced dexamethasone- or prednisolone-triggered cell death in T-cell and B-cell precursor ALL cell lines in a dose-dependent manner (Figure 1A-B). Calculation of CI showed that the drug interaction of BV6 with either dexamethasone or prednisolone is highly synergistic at multiple concentrations (supplemental Table 1). Because dexamethasone turned out to be superior to prednisolone to synergize with BV6 to trigger cell death in ALL cells, we focused subsequent studies on dexamethasone. To confirm that BV6 and dexamethasone induce apoptotic cell death, we also used another assay. Similarly, BV6 and dexamethasone acted in concert to trigger DNA fragmentation (supplemental Figure 1A). In addition, BV6 cooperated with dexamethasone to reduce significantly clonogenic growth of leukemia cells (Figure 1C).
Smac mimetic and dexamethasone cooperate to induce cell death in ALL cell lines. (A-B) Cells were treated for 72 hours with indicated concentrations of dexamethasone (A) or prednisolone (B) and/or BV6. Cell death was determined by forward/side scatter analysis and flow cytometry. (C) Clonogenic survival was assessed by colony assay after treatment with dexamethasone (Jurkat: 300 µM, Molt-4: 200 µM) and/or BV6 (Jurkat: 7 µM, Molt-4: 5 µM). The percentage of colonies relative to solvent control is shown. Mean and standard deviation (SD) of 3 experiments performed in triplicate are shown; *P < .05; **P < .01.
Smac mimetic and dexamethasone cooperate to induce cell death in ALL cell lines. (A-B) Cells were treated for 72 hours with indicated concentrations of dexamethasone (A) or prednisolone (B) and/or BV6. Cell death was determined by forward/side scatter analysis and flow cytometry. (C) Clonogenic survival was assessed by colony assay after treatment with dexamethasone (Jurkat: 300 µM, Molt-4: 200 µM) and/or BV6 (Jurkat: 7 µM, Molt-4: 5 µM). The percentage of colonies relative to solvent control is shown. Mean and standard deviation (SD) of 3 experiments performed in triplicate are shown; *P < .05; **P < .01.
To validate these results in primary ALL samples, we extended our study to freshly isolated leukemic blasts derived from children with ALL before the onset of chemotherapy (supplemental Table 2). Importantly, BV6 sensitized primary leukemic blasts for dexamethasone-triggered cell death in all analyzed patient samples, including children with poor prednisone response or high-risk disease (Figure 2A; supplemental Table 2). To evaluate the in vivo efficacy of BV6 and dexamethasone, we used a patient-derived xenograft model of childhood ALL established from a primary human sample. Importantly, BV6/dexamethasone cotreatment was significantly more effective to delay growth of xenografted human primary leukemia compared with dexamethasone or BV6 alone (Figure 2B) without causing additional side effects. These results in patient-derived clinical samples and in an in vivo mouse model of childhood ALL highlight the potential clinical relevance of our findings.
Smac mimetic and dexamethasone cooperate to induce cell death in primary ALL blasts but not in nonmalignant cells of the lymphohematopoietic system. (A) Primary leukemic blasts from children with ALL before the onset of chemotherapy were treated for 48 hours with indicated concentrations of dexamethasone and/or BV6. Cell death was determined by forward/side scatter analysis and flow cytometry. The percentage of specific cell death was calculated as follows: 100 × [experimental cell death (%) − spontaneous cell death (%)]/[100% − spontaneous cell death (%)]. Mean of 1 experiment performed in triplicate is shown. (B) One fragment each of the human ALL-SCID4 was subcutaneously transplanted into the flank of NSG mice. Treatment started on day 21 after tumor transplantation with 6 mg/kg BV6 intravenously every fourth day and/or 1 mg/kg dexamethasone orally 5 days/week. Mice were killed when tumors reached a volume exceeding 1.5 cm3. Because of complete remissions in the dexamethasone groups, treatment was stopped at day 33, and only BV6 therapy was continued until day 75. Tumor growth and body weight were monitored twice per week. Tumor volumes were calculated by [length × (width)2] × 0.5, and mean values per group were determined. (Left): Mean tumor volume with standard error of the mean; statistical comparisons between dexamethasone- and dexamethasone/BV6-treated groups were performed with the Mann-Whitney U test; *P < .05. (Right): Tumors of dexamethasone- and dexamethasone/BV6-treated groups were taken at day 75 and photographed. (C) PBLs of 2 distinct donors were treated for 48 hours with indicated concentrations of BV6 and/or 200 µM dexamethasone. Cell death was determined by forward/side scatter analysis and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (D) MSCs of 2 distinct donors were treated for 48 hours with 200 µM dexamethasone and/or 7 µM BV6. As positive control (PC), MSCs were treated with 10 µM Actinomycin D. Cell death was determined by PI uptake and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (E) Clonogenicity of human CD34+ hematopoietic cells treated with 300 µM dexamethasone and/or 7 µM BV6 was assessed by colony-forming assay. The percentage of colony-forming unit-granulomonocyte, colony-forming unit-multipotent, and burst-forming unit-erythroid relative to untreated controls with mean and SD of 3 experiments performed in triplicate are shown. (F) Monocyte-derived iDCs were generated by cultivation for 7 days with GM-CSF/IL4. iDCs were treated for 72 hours with 5 µM BV6 and/or 300 µM dexamethasone. As PC, iDCs were treated with 300 ng/mL TNFα as a potent trigger of iDCs maturation. Maturation was analyzed by fluorescence-activated cell sorter for cell surface expression of CD86 and is shown relative to untreated controls with mean and SD of 3 different experiments performed with iDCs from 3 different donors.
Smac mimetic and dexamethasone cooperate to induce cell death in primary ALL blasts but not in nonmalignant cells of the lymphohematopoietic system. (A) Primary leukemic blasts from children with ALL before the onset of chemotherapy were treated for 48 hours with indicated concentrations of dexamethasone and/or BV6. Cell death was determined by forward/side scatter analysis and flow cytometry. The percentage of specific cell death was calculated as follows: 100 × [experimental cell death (%) − spontaneous cell death (%)]/[100% − spontaneous cell death (%)]. Mean of 1 experiment performed in triplicate is shown. (B) One fragment each of the human ALL-SCID4 was subcutaneously transplanted into the flank of NSG mice. Treatment started on day 21 after tumor transplantation with 6 mg/kg BV6 intravenously every fourth day and/or 1 mg/kg dexamethasone orally 5 days/week. Mice were killed when tumors reached a volume exceeding 1.5 cm3. Because of complete remissions in the dexamethasone groups, treatment was stopped at day 33, and only BV6 therapy was continued until day 75. Tumor growth and body weight were monitored twice per week. Tumor volumes were calculated by [length × (width)2] × 0.5, and mean values per group were determined. (Left): Mean tumor volume with standard error of the mean; statistical comparisons between dexamethasone- and dexamethasone/BV6-treated groups were performed with the Mann-Whitney U test; *P < .05. (Right): Tumors of dexamethasone- and dexamethasone/BV6-treated groups were taken at day 75 and photographed. (C) PBLs of 2 distinct donors were treated for 48 hours with indicated concentrations of BV6 and/or 200 µM dexamethasone. Cell death was determined by forward/side scatter analysis and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (D) MSCs of 2 distinct donors were treated for 48 hours with 200 µM dexamethasone and/or 7 µM BV6. As positive control (PC), MSCs were treated with 10 µM Actinomycin D. Cell death was determined by PI uptake and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (E) Clonogenicity of human CD34+ hematopoietic cells treated with 300 µM dexamethasone and/or 7 µM BV6 was assessed by colony-forming assay. The percentage of colony-forming unit-granulomonocyte, colony-forming unit-multipotent, and burst-forming unit-erythroid relative to untreated controls with mean and SD of 3 experiments performed in triplicate are shown. (F) Monocyte-derived iDCs were generated by cultivation for 7 days with GM-CSF/IL4. iDCs were treated for 72 hours with 5 µM BV6 and/or 300 µM dexamethasone. As PC, iDCs were treated with 300 ng/mL TNFα as a potent trigger of iDCs maturation. Maturation was analyzed by fluorescence-activated cell sorter for cell surface expression of CD86 and is shown relative to untreated controls with mean and SD of 3 different experiments performed with iDCs from 3 different donors.
Smac mimetic does not increase the toxicity of dexamethasone against nonmalignant cells of the lymphohematopoietic system
To investigate whether BV6/dexamethasone cotreatment is cytotoxic to normal lymphohematopoietic cells, we extended our experiments to peripheral blood lymphocytes (PBLs), mesenchymal stromal cells (MSCs), G-CSF mobilized CD34+ (immature) hematopoietic cells, peripheral blood monocytes, and monocyte-derived immature dendritic cells (iDCs) that were all freshly isolated from healthy human donors. BV6 did not augment dexamethasone-induced cell death in PBLs or MSCs at equimolar concentrations that synergized to trigger apoptosis in ALL cells (Figure 2C-D) and did not increase the cytotoxicity of dexamethasone against human CD34+ hematopoietic cells (Figure 2E). BV6/dexamethasone cotreatment did not alter CD86 surface expression of iDCs, which was used as maturation marker, and did not cooperate to trigger apoptosis in peripheral blood monocytes, whereas BV6 alone moderately induced apoptosis (Figure 2F; supplemental Figure 2B), in line with a recent report.32 Together, this set of experiments demonstrates that the BV6 sensitizes ALL cells including primary leukemic blasts but not several normal lymphohematopoietic cell types to dexamethasone-induced cell death.
Smac mimetic and dexamethasone cooperate to induce caspase activation and mitochondrial perturbations
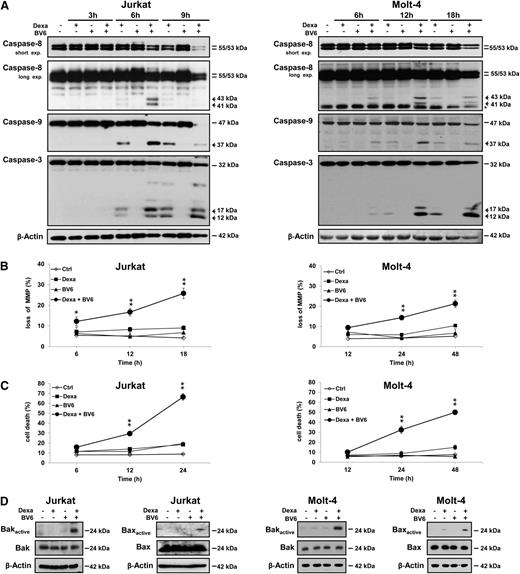
Next, we monitored activation of the caspase cascade. BV6 substantially enhanced dexamethasone-induced processing of caspase-8, caspase-9, and caspase-3 into active cleavage fragments accompanied by decreased expression of caspase proenzyme forms, a parameter of their proteolytic turnover (Figure 3A). Notably, BV6 and dexamethasone acted together to trigger caspase-3 cleavage in primary ALL blasts (supplemental Figure 1B), underlining the clinical relevance of this finding. Furthermore, BV6 and dexamethasone cooperated to induce loss of mitochondrial membrane potential (MMP) (Figure 3B). Kinetic analysis showed that BV6/GC-induced activation of caspases and mitochondrial perturbations preceded the time-dependent induction of apoptosis (Figure 3C). Also, BV6 cooperated with dexamethasone to cause Bak and Bax activation (Figure 3D) and cytochrome c release from mitochondria (supplemental Figure 2). These findings demonstrate that BV6 and dexamethasone cooperate to induce caspase activation and mitochondrial perturbations.
Smac mimetic and dexamethasone cooperate to induce caspase activation and mitochondrial perturbations. Cells were treated for indicated times (A-C) or for 5 hours (D, Jurkat) or 6 hours (D, Molt-4) with dexamethasone (Jurkat: 300 µM, Molt-4: 200 µM) and/or BV6 (Jurkat: 7 µM, Molt-4: 5 µM). (A) Caspase activation was analyzed by western blotting, active cleavage fragments are indicated by arrowheads, and unspecific bands by asterisk. (B) MMP was assessed by flow cytometry. (C) Cell death was determined by forward/side scatter analysis and flow cytometry. (D) Bak and Bax activation was determined by immunoprecipitation using active conformation-specific antibodies. (B-C) Mean and SD of 3 experiments performed in triplicate are shown; *P < .05; **P < .01 comparing BV6/dexamethasone-treated to control cells.
Smac mimetic and dexamethasone cooperate to induce caspase activation and mitochondrial perturbations. Cells were treated for indicated times (A-C) or for 5 hours (D, Jurkat) or 6 hours (D, Molt-4) with dexamethasone (Jurkat: 300 µM, Molt-4: 200 µM) and/or BV6 (Jurkat: 7 µM, Molt-4: 5 µM). (A) Caspase activation was analyzed by western blotting, active cleavage fragments are indicated by arrowheads, and unspecific bands by asterisk. (B) MMP was assessed by flow cytometry. (C) Cell death was determined by forward/side scatter analysis and flow cytometry. (D) Bak and Bax activation was determined by immunoprecipitation using active conformation-specific antibodies. (B-C) Mean and SD of 3 experiments performed in triplicate are shown; *P < .05; **P < .01 comparing BV6/dexamethasone-treated to control cells.
Smac mimetic and dexamethasone cooperate to trigger ROS production
Next, we monitored ROS production in viable cells before they succumb to BV6/dexamethasone-induced cell death using the fluorescent dye CellROX. BV6 acted together with dexamethasone to increase ROS generation significantly (supplemental Figure 3A). Similar results were obtained using the fluorescent dye MitoSOX, which detects mitochondrial ROS production (supplemental Figure 3B). Interestingly, the addition of MnTBAP, a cell-permeable superoxide dismutase mimetic and peroxynitrite scavenger,33 significantly decreased ROS generation and cell death upon cotreatment (supplemental Figure 3B-C). Also, MnTBAP reduced Bak activation and caspase-3 cleavage in cotreated cells (supplemental Figure 3D-E). Similarly, additional ROS scavengers (ie, catalase, N-acetyl-cysteine) significantly decreased BV6/dexamethasone-triggered cell death (supplemental Figure 3F-G). Together, this indicates that ROS production contributes to BV6/GC-induced cell death.
Smac mimetic and dexamethasone cooperate to downregulate IAP protein levels
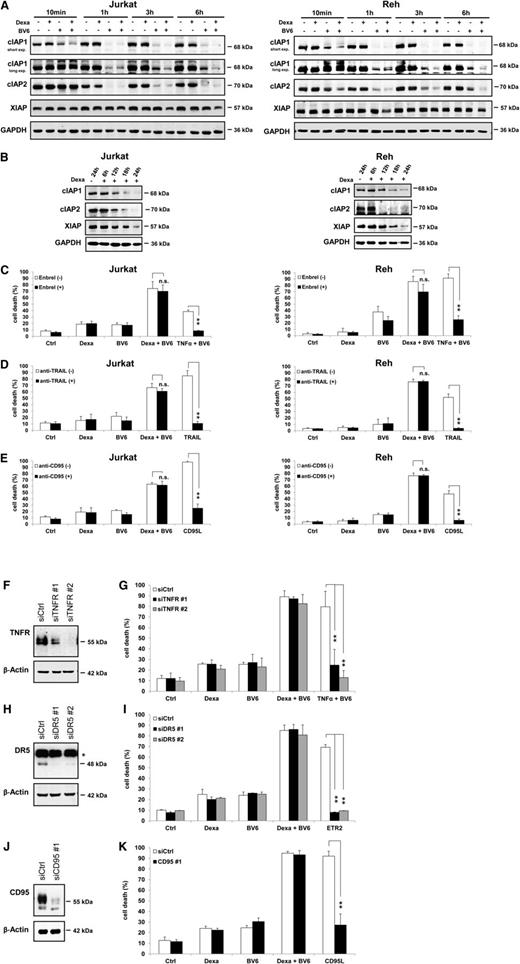
Next, we examined IAP protein levels, as BV6 and dexamethasone have been implicated in modulating IAP proteins.19-21,34-36 Intriguingly, we found that BV6 and dexamethasone acted in concert to downregulate cIAP1, cIAP2, and XIAP (Figure 4A). The addition of dexamethasone to BV6 further decreased cIAP1 protein levels after 10 minutes compared with BV6 alone, and this cooperative downregulation of cIAP1 by dexamethasone/BV6 was continuously observed throughout the subsequent hours (Figure 4A). BV6 alone downregulated cIAP1 and cIAP2 less efficiently than the BV6/dexamethasone combination (Figure 4A), dexamethasone alone reduced cIAP1, cIAP2, and XIAP levels on prolonged incubation (Figure 4B). To investigate whether this downregulation of IAP proteins by BV6/dexamethasone occurs via proteasomal degradation and/or caspase-mediated cleavage, we performed rescue experiments using the proteasome inhibitor bortezomib and/or the broad-range caspase inhibitor zVAD.fmk. Interestingly, bortezomib or bortezomib and zVAD.fmk attenuated the BV6/dexamethasone-stimulated depletion of cIAP1, cIAP2, and XIAP (supplemental Figure 4), indicating that proteasomal degradation is involved.
Smac mimetic and dexamethasone cooperate to downregulate IAP protein levels. (A-B) Cells were treated for indicated times with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) (A) or with 300 µM dexamethasone (B). Protein expression of cIAP1, cIAP2, and XIAP was analyzed by western blotting after short or long exposure (exp.) times of blots. (C-E) Cells were treated for 24 hours (Jurkat) or 72 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) in the presence or absence of 10 µg/mL Enbrel (C), 1 µg/mL anti-TRAIL antibody (D), or 5 µg/mL anti-CD95 antibody (E); treatments with 1 ng/mL TNFα and BV6 (Jurkat: 7 µM, Reh: 0.3 µM) (C), 5 ng/mL TRAIL (D), or 40 ng/mL hexameric CD95 ligand (CD95L) were used as PCs to demonstrate the effectiveness of blocking antibodies. (F-K) Jurkat cells were transfected with nonsilencing control siRNA (siCtrl) or siRNA against TNFR1 (siTNFR#1, siTNFR#2), DR5 (siDR5#1, siDR5#2), or CD95 (siCD95#1). Expression of death receptors was analyzed by western blotting, and asterisk indicates unspecific bands (F,H,J). Cells were treated for 24 hours with 300 µM dexamethasone and/or 7 µM BV6; treatments with 1 ng/mL TNFα and 1 µM BV6 (G), 1 μg/mL DR5 agonistic antibody Lexatumumab (ETR2) (I), or 40 ng/mL hexameric CD95 ligand (CD95L) (K) were used as PCs to demonstrate the efficacy of gene silencing. Cell death was determined by forward/side scatter analysis and flow cytometry. Mean and SD of 3 experiments performed in triplicate are shown; **P < .01; n.s., not significant.
Smac mimetic and dexamethasone cooperate to downregulate IAP protein levels. (A-B) Cells were treated for indicated times with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) (A) or with 300 µM dexamethasone (B). Protein expression of cIAP1, cIAP2, and XIAP was analyzed by western blotting after short or long exposure (exp.) times of blots. (C-E) Cells were treated for 24 hours (Jurkat) or 72 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) in the presence or absence of 10 µg/mL Enbrel (C), 1 µg/mL anti-TRAIL antibody (D), or 5 µg/mL anti-CD95 antibody (E); treatments with 1 ng/mL TNFα and BV6 (Jurkat: 7 µM, Reh: 0.3 µM) (C), 5 ng/mL TRAIL (D), or 40 ng/mL hexameric CD95 ligand (CD95L) were used as PCs to demonstrate the effectiveness of blocking antibodies. (F-K) Jurkat cells were transfected with nonsilencing control siRNA (siCtrl) or siRNA against TNFR1 (siTNFR#1, siTNFR#2), DR5 (siDR5#1, siDR5#2), or CD95 (siCD95#1). Expression of death receptors was analyzed by western blotting, and asterisk indicates unspecific bands (F,H,J). Cells were treated for 24 hours with 300 µM dexamethasone and/or 7 µM BV6; treatments with 1 ng/mL TNFα and 1 µM BV6 (G), 1 μg/mL DR5 agonistic antibody Lexatumumab (ETR2) (I), or 40 ng/mL hexameric CD95 ligand (CD95L) (K) were used as PCs to demonstrate the efficacy of gene silencing. Cell death was determined by forward/side scatter analysis and flow cytometry. Mean and SD of 3 experiments performed in triplicate are shown; **P < .01; n.s., not significant.
Smac mimetic/dexamethasone-induced cell death occurs independently of death receptor ligands
To investigate whether the observed cIAP protein depletion engages a TNFα-driven autocrine/paracrine loop that mediates BV6/dexamethasone-induced cell death,19,20,34,37 we used the TNFα-blocking antibody Enbrel. Enbrel failed to rescue BV6/dexamethasone-induced cell death, whereas it prevented TNFα/BV6-stimulated cell death used as a PC (Figure 4C). Similarly, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-blocking or CD95-blocking antibodies did not protect against BV6/dexamethasone-induced apoptosis, even at high concentrations and under conditions in which they inhibited TRAIL- or CD95 ligand-induced cell death (Figure 4D-E), confirming that these blocking antibodies were effective. Also, knockdown of TNFR1, DR5 (TRAIL receptor 2), or CD95 did not rescue BV6/dexamethasone-mediated cell death, although corresponding PCs confirmed that cells were protected against TNFα-, TRAIL-, or CD95 ligand-induced cell death (Figure 4F-K). This set of experiments indicates that BV6/dexamethasone-induced cell death occurs independently of death receptors and their ligands.
Because NF-κB has been implicated in BV6-induced cell death,38 we investigated the involvement of NF-κB using IKKγ-deficient Jurkat cells. NF-κB inhibition was confirmed by the lack of IκBα phosphorylation after TNFα stimulation and reduced expression of NF-κB target genes such as IκBα, RelB, and cIAP2 (supplemental Figure 5A). IKKγ-deficient cells showed no significant difference in BV6/dexamethasone-induced cell death (supplemental Figure 5B), demonstrating that NF-κB is not involved in BV6/dexamethasone-mediated cell death.
Smac mimetic and dexamethasone cooperate to trigger ripoptosome formation
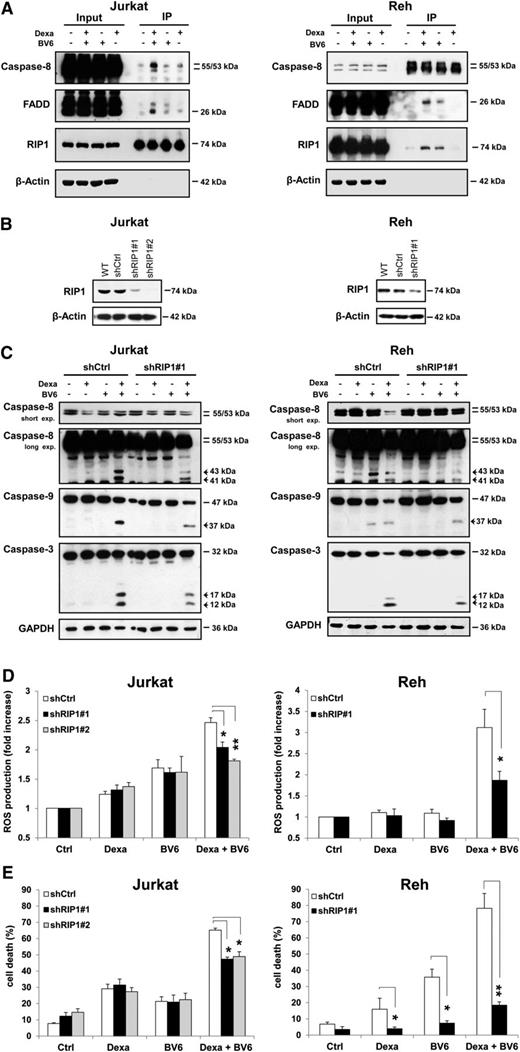
cIAP protein depletion by Smac mimetics has been shown to promote the interaction of RIP1 with FADD and caspase-8 to form a cell death signaling complex.19,20,34,37 Therefore, we examined by immunoprecipitation the interaction partners of RIP1 on BV6/dexamethasone cotreatment. Importantly, BV6 cooperated with dexamethasone to stimulate formation of a cytosolic complex containing RIP1, FADD, and caspase-8 (Figure 5A). To explore whether RIP1 is required for cell death, we silenced RIP1 (Figure 5B). Interestingly, RIP1 knockdown diminished BV6/dexamethasone-induced activation of caspase-8, caspase-9, and caspase-3 (Figure 5C). Also, RIP1 silencing significantly decreased BV6/dexamethasone-mediated ROS production (Figure 5D). Most importantly, RIP1 knockdown significantly reduced BV6/dexamethasone-induced cell death (Figure 5E). Similarly, caspase-8 deficiency significantly decreased BV6/dexamethasone-triggered ROS production and activation of caspase-9 and caspase-3 (supplemental Figure 6). This indicates that RIP1 is a critical mediator of BV6/dexamethasone-induced cell death by promoting assembly of a RIP1/FADD/caspase-8 complex that drives caspase-8 activation, ROS production, and cell death.
Smac mimetic and dexamethasone cooperate to trigger the formation of the ripoptosome, which is required for Smac mimetic-/dexamethasone-induced cell death. (A) Cells were treated for 6 hours with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) in the presence of 20 µM zVAD.fmk. Caspase-8 was immunoprecipitated (IP) using an anti-RIP1 antibody (Jurkat) or an anti-caspase-8 antibody (Reh), and western blotting detected the indicated proteins. (B-E) Cells were transduced with control vector (shCtrl) or vector containing shRNA sequences against RIP1 (shRIP1#1, shRIP1#2). Expression of RIP1 was analyzed by western blotting (B). In (C), cells were treated for 6 hours (Jurkat) or 12 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM), and caspase activation was analyzed by western blotting, active cleavage fragments are indicated by arrowheads. In (D), cells were treated for 4 hours (Jurkat) or 8 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM), and ROS production was determined by CellROX staining and flow cytometry and is shown as fold increase. In (E), cells were treated for 24 hours (Jurkat) or 72 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) and cell death was determined by forward/side scatter analysis and flow cytometry. In (D-E), mean and SD of 3 experiments performed in triplicate are shown; *P < .05; **P < .01.
Smac mimetic and dexamethasone cooperate to trigger the formation of the ripoptosome, which is required for Smac mimetic-/dexamethasone-induced cell death. (A) Cells were treated for 6 hours with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) in the presence of 20 µM zVAD.fmk. Caspase-8 was immunoprecipitated (IP) using an anti-RIP1 antibody (Jurkat) or an anti-caspase-8 antibody (Reh), and western blotting detected the indicated proteins. (B-E) Cells were transduced with control vector (shCtrl) or vector containing shRNA sequences against RIP1 (shRIP1#1, shRIP1#2). Expression of RIP1 was analyzed by western blotting (B). In (C), cells were treated for 6 hours (Jurkat) or 12 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM), and caspase activation was analyzed by western blotting, active cleavage fragments are indicated by arrowheads. In (D), cells were treated for 4 hours (Jurkat) or 8 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM), and ROS production was determined by CellROX staining and flow cytometry and is shown as fold increase. In (E), cells were treated for 24 hours (Jurkat) or 72 hours (Reh) with 300 µM dexamethasone and/or BV6 (Jurkat: 7 µM, Reh: 0.3 µM) and cell death was determined by forward/side scatter analysis and flow cytometry. In (D-E), mean and SD of 3 experiments performed in triplicate are shown; *P < .05; **P < .01.
Discussion
Evasion of apoptosis contributes to GC resistance and poor treatment response of pediatric high-risk ALL,39 highlighting the need for novel strategies to reactivate programmed cell death. In the present study, we discover that the Smac mimetic BV6 acts in concert with GC to trigger apoptosis in ALL cells. This synthetic lethal interaction occurs in a highly synergistic manner as demonstrated by the calculation of CI underlining the potency of this approach. In addition to increasing cell death in short-term assays, BV6 and GC act together to inhibit long-term clonogenic survival of ALL cells. The clinical relevance of this novel synergistic combination is underscored by parallel experiments in patient-derived primary leukemia samples, including poor prednisone and high-risk disease showing that BV6 and dexamethasone cooperate to induce cell death in primary leukemic blasts. Importantly, BV6 enhances dexamethasone’s antileukemic activity in vivo compared with dexamethasone alone in a patient-derived xenograft model of pediatric ALL without causing additional side effects.
The novelty of our study resides particularly in the following 2 points: First, we identify a new synergistic drug combination of the Smac mimetic BV6 together with GC to induce cell death in ALL cells. Because GCs represent a key element of current treatment protocols for ALL, the identification of this synergistic drug combination of BV6 and GC is particularly relevant for the design of future Smac mimetic-based combination therapies. Previously, we reported that small-molecule inhibitors of IAP proteins such as Smac mimetics act in concert with other cytotoxic stimuli, for example death receptor ligands such as TRAIL and CD95 ligand, chemotherapeutic drugs, or irradiation, in ALL or other cancer entities.24,25,38,40-43 Second, we identify a novel underlying molecular mechanism of this synergistic interaction by showing that BV6 and dexamethasone act in concert to trigger proteasomal degradation of IAP proteins, which in turn promotes the assembly of the ripoptosome, a cytosolic cell death complex (Figure 6). Several lines of evidence support this conclusion, as follows:
Cotreatment with BV6 and dexamethasone is more efficient than either treatment alone to stimulate degradation of cIAP1, cIAP2, and XIAP by the proteasome. This conclusion is supported by rescue experiments showing that the addition of the proteasome inhibitor bortezomib substantially attenuates the BV6/dexamethasone-stimulated depletion of IAP proteins.
BV6 and dexamethasone cooperate to trigger the depletion of cIAP proteins and the formation of the ripoptosome, a multimeric platform signaling cell death in the cytosol constituting RIP1, FADD, and caspase-8.
Formation of the ripoptosome is required for BV6/dexamethasone-induced cell death, because knockdown of RIP1 reduces caspase activation, ROS production, and induction of cell death. The notion that the assembly of this multiprotein complex is a proximal event during BV6/dexamethasone-induced cell death, which initiates activation of the caspase cascade, is further supported by data showing that RIP1 silencing reduces activation of both the initiator caspase-8 and the effector caspase-3.
Ripoptosome formation occurs independently of auto- or paracrine acting loops of death receptor ligands, because blocking antibodies for TNFα, TRAIL, or CD95 ligand or genetic silencing of their corresponding receptors fail to rescue BV6/dexamethasone-induced cell death under conditions in which blocking antibodies and knockdown of receptors fully prevent TNFα-, TRAIL-, or CD95 ligand-mediated cell death, thus confirming the efficacy of gene silencing.
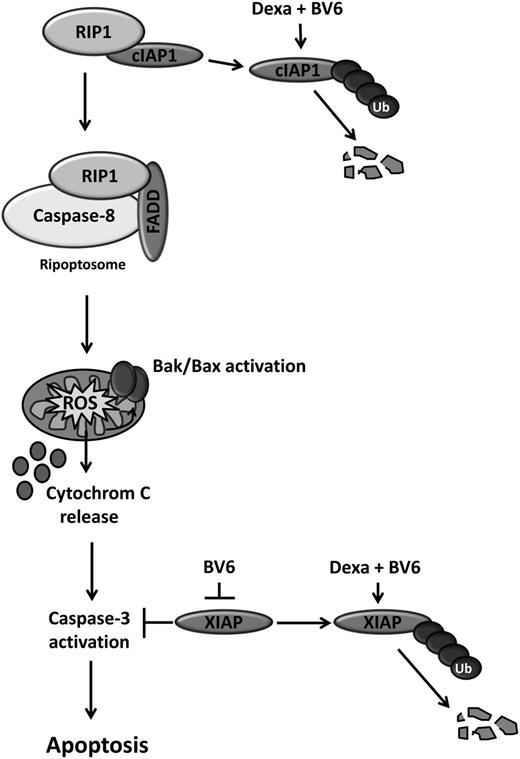
Scheme of synergistic induction of apoptosis by BV6/dexamethasone cotreatment. BV6 and dexamethasone cooperate to cause rapid downregulation of cIAP proteins, which in turn triggers the formation of the ripoptosome, a cytosolic complex of RIP1, FADD, and caspase-8. Ripoptosome assembly drives caspase-8 activation, leading to Bak activation, ROS production, cytochrome C release, caspase-3 activation, and cell death. Also, BV6 and dexamethasone act together to downregulate XIAP, which promotes caspase-3 activation and cell death. In addition, BV6 binds to and antagonizes XIAP.
Scheme of synergistic induction of apoptosis by BV6/dexamethasone cotreatment. BV6 and dexamethasone cooperate to cause rapid downregulation of cIAP proteins, which in turn triggers the formation of the ripoptosome, a cytosolic complex of RIP1, FADD, and caspase-8. Ripoptosome assembly drives caspase-8 activation, leading to Bak activation, ROS production, cytochrome C release, caspase-3 activation, and cell death. Also, BV6 and dexamethasone act together to downregulate XIAP, which promotes caspase-3 activation and cell death. In addition, BV6 binds to and antagonizes XIAP.
Smac mimetics such as BV6 have been shown to induce a conformational change in cIAP1 structure that promotes the E3 ubiquitin ligase activity of its RING domain, resulting in autoubiquitination and proteasomal degradation.44,45 Also, GCs have been implicated in regulating expression levels of IAP proteins; however, data are less consistent as to whether they positively or negatively affect IAP protein levels. Although dexamethasone was described to trigger proteasomal degradation of XIAP and cIAP1,46 GCs were also reported to upregulate cIAP2 levels in ovarian cancer cells35 and to act together with TNFα to induce cIAP2 expression in various cell types.36 Compared with these earlier reports, our study is the first to demonstrate the cooperativity of BV6 and GC to stimulate proteasomal degradation of cIAP1, cIAP2, and XIAP, thereby providing a new mechanistic explanation for the synergistic induction of cell death by both agents.
The assembly of the ripoptosome is constitutively prevented by cIAP proteins via K63-linked ubiquitination of RIP1, which inhibits the interaction of RIP1 with caspase-8 and FADD.34 Therefore, conditions that deplete cIAP proteins have been described as promoting ripoptosome formation, for example Smac mimetic, DNA-damaging drugs, cytokines exemplified by tumor necrosis factor-like weak inducer of apoptosis, or TLR3 stimulation.16,17,47,48 Besides these stimuli, little is currently known about additional signals that stimulate ripoptosome formation. Here, we identify GC as a trigger for ripoptosome formation together with the Smac mimetic BV6. By definition, the ripoptosome forms independently from death receptors and their ligands, such as TNFα, TRAIL, or CD95 ligand.16,17 Our data, showing that antagonistic antibodies against death receptor ligands or knockdown of death receptors fail to prevent BV6/dexamethasone-induced cell death, support the notion that the cotreatment indeed triggers ripoptosome assembly. The ripoptosome is critical to drive caspase activation during BV6/dexamethasone-induced cell death, because knockdown of the core component RIP1 reduces caspase activation and cell death. Also, neutralization of XIAP-imposed inhibition of effector caspases by BV6 might contribute to cell death induction, because BV6 antagonizes XIAP besides cIAP1 and cIAP2.19
Our findings have important implications for the future development of Smac mimetic-based treatment strategies in childhood ALL. First, IAP proteins represent promising molecular targets for therapeutic intervention in pediatric ALL, because high XIAP protein expression in leukemic blasts has been linked to poor prednisone response in T-ALL12 and because samples from children with acute leukemia have been reported to harbor high levels of cIAP1 protein.13 Second, the identification of this new synergistic drug interaction of the Smac mimetic BV6 together with GC is particularly relevant, because GCs constitute key components of current treatment protocols for children with ALL.3 The clinical relevance of this combination is underscored by our findings demonstrating that BV6/GC cotreatment synergizes to induce apoptosis in patient-derived primary leukemia samples, including poor prednisone response and high-risk disease, and cooperates to reduce the leukemic burden in a patient-derived xenograft mouse model of childhood ALL without causing additional side effects. Third, our data may point to a therapeutic window for Smac mimetic-mediated sensitization of leukemia cells, because BV6 differentially enhances GC-induced cell death in malignant leukemia cells, whereas it did not cooperate with dexamethasone to induce cell death in several normal cell types of the lymphohematopoietic system. The moderate induction of apoptosis by BV6 in monocytes might be a tolerable side effect, because monocytes can be reproduced from CD34+ cells, which we found were not affected by BV6/dexamethasone treatment, although it has to be considered for clinical studies. Monocytes have previously been described to undergo excessive apoptosis, for example on oxidative stress because of defective DNA repair mechanisms.49 Fourth, Smac mimetic may be used in combination regimens to lower the dose of GC required for antileukemic responses and thus may reduce GC-related toxicities, given the highly synergistic interaction of both drugs. Finally, in a broader perspective, Smac mimetic-based combination regimens together with GCs have implications for other cancers beyond childhood acute leukemia. Because Smac mimetics are currently tested in early clinical trials alone and in combinations,10 the identification of novel synergistic drug combinations is timely and clinically relevant and has important implications for apoptosis-targeted therapies for childhood ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank D. Vucic (Genentech Inc., South San Francisco, CA) for providing Smac mimetic, J. Leibacher for providing MSCs, and C. Hugenberg for expert secretarial assistance.
This work was partially supported by grants from the Deutsche Forschungsgemeinschaft, IAP VII, and Jose-Carreras Stiftung (S.F.).
Authorship
Contribution: K.B. performed and designed experiments, analyzed and interpreted data, and prepared the manuscript together with S.F.; H.S. performed experiments; S.W., H.B., and T.K. contributed to analysis of primary samples; A.W. contributed to experiments with monocytes and dendritic cells; I.F. designed and performed in vivo experiments; S.F. designed research, analyzed and interpreted data, supervised the project, and wrote the manuscript together with K.B.; and all authors approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simone Fulda, Institute for Experimental Cancer Research in Pediatrics, Goethe-University Frankfurt, Komturstrasse 3a, 60528 Frankfurt, Germany; e-mail: simone.fulda@kgu.de.


![Figure 2. Smac mimetic and dexamethasone cooperate to induce cell death in primary ALL blasts but not in nonmalignant cells of the lymphohematopoietic system. (A) Primary leukemic blasts from children with ALL before the onset of chemotherapy were treated for 48 hours with indicated concentrations of dexamethasone and/or BV6. Cell death was determined by forward/side scatter analysis and flow cytometry. The percentage of specific cell death was calculated as follows: 100 × [experimental cell death (%) − spontaneous cell death (%)]/[100% − spontaneous cell death (%)]. Mean of 1 experiment performed in triplicate is shown. (B) One fragment each of the human ALL-SCID4 was subcutaneously transplanted into the flank of NSG mice. Treatment started on day 21 after tumor transplantation with 6 mg/kg BV6 intravenously every fourth day and/or 1 mg/kg dexamethasone orally 5 days/week. Mice were killed when tumors reached a volume exceeding 1.5 cm3. Because of complete remissions in the dexamethasone groups, treatment was stopped at day 33, and only BV6 therapy was continued until day 75. Tumor growth and body weight were monitored twice per week. Tumor volumes were calculated by [length × (width)2] × 0.5, and mean values per group were determined. (Left): Mean tumor volume with standard error of the mean; statistical comparisons between dexamethasone- and dexamethasone/BV6-treated groups were performed with the Mann-Whitney U test; *P < .05. (Right): Tumors of dexamethasone- and dexamethasone/BV6-treated groups were taken at day 75 and photographed. (C) PBLs of 2 distinct donors were treated for 48 hours with indicated concentrations of BV6 and/or 200 µM dexamethasone. Cell death was determined by forward/side scatter analysis and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (D) MSCs of 2 distinct donors were treated for 48 hours with 200 µM dexamethasone and/or 7 µM BV6. As positive control (PC), MSCs were treated with 10 µM Actinomycin D. Cell death was determined by PI uptake and flow cytometry. Mean of 1 experiment performed in triplicate is shown. (E) Clonogenicity of human CD34+ hematopoietic cells treated with 300 µM dexamethasone and/or 7 µM BV6 was assessed by colony-forming assay. The percentage of colony-forming unit-granulomonocyte, colony-forming unit-multipotent, and burst-forming unit-erythroid relative to untreated controls with mean and SD of 3 experiments performed in triplicate are shown. (F) Monocyte-derived iDCs were generated by cultivation for 7 days with GM-CSF/IL4. iDCs were treated for 72 hours with 5 µM BV6 and/or 300 µM dexamethasone. As PC, iDCs were treated with 300 ng/mL TNFα as a potent trigger of iDCs maturation. Maturation was analyzed by fluorescence-activated cell sorter for cell surface expression of CD86 and is shown relative to untreated controls with mean and SD of 3 different experiments performed with iDCs from 3 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/2/10.1182_blood-2013-05-500918/4/m_240f2.jpeg?Expires=1767872775&Signature=umtMSZS8Y9Tha0wjl2H0oWMKq1gTFav4y-PXAGjad2pnHMb1oz2JOzJPD33eMuJnNGVTqZsHN7QqfkdPlrdk8OcQDMPIOhC883Y1RzNUIsTvu61BNK0YFwekjs9ITOwzdpasSyue82LTwEaOLimnHXnWJB2Ani8HN1RDFfnmYjLAe8DhllXIVFPR6psHHsCTtWI-xp8QWJWzfqu5QzGDyFuJYucVtRgO37RVfYjF1d-K7ZkVpyYvP5AKDHJM18ENXOuAvpGklfTnzTBnsDBDkvVe9BK6l5e8ichrkPuSSCBykbmHZZOiq7r3gm3rBQ0UWPDyxkPJeBQkPkH4ClU6yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal