Key Points
TP53 mutations are detected in 15.7% of patients with ALL and are correlated to a low hypodiploid karyotype and to MYC-translocations.
Disruption of both TP53 alleles is associated with adverse prognosis in ALL.
Abstract
TP53 is the most extensively studied gene in cancer. However, data on frequency and the prognostic impact of TP53 mutations in acute lymphoblastic leukemia (ALL) remain scarce. Thus, we aimed at identifying the mutation frequency of TP53, its association with cytogenetic subgroups, and its impact on survival in a large cohort of 625 patients with ALL. Our data revealed an overall mutation incidence of 15.7%, which increases with age. Correlation with cytogenetic subgroups showed that mutations were most frequent in ALL with low hypodiploidy or MYC-rearrangements. Furthermore, for a large number of patients, both TP53 alleles were altered, either by 2 TP53 mutations (12%) or by a TP53 mutation and a TP53 deletion in the second allele (39%). A high TP53 mutation load was correlated to low hypodiploidy, high hyperdiploidy, and a complex karyotype. Moreover, a higher mutation load was found in B-lineage ALL compared with T-lineage ALL. Similar to other cancers, the median overall survival was significantly shorter in patients with TP53 mutation compared with patients with wild-type TP53. This effect was especially pronounced when both TP53 alleles were affected, either by 2 TP53 mutations or by both a mutation and an accompanying TP53 deletion.
Introduction
Acute lymphoblastic leukemia (ALL) is a biologically and clinically heterogeneous disease that affects both children and adults. It is caused by uncontrolled proliferation of immature B- or T-lymphoid cells and occurs more frequently in the B-lineage (85% to 90% in children, ∼75% in adults) than in the T-lineage (10% to 15% in children, ∼25% in adults).1,2 ALL is the most common childhood tumor; however, progress in treatment development has led to a cure rate of >80% in children, in contrast to the situation in infants and adults, where prognosis remains poor.1 This is, among other factors, related to the types of genetic alterations of the disease, as the frequencies of particular genetic subtypes differ in children and adults and shift from aberrations with a favorable outcome to alterations associated with poor outcome with increasing age.3,4
Genetic alterations described in ALL often affect genes involved in cell proliferation, cell differentiation, or apoptosis and comprise activation of proto-oncogenes, inactivation of tumor suppressor genes, or creation of fusion genes, some of them encoding active kinases. The mechanisms underlying such alterations include chromosomal rearrangements, chromosome gains or losses, deletions, mutations, and (de)-methylation of the promoter regions of the respective genes.1,5,6 Alterations most commonly found in ALL include t(9;22)(q34;q11) BCR-ABL1 (mainly in adults), hyperdiploidy, t(12;21)(p13;q22) ETV6-RUNX1 (predominantly in children), and MLL- and MYC-rearrangements. In addition, multiple other genotypes have been described.1,4
The most frequently mutated gene in cancer is TP53, which is well characterized in other hematological malignancies, including acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL). However, the frequency and prognostic impact of TP53 mutations has only rarely been studied in ALL thus far. Mutations in or deletion of TP53 are generally associated with advanced stages of disease,7 insufficient response to therapy,8,9 and a poor prognosis.10,11 TP53 is a transcription factor and functions as key tumor suppressor and master regulator of various signaling pathways. Its importance is reflected by the fact that it is altered in ∼50% of human cancers.12,13 It is located on the short arm of chromosome 17 (17p13) and encodes for a nuclear phosphoprotein that is tetrameric in its functional form.14,15 The TP53 protein plays crucial roles in a variety of cellular processes, including cell cycle arrest, apoptosis, DNA repair, genomic stability, cell differentiation, and senescence.16-20 Inactivation of TP53 in tumors is supposed to be most frequently generated by missense mutations in the coding region or by deletions of the TP53 gene (17p13 deletion), although hypermethylation of the promoter region resulting in a decrease of TP53 transcription has also been reported.14,21 It was conclusively shown that TP53 deletions are often associated with TP53 mutations of the second allele.6,13 This supports the “two-hit” hypothesis implying that both copies of a tumor-suppressor gene have to be altered for cancer development.6 The cancer-associated TP53 mutations are very diverse in their locations within the TP53 sequence. However, they most frequently result in disruption of the specific DNA-binding ability of TP53.22
In ALL, TP53 mutations have so far been considered to be rather infrequent13 (2% to 3%), although other studies suggest a much higher percentage of TP53 alterations (30% to 40%), if analyses do not merely focus on TP53 mutations and deletions but also include promoter hypermethylation.6 Generally, TP53 mutations mainly have been investigated in children, where they most frequently occur at relapse and are associated with poor outcome.23,24 The proposed role of TP53 mutations in disease relapse might be due to the selective advantage of the respective cells caused by their resistance to therapy.6 For TP53 mutations in adult ALL, only a few studies are available that include mostly relapsed cases and small cohorts of patients.25,26 Thus far, the largest series of adult ALL investigated for TP53 mutations includes 98 adult patients2 and shows TP53 mutations in 8 patients (8.2%), which is similar to the TP53 mutation frequency in AML at diagnosis and in CLL at the time of progression.10,11 Furthermore, the results support previous findings of an increase of TP53 mutations at disease reappearance.23,25,26 Moreover, TP53 mutations were found to be more frequent in T-lineage ALL than in B-lineage ALL and were detected in 14% of cases negative for recurrent fusion genes.2
As data on incidence and prognostic significance of TP53 mutations in ALL remains scarce to date, we aimed to determine the TP53 mutation frequency, the association with cytogenetic subgroups and age, as well as the impact on survival in a large cohort of 625 ALL patients.
Methods
Patient cohort
A total of 625 patients newly diagnosed with ALL was studied. Patient samples were sent between August 2005 and May 2013 for diagnosis to the Munich Leukemia Laboratory, and patients agreed with the use of laboratory data for research studies. The study was approved by the institutional review board. The study was conducted in accordance with the Declaration of Helsinki. The cohort comprised 353 male and 272 female patients and the median age was 49.5 years (range 0.1-91.4 years; supplemental Figure 1, available on the Blood Web site). It has to be noted that the presented cohort comprises a relatively low proportion of childhood ALL, which is generally known to constitute a large number of ALL cases. This study includes 24 cases that were analyzed in a study on ALL with low hypodiploid/near-triploid karyotype.27 For analysis of the overall survival (OS), patients with an available detailed immunophenotype who were aged ≥18 years were selected from the total cohort (n = 374). The median follow-up time was 18.4 months.
Cytomorphology and immunophenotype
Cytomorphology of bone marrow and/or peripheral blood samples was carried out as previously described.28 Multiparameter flow cytometry analyses were performed using FC500 or Navios flow cytometers (Beckman Coulter, Miami, FL). List mode files were analyzed using CXP Software (version 2.0) and Kaluza (version 1.0) (Beckman Coulter). Diagnoses were assigned according to the European Group for the Immunological Characterization of Leukemias and World Health Organization classifications.29,30 Detailed data on immunophenotype were available for 408 patients.
Cytogenetics and FISH
Chromosome preparations and banding analysis were performed for all 625 cases as previously described according to standard methods.31 If required, cases with an aberrant karyotype in chromosome banding analysis were additionally investigated by 24-color fluorescence in situ hybridization (FISH; Metasystems, Altlussheim, Germany). For classification of abnormalities and karyotypes, the International System for Human Cytogenetic Nomenclature guidelines (2013) were used.32 In 383 patients, interphase FISH using probes for TP53 spanning a 167-kb region in 17p13 including the complete sequence of TP53 was performed to determine the copy number state of TP53 (Metasystems, Altlussheim, Germany). Moreover, interphase FISH was applied with probes for BCR-ABL1 (Metasystems) and MLL (Abbott, Wiesbaden, Germany). MYC (Abbott) was analyzed in cases in which respective rearrangements were suspected by chromosome banding analysis.
Mutation analysis
For the total cohort of 625 patients, deep-sequencing was applied to identify and quantify the TP53 mutation load as previously described.33 For this, DNA was extracted according to standard methods from bone marrow and peripheral blood cells obtained at the time of diagnosis. Next-generation amplicon deep-sequencing (Illumina, San Diego, CA; 454 Life Sciences, Branford, CT) was applied to investigate the mutational hotspot regions of TP5334 (ENST00000269305, exons 4-11). next generation sequencing data were analyzed using the GS Amplicon Variant Analyzer Software 2.6 (454 Life Sciences) and Sequence Pilot (version 4.1.1 Build 514 for the 454 platform; version 4.1.1 Build 510 for the Illumina platform, JSI Medicalsystems, Kippenheim, Germany).
Statistical analysis
SPSS (version 19.0.0) software (IBM Corporation, Armonk, NY) was used for statistical analysis. OS curves were calculated according to Kaplan-Meier and compared using the 2-sided log-rank test. All reported P values are 2-sided and were considered significant at P ≤ .05. OS was measured from the date of diagnosis until last follow-up or death.
Results
Frequency, type of TP53 mutations, and TP53 mutation load
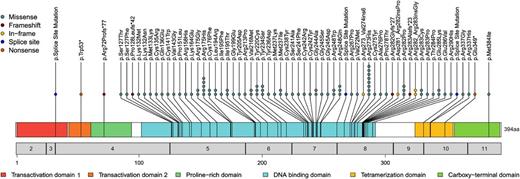
Analysis of the TP53 mutation frequency in the total cohort revealed 110 mutations in 98/625 (15.7%) of all patients. In 12 patients, 2 concomitant TP53 mutations were found. Investigation of the type of TP53 mutation showed that the vast majority of mutations were missense mutations (90/110; 81.8%), followed by frameshift (8/110; 7.2%), in-frame (5/110; 4.5%), splice-site (3/110; 2.7%), and nonsense mutations (4/110; 3.6%) (Figure 1). The mutations were found to be quite diverse with respect to the location across the coding region of the gene; however, they occurred preferentially in the DNA-binding domain of TP53 (amino acids 101-300). Moreover, many mutations affected codons described to code for the “hotspot” residues Arg273, Arg175, Arg282, and Arg248 (Table 1). Whereas single heterozygous TP53 mutations were present in 78.6% (77/98) of patients with TP53 mutation, 2 heterozygous TP53 mutations were observed in 12.2% (12/98), and for 9.2% of patients (9/98), a homozygous TP53 mutation was detected.
Location, frequency, and type of TP53 mutations in ALL. The locations and mutation types are depicted. Each circle represents a detected mutation. Numbers in the gray bar denote the respective exon, and numbers below correlate to the amino acid sequence. Functional TP53 domains are color-coded.
Location, frequency, and type of TP53 mutations in ALL. The locations and mutation types are depicted. Each circle represents a detected mutation. Numbers in the gray bar denote the respective exon, and numbers below correlate to the amino acid sequence. Functional TP53 domains are color-coded.
Amino acid residues of TP53 affected by mutations
| Site of respective mutation . | Number of patients with respective mutation . |
|---|---|
| Arg273* | 9 |
| Arg175*, Arg282* | 7 |
| Arg248*, Ile195 | 5 |
| Arg283 | 4 |
| Tyr220, Tyr234, Met237, Gly245*, Ile332 | 3 |
| Ser127, Lys132, Arg158, Cys176, Leu194, Val216, Cys238, Ser241, Cys242, Gly244, Cys275, Glu285, Arg337 | 2 |
| Trp53, Arg72, Pro128, Met133, Cys135, Gln136, Cys141, Val143, Thr150, Pro151, Lys164, Gly199, Tyr205, Arg213, Tyr236, Met246, Arg267, Phe270, Val272, Ala276, Pro278, Gly279, Asp281, Thr284, Glu286, Arg290, Lys292, Ile331, Glu349, Met384, Phe385 | 1 each |
| Site of respective mutation . | Number of patients with respective mutation . |
|---|---|
| Arg273* | 9 |
| Arg175*, Arg282* | 7 |
| Arg248*, Ile195 | 5 |
| Arg283 | 4 |
| Tyr220, Tyr234, Met237, Gly245*, Ile332 | 3 |
| Ser127, Lys132, Arg158, Cys176, Leu194, Val216, Cys238, Ser241, Cys242, Gly244, Cys275, Glu285, Arg337 | 2 |
| Trp53, Arg72, Pro128, Met133, Cys135, Gln136, Cys141, Val143, Thr150, Pro151, Lys164, Gly199, Tyr205, Arg213, Tyr236, Met246, Arg267, Phe270, Val272, Ala276, Pro278, Gly279, Asp281, Thr284, Glu286, Arg290, Lys292, Ile331, Glu349, Met384, Phe385 | 1 each |
The number of patients showing 1 of the respective mutations indicated is depicted.
These are the known hotspot mutation residues located in the DNA-binding domain of TP53.40
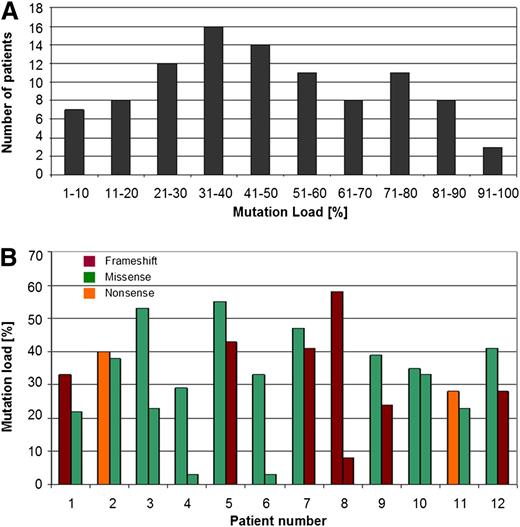
In addition, because next-generation sequencing was used, we were able to investigate the TP53 mutation load and observed a range between 2% and 98% (median: 41%) (Figure 2A). For some patients with 2 TP53 mutations, the respective mutation load differed considerably between the 2 mutations, whereas in other patients the respective mutation loads were found to be almost identical (Figure 2B). Comparison of the type and localization of the mutations with the respective mutation load did not reveal any correlation between these parameters (supplemental Table 1).
Mutation load in patients with TP53 mutations. (A) Numbers of patients with the respective TP53 mutation load percentages are depicted. It was investigated by next-generation deep-sequencing and varied between 2% and 98% (median: 41%). (B) TP53 mutation load percentages in the 12 patients that were identified to carry 2 TP53 mutations. The mutation type is depicted as well.
Mutation load in patients with TP53 mutations. (A) Numbers of patients with the respective TP53 mutation load percentages are depicted. It was investigated by next-generation deep-sequencing and varied between 2% and 98% (median: 41%). (B) TP53 mutation load percentages in the 12 patients that were identified to carry 2 TP53 mutations. The mutation type is depicted as well.
Correlation of TP53 mutations with TP53 deletion
The TP53 deletion status was investigated in a subset of 383 patients by interphase FISH. It was revealed that 64/383 (16.7%) patients with available TP53 deletion status harbored a TP53 mutation, whereas the remaining 83.3% were TP53 wild-type (wt). Of the TP53 mutated patients, 25/64 (39.1%) showed a deletion of the second allele, whereas no TP53 deletion was found in 39/64 (60.9%) patients with TP53 mutation. Moreover, in only 17/319 (5.3%) TP53wt patients with available TP53 deletion status, a TP53 deletion was detected (P < .001). Consequently, no TP53 deletion was observed in 302/319 TP53wt patients (94.7%) (supplemental Figure 2A-B).
Correlation of TP53 mutations with cytogenetic subgroups
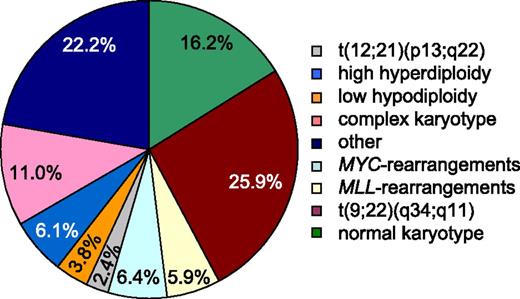
The distribution of cytogenetic subgroups in the total cohort of 625 patients resembles the frequencies of specific genotypes previously reported in patients with ALL,1 although it has to be noted that the cohort used in our study includes both children and adults encompassing relatively few childhood cases (0.1-91.4 years) (Figure 3 and supplemental Figure 3). As indicated above, TP53 mutations were found in 15.7% of all patients analyzed in the total cohort. However, when TP53 mutations were analyzed in the different cytogenetic subgroups, a clear correlation to ALL with low hypodiploidy (22/24; 91.7%) and MYC-translocated ALL (25/40; 62.5%) was observed (Table 2). Additionally, TP53 mutations were found to appear above-average in ALL with complex karyotype (16/69; 23.2%) and in MLL-translocated ALL (6/37; 16.2%). In contrast, TP53 mutations were clearly underrepresented in ALL with t(9;22)(q34;q11) (7/162; 4.3%), rare in ALL with high hyperdiploidy (3/38; 6.1%), ALL with normal karyotype (13/101; 12.9%) and ALL with other cytogenetic abnormalities (6/139; 4.3%), and were even absent in ALL with the ETV6-RUNX1 translocation t(12;21) (p13;q22) (0/15; 0%). Taken together, statistically significant correlations of TP53 mutations with a low hypodiploid karyotype and also with MYC-translocations were observed.
Frequency of specific karyotypes of patients with ALL. Analysis of karyotypes in the total cohort of 625 patients. Percentages are given for each cytogenetic subgroup. BCR-ABL1 t(9;22)(q24;q11) translocation (n = 162), normal karyotype (n = 101), complex karyotype (≥4 aberrations) (n = 69), MYC-rearrangements (n = 40), high hyperdiploidy (51-68 chromosomes) (n = 38), MLL-rearrangements (n = 37), low hypodiploidy (<40 chromosomes) (n = 24), t(12;21)(p13;q22) (n = 15), and other cytogenetic abnormalities (n = 139).
Frequency of specific karyotypes of patients with ALL. Analysis of karyotypes in the total cohort of 625 patients. Percentages are given for each cytogenetic subgroup. BCR-ABL1 t(9;22)(q24;q11) translocation (n = 162), normal karyotype (n = 101), complex karyotype (≥4 aberrations) (n = 69), MYC-rearrangements (n = 40), high hyperdiploidy (51-68 chromosomes) (n = 38), MLL-rearrangements (n = 37), low hypodiploidy (<40 chromosomes) (n = 24), t(12;21)(p13;q22) (n = 15), and other cytogenetic abnormalities (n = 139).
Patients with TP53 mutations within cytogenetic subgroups
| Cytogenetic subtype (patients mutated/studied) . | TP53 mutation (% of respective subgroup) . | P value (TP53 mutated patients vs total cohort) . |
|---|---|---|
| Low hypodiploid (n = 22/24) | 91.7 | <.0001 |
| MYC-rearrangement (n = 25/40) | 62.5 | <.0001 |
| Complex karyotype (n = 16/69) | 23.2 | <.0001 |
| BCR-ABL1 fusion gene (n = 7/162) | 4.3 | <.0001 |
| Normal karyotype (n = 13/101) | 12.9 | n.s. |
| MLL-rearrangement (n = 6/37) | 16.2 | n.s. |
| High hyperdiploid (n = 3/38) | 6.1 | n.s. |
| Other cytogenetic abnormalities (n = 6/139) | 4.3 | n.s. |
| ETV6-RUNX1 fusion gene (n = 0/15) | 0.0 | n.s. |
| Cytogenetic subtype (patients mutated/studied) . | TP53 mutation (% of respective subgroup) . | P value (TP53 mutated patients vs total cohort) . |
|---|---|---|
| Low hypodiploid (n = 22/24) | 91.7 | <.0001 |
| MYC-rearrangement (n = 25/40) | 62.5 | <.0001 |
| Complex karyotype (n = 16/69) | 23.2 | <.0001 |
| BCR-ABL1 fusion gene (n = 7/162) | 4.3 | <.0001 |
| Normal karyotype (n = 13/101) | 12.9 | n.s. |
| MLL-rearrangement (n = 6/37) | 16.2 | n.s. |
| High hyperdiploid (n = 3/38) | 6.1 | n.s. |
| Other cytogenetic abnormalities (n = 6/139) | 4.3 | n.s. |
| ETV6-RUNX1 fusion gene (n = 0/15) | 0.0 | n.s. |
n.s., not significant.
Frequency of TP53 mutations in Burkitt leukemia, B-lineage ALL, and T-lineage ALL
For 408 patients, detailed data on immunophenotype was available (T-lineage: n = 105, 25.7%; B-lineage: n = 267, 65.4%; Burkitt: n = 36, 8.8%); hence, analysis of the frequency of TP53 mutations in the respective subgroups was performed. Mutations of TP53 occurred in 58.3% of all Burkitt leukemias analyzed (21/36) and were also detected in a substantial fraction of B-lineage ALL (41/267; 15.4%). Compared with these findings, TP53 mutations were less frequent in T-lineage ALL (8/105; 7.6%).
Age dependence of TP53 mutations and TP53 deletions
The cohort was split into subgroups of patients <60 years (n = 417) and patients ≥60 years (n = 208). A clear interrelation of TP53 mutation frequency with increasing age was observed (TP53mut <60 years = 10.8% (45/417) vs ≥60 years = 25.5% (53/208); P < .0001). To analyze the TP53 mutation frequency in childhood ALL compared with adult ALL in more detail, the cohort was further split into the following subgroups: infant ALL (<1 years), childhood ALL (1-14 years), TYA ALL (teenage and young adults; 15-24 years), adult ALL (25-59 years), and older ALL (>60 years) (supplemental Figure 4A-B). The highest frequency of TP53 mutations was observed for patients >60 years (25.2%), although a relatively high incidence was also detected for adult ALL (12.7%) and childhood ALL (9.6%). Moreover, the frequency of TP53 deletions was analyzed in patients with available TP53 deletion status <60 years (n = 249) and in patients ≥ 60 years (n = 134). A similar correlation was observed, although it has to be noted that TP53 deletions were generally found to occur less frequently than TP53 mutations (TP53del <60 years vs ≥60 years = 8.4% [21/249] vs 15.7% [21/134; P < .0001]). Taken together, our data suggested an age dependence of TP53 mutation/deletion frequency.
Impact of TP53 mutations and their mutation load and TP53 deletions on survival
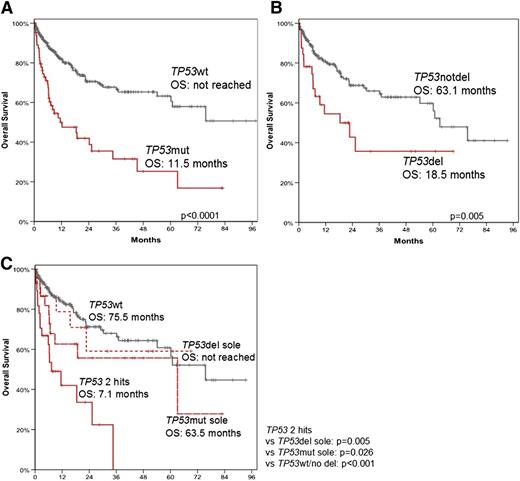
OS was significantly shorter in patients with a TP53 mutation (TP53mut) compared with patients without (TP53wt) (median 11.5 months vs not reached, P < .0001) (Figure 4A). Similarly, OS was clearly shorter in patients with a TP53 deletion (TP53del) compared with patients without (TP53notdel) (median 18.5. months vs 63.1 months, P = .005) (Figure 4B). Next, it was investigated whether both TP53 alleles have to be altered to cause the negative effect on prognosis. For this purpose, the OS was analyzed in more detail by dividing the cohort in (1) patients with mutations and/or deletions in both alleles (including: TP53mut + TP53del, homozygous TP53mut, 2 TP53mut; TP53 2hits, n = 29), (2) patients with TP53del only without TP53mut (TP53del sole; n = 16), (3) patients with one TP53mut (n = 22), and (4) patients with TP53wt (n = 188). The results revealed that for patients with TP53 2hits, a drastically reduced OS was observed compared with patients with TP53del, one TP53mut, and TP53wt patients (TP53 2hits [7.1 months] vs TP53del sole [not reached], P = .005; TP53 2hits vs TP53mut sole [63.5 months], P = .026; TP53 2hits vs TP53wt [75.5 months]; P = .001) (Figure 4C). To exclude a bias of the differently treated patients with Burkitt/MYC+ ALL and t(9;22) ALL (Ph+), we performed additional OS analyses excluding these patients. The impact of TP53 alterations on OS in this subset was comparable with the total cohort. The respective data for median OS were: 12.2 vs 75.5 months for TP53mut patients vs TP53wt patients (P < .0001); 18.5 vs 60.2 months in patients with TP53del compared with patients without TP53del (P = .02); 11.5 months for patients with 2 TP53 alterations (2hits) vs not reached for patients with TP53del sole (P = .007); vs 63.1 months for cases with TP53mut sole (P = .030); vs 60.2 months for TP53wt patients (P = .002) (supplemental Figure 5). The results clearly show that the negative effect on prognosis is dependent on alterations of both TP53 alleles.
OS of patients with TP53 mutation and/or deletion. OS was analyzed in patients with (TP53mut; n = 67) and without (TP53wt; n = 322) TP53 mutation (A); in patients with (TP53del; n = 34) and without (n = 217) TP53 deletion (B); and in patients with TP53 mutation without TP53 deletion (TP53mut sole; n = 22), patients with TP53 deletion without TP53 mutation (TP53del sole; n = 16), in patients that showed alterations in both alleles (TP53 2hits, n = 29) and in patients without aberrations in TP53 (TP53wt; n = 188) (C).
OS of patients with TP53 mutation and/or deletion. OS was analyzed in patients with (TP53mut; n = 67) and without (TP53wt; n = 322) TP53 mutation (A); in patients with (TP53del; n = 34) and without (n = 217) TP53 deletion (B); and in patients with TP53 mutation without TP53 deletion (TP53mut sole; n = 22), patients with TP53 deletion without TP53 mutation (TP53del sole; n = 16), in patients that showed alterations in both alleles (TP53 2hits, n = 29) and in patients without aberrations in TP53 (TP53wt; n = 188) (C).
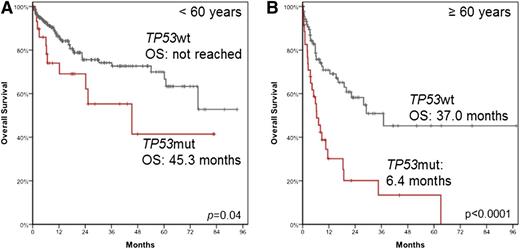
When the OS was investigated with respect to the age of the patients, it was observed that in patients <60 years, the median OS was not reached in TP53wt patients (n = 231) compared with 45.3 months in patients harboring the TP53mut (n = 31; P = .04). For patients ≥60 years, the median OS was found to be 37.0 months vs 6.4 months (n = 91 and n = 36; P < .0001) (Figure 5A-B).
OS of patients with TP53 mutation according to age. OS was analyzed in patients <60 years (A) and in patients ≥60 years (B).
OS of patients with TP53 mutation according to age. OS was analyzed in patients <60 years (A) and in patients ≥60 years (B).
In univariate Cox regression analysis, the following factors had an adverse impact on OS: TP53 2hits (hazard ratio [HR] = 3.7; P < .0001), age (<60 years vs ≥60 years, HR = 2.9; P = .0001), MLL-translocation (HR = 2.3; P = .005), MYC-translocation (HR = 3.3; P < .0001), and low hypodiploid ALL (HR = 2.5; P = .004) (Table 3). Multivariate Cox regression analysis, including the parameters identified to be associated with shorter OS in univariate analysis, revealed an independent adverse impact on OS for TP53 2hits (HR = 2.4; P < .0001), age (<60 years vs ≥60 years, HR = 2.8; P < .0001), MLL-rearrangement (HR = 2.1; P = .017), and MYC-rearrangement (HR = 2.1; P = .025) (Table 3). Taken together, it can be concluded that a complete loss of wt TP53 is associated with short OS in patients with ALL, independent of age or the cytogenetic subgroups analyzed.
Univariate and multivariate Cox regression analysis for OS
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR . | P value . | HR . | P value . | |
| TP53 2hits | 3.7 | <.0001 | 2.4 | <.0001 |
| Age <60 vs ≥60 y | 2.9 | <.0001 | 2.8 | <.0001 |
| MYC translocated | 3.3 | <.0001 | 2.1 | .025 |
| MLL translocated | 2.3 | .005 | 2.1 | .017 |
| Low hypodiploid | 2.5 | .004 | — | n.s. |
| Complex karyotype | — | n.s. | — | — |
| BCR-ABL1 rearrangement | 0.5 | .009 | 0.6 | .03 |
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR . | P value . | HR . | P value . | |
| TP53 2hits | 3.7 | <.0001 | 2.4 | <.0001 |
| Age <60 vs ≥60 y | 2.9 | <.0001 | 2.8 | <.0001 |
| MYC translocated | 3.3 | <.0001 | 2.1 | .025 |
| MLL translocated | 2.3 | .005 | 2.1 | .017 |
| Low hypodiploid | 2.5 | .004 | — | n.s. |
| Complex karyotype | — | n.s. | — | — |
| BCR-ABL1 rearrangement | 0.5 | .009 | 0.6 | .03 |
n.s., not significant.
TP53 2hits: TP53 mutation + TP53 deletion, homozygous TP53 mutation, 2 TP53 mutations; low hypodiploid: 32-39 chromosomes; complex: >3 cytogenetic aberrations.
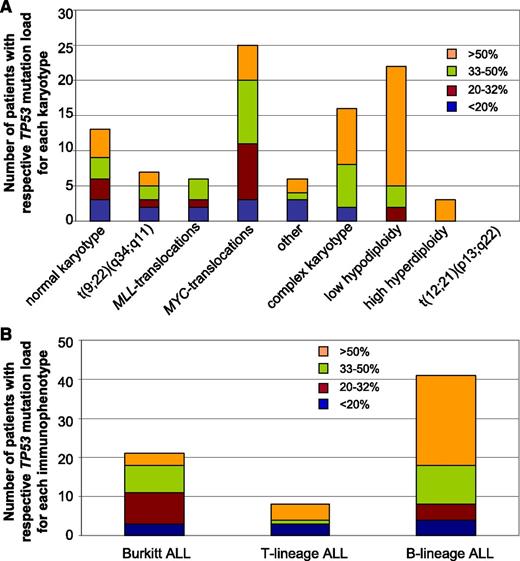
Correlation of TP53 mutation load with cytogenetic subgroups and immunophenotype
Analysis of the TP53 mutation load in regard to the respective karyotypes revealed a clear correlation with a high mutation load of >50% for patients with a complex karyotype, ALL with low hypodiploidy, and ALL with high hyperdiploidy (median 51.6%, 66.9%, and 67.0%, respectively). In contrast, the mutation load of patients with MLL-translocated ALL was found to be <50% in all cases (median: 26.7%) (Figure 6A). When the mutation load was analyzed in relation to the immunophenotype, a high mutation load was detected in patients with B-lineage ALL (median: 54.8%) (Figure 6B). In contrast to this, patients with Burkitt ALL showed a comparably low TP53 mutation load (median: 33.6%). For T-lineage ALL, a median mutation load of 43.6% was observed. However, it has to be noted that the mutation load in this subgroup showed a wide variation (2% to 82%), and the number of patients with T-lineage ALL and TP53 mutation was too low (n = 8) to draw definite conclusions.
Correlation of TP53 mutation load with cytogenetic subgroups and with immunophenotype. (A) For each patient, the mutation load was determined and correlated to the respective karyotype. The number of patients with the respective mutation load is shown in regard to cytogenetic aberrations. (B) The TP53 mutation load was determined in each patient and the obtained value was correlated to the respective immunophenotype. The number of patients with the respective mutation load is shown in regard to Burkitt-ALL, T-lineage ALL, or B-lineage ALL.
Correlation of TP53 mutation load with cytogenetic subgroups and with immunophenotype. (A) For each patient, the mutation load was determined and correlated to the respective karyotype. The number of patients with the respective mutation load is shown in regard to cytogenetic aberrations. (B) The TP53 mutation load was determined in each patient and the obtained value was correlated to the respective immunophenotype. The number of patients with the respective mutation load is shown in regard to Burkitt-ALL, T-lineage ALL, or B-lineage ALL.
Discussion
To our knowledge, this study comprises the largest cohort of patients with ALL analyzed for TP53 alterations so far. The data clearly showed that (1) TP53 mutations occur more frequently thanpreviously published (15.7% vs 2% to 3%13 ; 8% to 9%2,6 ), (2) TP53 mutations are predominantly associated with ALL with low hypodiploidy and MYC-translocated ALL, (3) the TP53 mutation/deletion frequency increases with age, (4) TP53 mutations are associated with short survival independent of age and specific cytogenetic alterations, and (5) the adverse impact on survival occurs only when the wt TP53 is lost. The reason why TP53 mutations were found at a higher frequency in ALL patients than in earlier studies might be due to the cohort analyzed, as previous studies mainly focused on children and our cohort includes a large variety of ALL subgroups, including, eg, Burkitt/MYC+ ALL. Alternatively, the higher mutation frequency might be caused by differences in sensitivity of the sequencing methods used (in our study, the limit-of-detection for TP53 mutations was approximately 3% using the 454 Life Sciences technology and approximately 5% when using the Illumina assay). Moreover, previous analyses often focused on sequencing exons 5 to 8 of TP53 (the DNA-binding domain), whereas 10% to 30% of the cancer-associated mutations in TP53 are known to be located outside this hotspot region.6,35 7.3% [8/110] of TP53 mutations detected were not located in exons 5 to 8, indicating a very high preference for TP53 mutations to occur in the hotspot region for ALL. The overall mutation frequency observed in our study clearly remains lower compared with the landscape in many solid tumors, eg, ∼50% in gastric cancer,36 and almost 100% in high-grade serous carcinoma of the ovary.12 However, the incidence of TP53 mutations found in our analyses is in the range of other hematologic neoplasms, including AML10,37 or CLL.11,38 TP53 was found to be predominantly inactivated by missense mutations, whereas deletions and insertions occurred rarely. This corroborates previous results regarding TP53 mutations in ALL and other human cancers.21,39,40 It has to be noted that a high number of mutations (31/110; 28.2%) detected in our study affect the 6 known hotspot residues (R175, G245, R248, R249, R273, and R282). These results nicely resemble previous data from different types of cancer, proposing that ∼30% of TP53 mutations cluster in these residues.12,41
Further, analysis of TP53 deletions revealed a frequent correlation with TP53 mutations in the second allele, thus underlying the “2-hit” idea for tumor-suppressor genes and their function in cancer development.6 Accordingly, previous data for patients with AML further support this hypothesis, as >40% of patients with a TP53 mutation were found to carry an additional TP53 deletion of the second allele.37,42 On the other hand, for patients with AML, a high coincidence of TP53 mutations with a complex aberrant karyotype was observed, in contrast to the present study, in which TP53 mutations were most frequently found in ALL with low hypodiploidy (91.7%) and MYC-translocations (62.5%). However, in ALL with complex karyotype, TP53 mutation frequency was still above average (23.2% vs 15.7%).
Other studies suggested a much higher percentage of TP53 alterations in ALL (30% to 40%), if analyses do not merely focus on TP53 mutations and deletions but include promoter hypermethylation.14 In this case, methylation of the TP53 promoter was even found to constitute the most frequent molecular event in ALL (32% methylated samples vs 8.8% missense mutations), hypothesizing that promoter methylation in different genes is common in ALL. However, an impact of this on OS and prognosis could not be demonstrated to date. Because we focused on TP53 mutations/deletions and not on methylation, the role of this potentially common event in ALL remains to be investigated. It was proposed that hypermethylation of the promoter regions of tumor-suppressor genes such as TP53 induces transcriptional silencing and is related to tumor progression. Thus, further analyses of the clinical and biological role of methylated genes in ALL might support the development of new markers and therapies.6
Moreover, our data suggest a correlation of TP53 mutations with Burkitt ALL and B-lineage ALL compared with T-lineage ALL, in which TP53 mutations were found to be rather infrequent. These results are, however, in contrast to a previous study in which TP53 mutations were found to be more frequent in T-lineage than in B-lineage ALL (4/36, 11.1% vs 4/62, 6.4%), although the different size and composition of the cohort has to be considered.2
Interestingly, our results demonstrate a clear association of TP53 mutations with ALL with low hypodiploidy (22/24; 91.7%) and MYC-translocated ALL (25/40; 62.5%). This corroborates recent studies demonstrating a very high frequency (91.2% and 93.1%, respectively) of TP53 alterations in low-hypodiploid ALL (32-39 chromosomes).27,43 In contrast, patients with near-haploid ALL (24-31 chromosomes) were found to show a different genomic profile, namely alterations in genes involved in the RAS signaling pathway and the lymphoid transcription factor gene IKZF3.43 Regarding MLL-translocations (16.2% TP53 mutation in our study), previous studies proposed a correlation to TP53 mutations in a cohort of children with relapsed ALL.23 Moreover, we showed that TP53 mutations are rare in patients with BCR-ABL1-positive ALL (7/162; 4.3%), which are known to constitute the largest cytogenetic subgroup in adult ALL1 (∼25%) and are associated with poor prognosis, although higher remission rates can be achieved by treatment with tyrosine-kinase inhibitors.44 As TP53 mutations seem to be almost mutually exclusive of BCR-ABL1 rearrangements in ALL, these 2 aberrations might function as independent prognostic markers, both defining genetic subgroups with a high prognostic significance.
In addition, analysis of the TP53 mutation load in regard to cytogenetic subgroups revealed a rather low load for patients with MLL- and BCR-ABL1-translocations, indicating that in these cases, not the TP53 mutation but rather the MLL- and BCR-ABL1-translocations promote the impact on prognosis. By contrast, patients with a complex karyotype, ALL with low hypodiploidy and ALL with high hyperdiploidy show a drastically higher TP53 mutation load. For patients with a low hypodiploid karyotype, this could be explained by the frequent loss of chromosome 17 in these cells (20/22 in the present series). Moreover, analysis of the mutation load in regard to the respective immunophenotype revealed a high load in patients with B-lineage ALL; however, the reason for this remains to be investigated.
The frequency of TP53 mutations/deletions was clearly found to increase with age. It has been noted before that patients harboring a hypodiploid ALL have a high risk of treatment failure,4,45 and it can be speculated that this is at least partly related to the high association with TP53 mutations. Additionally, it is known that TP53 mutations are generally associated with poor prognosis in all types of cancer. The higher TP53 mutation frequency in older patients might be one reason for the general poor outcome of adult ALL. However, MYC-translocations and low hypodiploid ALLs were not found to occur predominantly in older patients in contrast to MLL-translocations that have only been observed in age decades 4 to 8 of TP53-mutated patients (supplemental Figure 6). Regarding the OS, a negative effect of TP53 alterations was observed, which was still clearly detectable if patients with Burkitt/MYC+ ALL or Ph+ ALL were excluded from the analyses who receive different treatment protocols compared with other ALL patients. Moreover, patients with alterations in both TP53 alleles showed the shortest OS of all patients analyzed and hence define in the best way the subgroup with a very poor prognosis. Thus, the presence of TP53 mutations/deletions is an important prognostic parameter for patients with ALL, which has to be considered to be analyzed in all patients receiving therapy according to standard protocols for ALL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all coworkers at the Munich Leukemia Laboratory for approaching together many aspects in the field of leukemia diagnostics and research by their dedicated work. The authors would like to thank all physicians for providing and caring for patients as well as collecting data. Centers and investigators (contributing more than 10 cases) are listed in order of number of cases provided: Städtisches Klinikum München Schwabing (C. Wendtner), Klinikum Nürnberg (K. Schäfer-Eckart), Universitätsklinikum Köln (K.-A. Kreuzer), Klinikum Augsburg (C. Schmid), Universitätsklinikum Frankfurt (H. Serve), Universitätsklinikum Freiburg (J. Duyster), Asklepios Klinik Altona Hamburg (C. Meyer zum Büschenfelde), St. Antonius Krankenhaus Eschweiler (P. Staib), Universitätsklinikum des Saarlandes Homburg (M. Pfreundschuh), Universitätsklinikum Marburg (A. Neubauer), Rechts der Isar der Technischen Universität München (C. Peschel), and St. Marien-Krankenhaus Siegen (W. Gassmann).
Authorship
Contribution: C.H. designed the study; C.H. and A.S. interpreted the data; A.S. wrote the manuscript; S.W., S.Z., and A.K. did molecular analyses; C.H. was responsible for chromosome banding and FISH analyses; S.S. was responsible for molecular genetic analyses; W.K. was responsible for immunophenotyping; and T.H. was responsible for cytomorphologic analyses. All authors read and contributed to the final version of the manuscript.
Conflict-of-interest disclosure: C.H., S.S., W.K., and T.H. declare part ownership of MLL Munich Leukemia Laboratory. A.S., S.W., S.Z., and A.K. are employed by the Munich Leukemia Laboratory. The remaining author declares no competing financial interests.
Correspondence: Claudia Haferlach, Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 München, Germany; e-mail: claudia.haferlach@mll.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal