Key Points
Donor treatment with AAT suppresses GVHD in the transplant recipient while enhancing the GVL effect.
AAT effects are mediated via cell type–specific alterations of mitochondrial bioenergetics.
Abstract
Hematopoietic cell transplantation is curative in many patients. However, graft-versus-host disease (GVHD), triggered by alloreactive donor cells, has remained a major complication. Here, we show an inverse correlation between plasma α-1-antitrypsin (AAT) levels in human donors and the development of acute GVHD in the recipients (n = 111; P = .0006). In murine models, treatment of transplant donors with human AAT resulted in an increase in interleukin-10 messenger RNA and CD8+CD11c+CD205+ major histocompatibility complex class II+ dendritic cells (DCs), and the prevention or attenuation of acute GVHD in the recipients. Ablation of DCs (in AAT-treated CD11c-DTR donors) decreased CD4+CD25+FoxP3+ regulatory T cells to one-third and abrogated the anti-GVHD effect. The graft-versus-leukemia (GVL) effect of donor cells (against A20 tumor cells) was maintained or even enhanced with AAT treatment of the donor, mediated by an expanded population of NK1.1+, CD49B+, CD122+, CD335+ NKG2D-expressing natural killer (NK) cells. Blockade of NKG2D significantly suppressed the GVL effect. Metabolic analysis showed a high glycolysis–high oxidative phosphorylation profile for NK1.1+ cells, CD4+CD25+FoxP3+ T cells, and CD11c+ DCs but not for effector T cells, suggesting a cell type–specific effect of AAT. Thus, via altered metabolism, AAT exerts effective GVHD protection while enhancing GVL effects.
Introduction
Allogeneic hematopoietic stem cell transplantation is curative in many patients with leukemia and other lymphohematopoietic disorders. However, the immune response of donor cells that mediate the graft-versus-leukemia (GVL) effect, leading to disease eradication,1 also triggers graft-versus-host disease (GVHD).2 Preventing GVHD while maintaining the GVL effect would be a major advance. Several recent studies suggest that this should be possible.3,4 Donor T-cell activation, initiated by host antigen-presenting cells (APCs),5 is enhanced by proinflammatory cytokines, released from sites of tissue injury following transplant conditioning. These cytokines, including tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), and interferon γ (IFN-γ), promote T-helper 1 (Th-1) cell differentiation and enhance their proliferation and reactivity against host tissues.
The administration of α-1-antitrypsin (AAT), used therapeutically in patients with genetically determined AAT deficiency-related emphysema,6 profoundly alters cytokine profiles and has been shown to suppress GVHD.7-9 AAT is a serine protease inhibitor, which in addition to changes in cytokine profiles, also affects the redox status of cells and cell-mediated immunity, among other functions.6,10-15 Taken together, available data indicate that AAT therapy is beneficial in a broad spectrum of inflammatory and immune-mediated diseases not related to genetic AAT deficiency. Therefore, it is of interest that ancillary data suggest that patients transplanted from donors with higher AAT levels were less likely to develop acute GVHD. Hence, we investigated whether exposure of donor cells to (exogenous) AAT would modify cell function and thereby affect GVHD in recipients. However, because AAT also increases expression of cytoprotective factors such as IL10 and IL1Ra, we had to address the concern that AAT exposure might interfere with the desired GVL effect.
Materials and methods
Patients, sample collection, and follow-up
We analyzed retrospectively the association between donor plasma AAT levels and risk of acute GVHD among 111 recipients with acute myeloid leukemia (AML) in first complete remission who were treated with allogeneic hematopoietic cell transplantation (HCT) following high-intensity conditioning. Among 111 recipients, 20 received bone marrow grafts and 91 received mobilized peripheral blood stem cells (PBSCs) (see Table 1 for demographics). Marrow and PBSCs were volume reduced. Plasma donor samples were obtained from the Infectious Disease Sciences Biospecimen Repository, Fred Hutchinson Cancer Research Center (FHCRC). Donors were human leukocyte antigen (HLA)–identical siblings. GVHD prophylaxis consisted of cyclosporine or tacrolimus, plus methotrexate or mycophenolate mofetil. All patients and donors had given informed consent to participate in research studies as required by the institutional review board of the FHCRC and the Declaration of Helsinki.
Demographics of patients and sibling donors
| Characteristics . | No. . | % . |
|---|---|---|
| Patients, n = 111 | ||
| Age, y | ||
| Median | 49.05 | |
| Range | 19-72 | |
| Male sex | 51 | 45.9 |
| Karnofsky performance score | ||
| Median | 90 | |
| Range | 70-100 | |
| Diagnosis | ||
| AML | 111 | 100 |
| Myeloablative conditioning | ||
| Busulfan/cyclophosphamide | 61 | 55 |
| Cyclophosphamide/TBI | 36 | 32.5 |
| Fludarabine/treosulfan | 14 | 12.5 |
| GVHD prophylaxis | ||
| Tacrolimus/MTX | 85 | 76.5 |
| Cyclosporine/MMF | 16 | 14.4 |
| Cyclosporine/MTX | 10 | 9.1 |
| Average GVHD onset, d | ||
| Cyclosporine | 29 | |
| Range | 0-95 | |
| Donors, n = 111 | ||
| Age, y | ||
| Median | 47.5 | |
| Range | 24-79 | |
| Male sex | 45 | 40.5 |
| Stem cell product | ||
| Peripheral blood | 91 | 81.9 |
| Bone marrow | 20 | 18.1 |
| AAT plasma levels, mg/mL | ||
| Mean | 3.31 | |
| Range | 0.4-12.5 |
| Characteristics . | No. . | % . |
|---|---|---|
| Patients, n = 111 | ||
| Age, y | ||
| Median | 49.05 | |
| Range | 19-72 | |
| Male sex | 51 | 45.9 |
| Karnofsky performance score | ||
| Median | 90 | |
| Range | 70-100 | |
| Diagnosis | ||
| AML | 111 | 100 |
| Myeloablative conditioning | ||
| Busulfan/cyclophosphamide | 61 | 55 |
| Cyclophosphamide/TBI | 36 | 32.5 |
| Fludarabine/treosulfan | 14 | 12.5 |
| GVHD prophylaxis | ||
| Tacrolimus/MTX | 85 | 76.5 |
| Cyclosporine/MMF | 16 | 14.4 |
| Cyclosporine/MTX | 10 | 9.1 |
| Average GVHD onset, d | ||
| Cyclosporine | 29 | |
| Range | 0-95 | |
| Donors, n = 111 | ||
| Age, y | ||
| Median | 47.5 | |
| Range | 24-79 | |
| Male sex | 45 | 40.5 |
| Stem cell product | ||
| Peripheral blood | 91 | 81.9 |
| Bone marrow | 20 | 18.1 |
| AAT plasma levels, mg/mL | ||
| Mean | 3.31 | |
| Range | 0.4-12.5 |
Determination of plasma AAT levels
Human AAT levels were determined by enzyme-linked immunosorbent assay (ELISA) using 96-well polystyrene plates (Costar) coated overnight at 4°C with 0.5 μg/mL mouse anti-human AAT (R&D Systems) in 50 mM Na carbonate, pH 9.5. The reaction was stopped with 1 M H3PO4. Optical density was determined at 450 nm using a microplate reader (Vmax; Molecular Devices). AAT concentrations were calculated by standard methods (SoftMax Pro; Molecular Devices). Interassay and intraassay coefficient of variability (CV) were determined to be <10% with an assay sensitivity for <2 pg/mL. All human samples, standards, and controls were run in triplicates.
Murine models
Minor mismatch model.
Recipient C57BL/6 (H-2b, Thy-1.2) mice (The Jackson Laboratory), 10- to 14-weeks-old, received 1000 cGy single-dose total body irradiation (TBI) followed by tail vein injection of 5 × 106 T-cell–depleted marrow cells, and 0.2 × 10 6 CD8+ splenic lymphocytes from C3H.SW-H2b/SnJ donors (H-2bc) (The Jackson Laboratory).
Major mismatch model.
Recipient Balb/c ([H-2d]) mice received 800 cGy single-dose TBI followed by tail vein injection of 5 × 106 T-cell–depleted marrow cells, and 0.5 × 10 6 CD8+ splenic lymphocytes from C57BL/6 (H-2b) donors.
Marrow cells were depleted of T cells with the CD8a+ T-Cell Isolation kit, mouse (Miltenyi Biotec). T cells were isolated from splenocytes using the Pan T-Cell Isolation kit II, mouse, as directed by the manufacturer (Miltenyi Biotec).
Donors were given intraperitoneal (i.p.) AAT, 3 mg per dose every 2 days, for 6 doses (schema, see Figure 2A). Controls received i.p. albumin, 125 μL per dose, on the same schedule. Either only the donor (AAT/albumin) or the recipient (albumin/AAT) or both donor and recipient were given AAT (AAT/AAT) or neither one received AAT (albumin/albumin). Each group consisted of 12 pairs.
Cell ablation.
To determine the effect of ablation of CD11c+ dendritic cells (DCs) or T-regulatory cells (Tregs), we used male CD11c-DTR mice (B6.FVB-Tg[Itgax-DTR/EGFP]57Lan/J; The Jackson Laboratory) and FOXP3-DTR mice, a gift from Dr Alexander Rudensky (Rockefeller University, New York, NY),16 respectively. At 72 and 24 hours before sacrifice and cell harvesting for HCT, donors were injected i.p. with diphtheria toxin (DT; Sigma) in phosphate-buffered saline (PBS), 30 ng/g on day −3, and 10 ng/g on day −1, to deplete CD11c+ DCs or Tregs, respectively (Figure 3B; supplemental Figure 1B, see supplemental Data available at the Blood Web site).
Recipient treatment.
Recipients received 3 mg of AAT, suspended in 125 μL of PBS, i.p. before TBI and donor cell infusion, and every 2 days post-HCT (schema, see Figure 2A) for 10 injections.
Blood was collected sequentially for cytokine assays. Body weight and clinical GVHD score (5 criteria: percentage of weight change, posture [hunching], activity, fur texture, and skin integrity; maximum score = 10)17 were recorded sequentially. Mice reaching scores of 6.5 or higher were killed using CO2 euthanasia.
AAT preparation
Purified pooled human plasma AAT (Glassia) was provided by Baxter-Kamada. Glassia, commercially available, is dissolved in sterile water provided by the manufacturer.
Cytotoxicity assays
A20 B-cell lymphoma cells (gift from Dr R. Negrin, Stanford University, Stanford, CA) and EL4 (gift from Dr J. Pagel, Fred Hutchinson Cancer Research, Seattle, WA) served as targets. A20 is a BALB/c B-cell lymphoma line derived from a spontaneously occurring reticulum cell neoplasm in an old BALB/c mouse (H-2d)18 ; EL4 is a T-cell lymphoma induced in a C57BL/6 (H-2b) mouse by 9,10-dimethyl-1,2-benzanthracene. CD56+CD3− splenocytes were added to A20 cells or EL4 at effector/target ratios of 10:1, 5:1, and 1:1 for 4 hours at 37°C. Cells were stained with propidium iodide (PI; Molecular Probes) and analyzed by flow cytometry. Experiments were performed in triplicates. Standard errors were within 5% of the mean. For selected experiments, the following blocking antibodies were used: anti-CD314 (NKG2D), purified, clone MI-6, and anti-mouse RAE1 γ, purified, clone CX1, both purchased from eBioscience.
Analysis of the GVL effect in vivo
Briefly, Balb/c mice, syngeneic with the A20 tumor, were given 800 cGy TBI and injected IV with 2 × 104 A20-luc/yfp cells. These cells migrate to bone marrow, spleen, and other lymphoid organs.18 The presence of the firefly luciferase gene in A20 allows for detection of viable tumor cells following luciferin injection. The tumor was allowed 4 days to get established. On day 4, 5 × 106 T-cell–depleted bone marrow cells, and 0.5 × 106 CD8+ splenic lymphocytes from C57BL/6 (H-2b, Thy-1.2) donors were injected as described in “Murine models.” Tumor growth was assessed by bioluminescence imaging following luciferin injection (see “Bioluminescence imaging”). For the BCL1 tumor model, 10 × 103 BCL1 cells were given IV on day −5 before transplantation.18 In this model, tumor cells initially infiltrate the spleen and then metastasize to the liver and lung.
Flow cytometric analysis
Fycoll-Hypaque–separated marrow or spleen cells were washed twice in chilled PBS, and resuspended in ice-cold fluorescence-activated cell sorter (FACS) buffer (PBS, 0.5% bovine serum albumin, 0.1% sodium azide) before labeling with antibody.
The following antibodies were used for immunophenotypic characterization: anti-CD3 (eFluor 450, clone 17A2), anti-CD4 (Alexa Fluor 700, clone RM4-5), anti-CD8 (APV, clone 53-6.7), anti-FoxP3 (phycoerythrin [PE], clone FJK-16s), anti-CD11c (PE, clone N418), anti-CD45R (B220) (allophycocyanin [APC], clone RA3-6B2), anti-NK1.1 (clone PK136), anti-CD335 (NKp46, clone29A1.4), anti-CD122 (clone M-b1 [TM-beta1]), all purchased from eBioscience.
MITOSOX and tramethylrhodamine (TMRM) were purchased from Invitrogen. After washing with PBS, MITOSOX- and TMRM-labeled cells were resuspended in 100 µL of ice-cold FACS buffer and analyzed immediately on a FACSCalibur (Becton Dickinson).
Metabolic flux analysis
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using a Seahorse XF24 extracellular flux analyzer (Seahorse Bioscience). Twenty-four hours before analysis, CD4+, CD8+, CD11c+, NK1.1 natural killer (NK) cells and CD4+CD25+ T lymphocytes were obtained from the spleens of AAT- (and albumin-) treated donors and cultured on XF-24 plates at 8 × 105 cells per well. Immediately before analysis, the culture medium was replaced by 675 µL of unbuffered Dulbecco modified Eagle medium (DMEM; DMEM supplemented with 25 mM glucose, 1 mM sodium pyruvate, 31 mM NaCl, 2 mM GlutaMax, phenol red, pH 7.4), and plates were incubated at 37°C in a CO2-free incubator for 1 hour. All reagents were adjusted to pH 7.4. Baseline rates were measured ×4 at 37°C before sequentially adding the following mitochondrial inhibitors: rotenone, thenoyltrifluoroacetone (TTFA; to inhibit electron entry to ubiquinone), and antimycin A (AA, a blocker of ubisemiquinone). Respiratory assessment was followed by sequential addition of oligomycin, FCCP (carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone), and rotenone plus AA. OCR was converted from nmol O2/min/mL to pmol O2/min/µg mitochondrial protein. OCR and ECAR were calculated by Seahorse XF-24 software. Every point represents the average of 7 different wells.
RNA isolation, cDNA synthesis, and real-time polymerase chain reaction (PCR)
Splenocytes and marrow cells were isolated from AAT-treated and albumin-treated mice. RNA was purified with the RNeasy Micro kit (Qiagen), and RNA purity determined on 1.2% agarose gels. Complementary DNA (cDNA) was synthesized with the RT2 First Strand kit (Qiagen) and analyzed for expression of signaling-related genes transcribed by nuclear factor-κB (NF-κB), using Human NF-κB Signaling Pathway RT2 Profiler PCR Arrays (SABiosciences; Qiagen) according to the manufacturer’s protocols. Quantitative PCR was carried out on an ABI 7100 thermocycler (Applied Biosystems). Relative gene expression was determined using the ∆∆ cycle threshold methods on the SAB web portal (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). As endogenous references, messenger RNA (mRNA) levels of TNFα (Mm00443258), IL-1Ra (Mm01337566), FOXp3 (Mm00475162), IL-10 (Mm00439614), and HO-1 (Mm01337566_m1) were used. β-actin (Mm00607939) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were included as controls.
Bioluminescence imaging
In vivo bioluminescence imaging was performed as described.18 Briefly, mice were injected i.p. with luciferin (10 μg/g body weight). After 10 minutes, images were acquired using an IVIS200 charge-coupled device imaging system (Xenogen). Tumor volume was reported as photons/second/cm2. Imaging data were analyzed and quantified with Living Image Software (Xenogen) and IgorProCarbon (WaveMetrics).
Statistical analysis
Values of gene expression were expressed as the mean ± standard error of the mean (SEM). The Student t test was used to compare continuous variables between 2 groups; 1-way analysis of variance (ANOVA) was applied to compare continuous variables among 3 or more groups. Linear regression from GraphPad Prism 5 was used for AAT correlations. Levels of plasma AAT were compared across GVHD grades (as a nominal variable), thereby testing for a trend between AAT and GVHD using linear regression.
Results
AAT plasma levels in human donors and acute GVHD
Anecdotal data suggested an effect of donor plasma AAT levels on the development of GVHD. Thus, we measured plasma AAT levels in 111 matched allogeneic donors and determined the development of acute GVHD in the respective HLA-identical sibling recipients. All transplant recipients enrolled in this prospective analysis did receive high-intensity (myeloablative) conditioning for AML in first complete remission (see Table 1). Results showed an inverse relationship between donor AAT levels and the occurrence of GVHD in the recipients (at day 20, P = .01; at day 30, P = .002; and at day 100, P = .0006; Figure 1). Because the volume of donor plasma transferred with a standard transplant is minimal, it is likely that beneficial effects of AAT were indirect, mediated by the AAT-exposed donor cells. This hypothesis is supported by murine data showing profound alterations of cellular cytokine profiles following exposure to exogeneous AAT.7 We proceeded, therefore, to test our hypothesis in murine transplant models.
Donor AAT levels and acute GVHD (n = 111) by day 100. Plasma AAT levels in human allogeneic transplant donors. Donor AAT levels and the development of GVHD (by grade) in the respective recipients by day 100. GVHD severity increased with decreasing AAT levels. Modeling GVHD severity as a linear variable, a trend test showed decreasing average AAT plasma levels with increasing GVHD scores (P = .0006). This same conclusion held for GVHD scores by day 20 (P = .01) and by day 30 (P = .002) (not shown).
Donor AAT levels and acute GVHD (n = 111) by day 100. Plasma AAT levels in human allogeneic transplant donors. Donor AAT levels and the development of GVHD (by grade) in the respective recipients by day 100. GVHD severity increased with decreasing AAT levels. Modeling GVHD severity as a linear variable, a trend test showed decreasing average AAT plasma levels with increasing GVHD scores (P = .0006). This same conclusion held for GVHD scores by day 20 (P = .01) and by day 30 (P = .002) (not shown).
Donor pretreatment with AAT and GVHD in minor and major antigen mismatched recipients
The effect of donor AAT treatment on GVHD was tested in minor mismatch (C3H.SW [H 2bc] into C57BL/6 [H-2b]) and major mismatch (C57BL/6 [H-2b]) into Balb/c ([H-2d]) murine transplant recipients. As shown in Figure 2, in both models, recipients given cells from AAT-treated donors experienced less weight loss, had lower GVHD incidence and severity, and reduced mortality compared with albumin controls. The effect of donor AAT treatment on GVHD was comparable or superior to that of direct treatment of the recipient with AAT post-HCT; the benefit was further enhanced when donor and recipient were treated.
Effects of AAT treatment of donor, recipient, or both on GVHD and posttransplant outcome. (A) Treatment scheme for both donor (blue) and recipient (red). (B) Results with C3H.SW (H-2b) donors and C57BL/6 (H 2bc) recipients (minor mismatch) and (C) results with C57BL/6 (H-2bKb) donors, and Balb/c (H-2d Kd) recipients (major mismatch). Either donor (AAT/albumin) or recipient (albumin/AAT), both (AAT/AAT) or neither (albumin/albumin) were treated with AAT; albumin served as control. Left panels show survival; middle and right panels show body weight changes and GVHD scores (n = 12 for each transplant condition). (D) Changes in cytokine levels in donor marrow and spleen following AAT treatment relative to albumin controls (determined by RT-PCR; n = 7 mice per group). Results are expressed as mean ± SEM (log2) from pooled tissue extracts (marrow and spleen preparations). Ω indicates P values of at least = .01 when compared with albumin-treated control cells.
Effects of AAT treatment of donor, recipient, or both on GVHD and posttransplant outcome. (A) Treatment scheme for both donor (blue) and recipient (red). (B) Results with C3H.SW (H-2b) donors and C57BL/6 (H 2bc) recipients (minor mismatch) and (C) results with C57BL/6 (H-2bKb) donors, and Balb/c (H-2d Kd) recipients (major mismatch). Either donor (AAT/albumin) or recipient (albumin/AAT), both (AAT/AAT) or neither (albumin/albumin) were treated with AAT; albumin served as control. Left panels show survival; middle and right panels show body weight changes and GVHD scores (n = 12 for each transplant condition). (D) Changes in cytokine levels in donor marrow and spleen following AAT treatment relative to albumin controls (determined by RT-PCR; n = 7 mice per group). Results are expressed as mean ± SEM (log2) from pooled tissue extracts (marrow and spleen preparations). Ω indicates P values of at least = .01 when compared with albumin-treated control cells.
The data with donor-only treatment provided the opportunity to determine modulations of transplanted cells without contributions by the recipient environment (eg, host antigen-presenting cells). Flow cytometric analysis of marrow and spleen cells from AAT-treated donors showed an increase in CD8+CD205+ major histocompatibility class (MHC) II+ DCs (P = .0001) in spleen and marrow (Figure 3A). The proportions of CD4+CD25+FoxP3+ Tregs (ratios normalized to CD4+FoxP3− cells) in spleen and bone marrow were also increased (supplemental Figure 1A). Concurrently, there was a 50 log2 fold increase of IL-10 in the marrow of AAT-treated donors (n = 12, P = .00001) (Figure 2D). IL-10 has been shown to be involved in DC homeostasis and to contribute to Treg expansion.19 Thus, the upregulation of IL-10 by AAT suggested a link between IL-10, DC proliferation, and Treg expansion. In parallel, TNFα and IL-1β were downregulated, in agreement with our previous work.7
Effect of AAT on donor cell subpopulations. (A) Increase in the proportion of DCs in spleens of AAT-treated (AAT) and albumin-treated (controls) donors. Shown are spleen cells labeled for CD11c+CD205+CD8+ (left panels), and for MHC class II+CD205+CD86+ (right panels); data on all mice are summarized in the graph. (B) Treatment of CD11c-DTR mice expressing the DT receptor with DT or PBS (control). Bone marrow and spleen cells were collected and stained for CD11c. Following 2 injections of DT, CD11c-expressing cells were 80% to 90% depleted (see “Materials and methods”). (C) Survival of C3H.SW (H 2bc) recipients of marrow plus spleen cells from C57BL/6 [H-2b] donors that had been treated with AAT (AAT) only or treated with AAT and also injected with DT. Recipients of DC-depleted donor cells had greater weight loss and higher GVHD scores (lower panels) than mice transplanted from donors not injected with DT (n = 7, each arm). (D) Treatment of donor mice transgenic for CD11c DTR with DT also significantly reduced the proportion of CD4+CD25+FoxP3+ Tregs.
Effect of AAT on donor cell subpopulations. (A) Increase in the proportion of DCs in spleens of AAT-treated (AAT) and albumin-treated (controls) donors. Shown are spleen cells labeled for CD11c+CD205+CD8+ (left panels), and for MHC class II+CD205+CD86+ (right panels); data on all mice are summarized in the graph. (B) Treatment of CD11c-DTR mice expressing the DT receptor with DT or PBS (control). Bone marrow and spleen cells were collected and stained for CD11c. Following 2 injections of DT, CD11c-expressing cells were 80% to 90% depleted (see “Materials and methods”). (C) Survival of C3H.SW (H 2bc) recipients of marrow plus spleen cells from C57BL/6 [H-2b] donors that had been treated with AAT (AAT) only or treated with AAT and also injected with DT. Recipients of DC-depleted donor cells had greater weight loss and higher GVHD scores (lower panels) than mice transplanted from donors not injected with DT (n = 7, each arm). (D) Treatment of donor mice transgenic for CD11c DTR with DT also significantly reduced the proportion of CD4+CD25+FoxP3+ Tregs.
AAT treatment of CD11c DTR donor mice
To further determine the role of CD11c+ DCs, we used CD11c-DTR donor mice in which DCs can be ablated by DT. Figure 3B shows the presence of green fluorescent protein (GFP)+ CD11c+ DCs in the spleen of untreated CD11c-DTR mice and depletion by 80% to 90% following injections of DT. Therefore, CD11c DTR/C57BL/6 donor mice were treated with AAT (schema in Figure 2A and “Methods”), and were injected twice, 48 hours apart, with DT to ablate DCs. Twenty-four hours after the second DT injection, mice were killed and cells were harvested for transplantation. As shown in Figure 3C, depletion of CD11c+ DCs from the graft was associated with increased severity of GVHD and decreased survival compared with transplantation from AAT-treated donors not given DT (Figure 3C; P = .05). Of note, the threefold increase in CD4+CD25+FoxP3+ Tregs (n = 4, P = .0002) in AAT-treated donors was abrogated in donors injected with DT to ablate DCs (Figure 3D). These data imply that AAT-induced Treg expansion was DC-dependent.
We next determined the effect of direct ablation of FoxP3+ Tregs. As shown in supplemental Figure 1A, GFP+CD4+CD25+FoxP3+ T lymphocytes were present in the spleens of donor mice treated with AAT but not given DT. Two injections of DT, 48 hours apart, depleted CD4+CD25+FoxP3+ cells to <1% (P = .01) (supplemental Figure 1B). Therefore, next, FoxP3 DTR/C57BL/6 donors (with conditionally ablatable FoxP3+ Tregs) were treated twice with DT, 48 hours apart, and 24 hours after the second dose of DT cells were harvested. As shown in supplemental Figure 1C, transplantation of cells from DT-treated donors resulted in day 60 survival of 83%, not significantly different from controls (100%; P = .55), suggesting that the impact of donor AAT treatment on transplant outcome was primarily dependent upon DCs. Although a contribution of Tregs cannot be categorically excluded, the present data are in agreement with other studies, which show the requirement of donor DCs, as a means of mitigating GVHD.5,20
NK-cell expansion and GVL activity
We then asked whether donor AAT exposure was associated with a loss of the GVL effect (tumor transplant scheme, Figure 4A). As illustrated in Figure 4B-C, mice transplanted with cells from AAT-treated donors showed significantly lower A-20 tumor growth than mice transplanted from albumin treated donors as measured by luciferase signals (n = 6 per group, mean 4.8 ×106 vs 7 × 106 photons/second/mouse; P = .03). Thus, AAT treatment of the donor, in fact, provided enhanced antitumor activity, and donor pretreatment with AAT provided a survival benefit (Figure 4D). This prompted a reanalysis of transplanted cells, which revealed a fourfold higher proportion of NK cells (NK1.1+, CD49B+, CD122+, p46NK [CD335]+) in AAT-treated mice than in albumin-treated controls (Figure 4E). Furthermore, NK cells in AAT-treated donors showed upregulation of the activation receptor NKG2D (n = 4, P = .001) (Figure 5A). Consistent with in vivo data, NK cells from AAT-treated donors exhibited potent anti-A-20 cytotoxicity in vitro (Figure 4F). Similar results were obtained with a second murine cell line, BCL1 (supplemental Figure 2A-C). Additionally, in vitro results with a third cell line, T-cell lymphoma–derived EL4, showed potent anti-EL4 cytotoxicity of NK cells derived from AAT-pretreated donors (supplemental Figure 2D), supporting a broad applicability of AAT-enhanced NK-cell–mediated antitumor activity.
Effect of AAT on NK cells and antitumor activity. (A) Treatment scheme: injection of A20 tumor cells (A20 luc tumor) and transplantation; C57BL/6 (H-2bKb) donors, Balb/c (H-2d Kd) recipients (major mismatch). (B) Either donors (D) or recipients (R) or both were treated with AAT (controls received albumin), resulting in four donor/recipient combinations: transplantation of cells from AAT-treated donors into recipients treated with albumin (AAT/albumin; red); transplantation of cells from albumin-treated donors into AAT-treated recipients (albumin/AAT; black); transplantation of cells from AAT-treated donors into AAT-treated recipients (AAT/AAT; green), and transplantation of cells from albumin-treated donors into albumin-treated recipients (albumin/albumin; purple). Photon reading of luciferase activity; higher readings indicate greater tumor volume. (C) Transplantation with either donors (D) or recipients (R) given AAT while the other partner received albumin: AAT-treated donors (D:AAT; red) into albumin-treated recipients (R:albumin), and albumin-treated donors (D:albumin) into AAT-treated recipients (R:AAT; black). Tumors were imaged 3, 7, and 14 days after donor cell transplantation (ie, 7, 11, and 18 days after tumor cell injection). Tumor presence and size were determined using the luciferase reporter signal. Tumor progression was more delayed with transplantation of cells from AAT-treated donors than with direct recipient treatment. (n = 12, each condition, results presented as the mean of transplantations). (D) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors after treatment of donors and recipients with the various AAT/albumin combinations (P values displayed in the figure). (E) Proportions of spleen-derived NK cells, determined by flow cytometry (FACS) using several immunophenotypic markers, in C57BL/6 donor mice treated with albumin (left, control) or AAT (right), respectively; NK cells were increased in AAT-treated mice (right panel) (horizontal axis, CD49B; vertical axis, NKp46). (F) Cytolytic function of NK cells obtained from the spleens of AAT (AAT) or albumin treated (control) mice (n = 7, each condition), and assayed at effector/target ratios of 1:1; 5:1, 10:1, and 50:1 against A20 cells. Lytic activity (percent of CFSE+, PI+ cells) was measured after 18 hours of incubation of the effector cells. Shown are the mean ± SEM of 5 experiments.
Effect of AAT on NK cells and antitumor activity. (A) Treatment scheme: injection of A20 tumor cells (A20 luc tumor) and transplantation; C57BL/6 (H-2bKb) donors, Balb/c (H-2d Kd) recipients (major mismatch). (B) Either donors (D) or recipients (R) or both were treated with AAT (controls received albumin), resulting in four donor/recipient combinations: transplantation of cells from AAT-treated donors into recipients treated with albumin (AAT/albumin; red); transplantation of cells from albumin-treated donors into AAT-treated recipients (albumin/AAT; black); transplantation of cells from AAT-treated donors into AAT-treated recipients (AAT/AAT; green), and transplantation of cells from albumin-treated donors into albumin-treated recipients (albumin/albumin; purple). Photon reading of luciferase activity; higher readings indicate greater tumor volume. (C) Transplantation with either donors (D) or recipients (R) given AAT while the other partner received albumin: AAT-treated donors (D:AAT; red) into albumin-treated recipients (R:albumin), and albumin-treated donors (D:albumin) into AAT-treated recipients (R:AAT; black). Tumors were imaged 3, 7, and 14 days after donor cell transplantation (ie, 7, 11, and 18 days after tumor cell injection). Tumor presence and size were determined using the luciferase reporter signal. Tumor progression was more delayed with transplantation of cells from AAT-treated donors than with direct recipient treatment. (n = 12, each condition, results presented as the mean of transplantations). (D) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors after treatment of donors and recipients with the various AAT/albumin combinations (P values displayed in the figure). (E) Proportions of spleen-derived NK cells, determined by flow cytometry (FACS) using several immunophenotypic markers, in C57BL/6 donor mice treated with albumin (left, control) or AAT (right), respectively; NK cells were increased in AAT-treated mice (right panel) (horizontal axis, CD49B; vertical axis, NKp46). (F) Cytolytic function of NK cells obtained from the spleens of AAT (AAT) or albumin treated (control) mice (n = 7, each condition), and assayed at effector/target ratios of 1:1; 5:1, 10:1, and 50:1 against A20 cells. Lytic activity (percent of CFSE+, PI+ cells) was measured after 18 hours of incubation of the effector cells. Shown are the mean ± SEM of 5 experiments.
NKG2D is upregulated by AAT and is required for tumor cell kill. (A) NKG2D expression on NK cells from spleens of AAT-treated (blue) or albumin-treated (green) control mice; red = isotype control. Histograms are gated on NK1.1+CD3− populations (n = 5, P = .001). (B) Cytolytic function of NK cells obtained from spleens of AAT-treated donors (n = 7) and assayed at effector to target ratios of 1:1; 5:1, 10:1, and 50:1 against A-20 cells. NK 1.1 cells purified from AAT-treated donors were incubated with isotype control antibodies (Iso IgG2b), with anti-NKG2D antibody or with anti-Rae, the cognate receptor on A-20 cells. Lytic activity was measured after 18 hours of incubation with the effector cells (error bars indicate standard deviation of 5 independent experiments). (C) Recipient mice received 800 cGy TBI and were injected with 104 A-20 cells IV 4 days before transplantation of allogeneic cells from AAT-treated C57BL/6 donors. Tumors were imaged at 6, 10, and 14 days after HCT using the luciferase reporter signal. Tumor growth as determined by bioimaging was more aggressive in mice treated with NKG2D blocking antibody (anti-CD314 [NKG2D] left panel), leading to death or requiring sacrifice. In mice not given the blocking antibody (control, right panel) the tumor regressed by day 14. (n = 12, each condition, transplant experiments were done in triplicates, results presented as the sum of transplantations). (D) Tumor size (luciferase activity) with NKG2D blockade (red), Fc Isotype control IgG2b (black); photon reading of luciferase activity; higher readings indicate greater tumor volume (values represent mean ± SEM of 12 mice, P = .0043). (E) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors that had been treated with the above conditions, P values displayed in the figure, (n = 12 per arm).
NKG2D is upregulated by AAT and is required for tumor cell kill. (A) NKG2D expression on NK cells from spleens of AAT-treated (blue) or albumin-treated (green) control mice; red = isotype control. Histograms are gated on NK1.1+CD3− populations (n = 5, P = .001). (B) Cytolytic function of NK cells obtained from spleens of AAT-treated donors (n = 7) and assayed at effector to target ratios of 1:1; 5:1, 10:1, and 50:1 against A-20 cells. NK 1.1 cells purified from AAT-treated donors were incubated with isotype control antibodies (Iso IgG2b), with anti-NKG2D antibody or with anti-Rae, the cognate receptor on A-20 cells. Lytic activity was measured after 18 hours of incubation with the effector cells (error bars indicate standard deviation of 5 independent experiments). (C) Recipient mice received 800 cGy TBI and were injected with 104 A-20 cells IV 4 days before transplantation of allogeneic cells from AAT-treated C57BL/6 donors. Tumors were imaged at 6, 10, and 14 days after HCT using the luciferase reporter signal. Tumor growth as determined by bioimaging was more aggressive in mice treated with NKG2D blocking antibody (anti-CD314 [NKG2D] left panel), leading to death or requiring sacrifice. In mice not given the blocking antibody (control, right panel) the tumor regressed by day 14. (n = 12, each condition, transplant experiments were done in triplicates, results presented as the sum of transplantations). (D) Tumor size (luciferase activity) with NKG2D blockade (red), Fc Isotype control IgG2b (black); photon reading of luciferase activity; higher readings indicate greater tumor volume (values represent mean ± SEM of 12 mice, P = .0043). (E) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors that had been treated with the above conditions, P values displayed in the figure, (n = 12 per arm).
Blockade of NKG2D/Pan-RAE interferes with AAT-enhanced NK cytotoxicity
We next determined the impact of NKG2D/Pan-RAE blockade on NK-mediated cytotoxicity against A-20 (Figure 5A). Blockade of NKG2D (Pan-RAE) significantly reduced the cytolytic activity of NK cells (Figure 5B). Mice were given 2 i.p. injections of anti-NKG2D antibody, 96 hours apart, before transplantation. Mice so treated exhibited greater tumor growth than did controls (Figure 5C-D), in support of the concept that NKG2D receptor-dependent effects were instrumental for the GVL effect as reported by Diefenbach et al,21 an activity apparently enhanced by AAT exposure.
AAT alters mitochondrial biogenesis in a cell-specific manner
NKG2D expression is enhanced by increased production of reactive oxygen species (ROS), a marker of activation and proliferation.22 Available data indicate that cellular redox potential was profoundly altered by treatment with AAT.23 We, therefore, tested the hypothesis that AAT-dependent oxidative alterations occurred in a cell type–specific fashion, that is, involving DC, Treg, and NK cells, but probably not cytotoxic T lymphocytes.
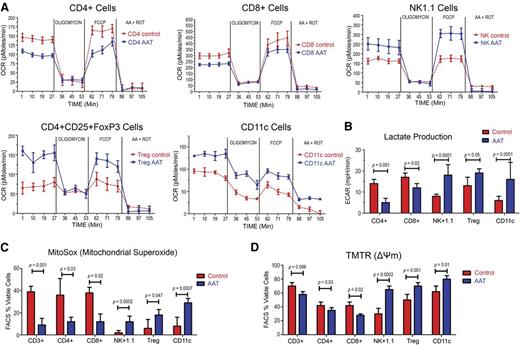
As shown in Figure 6A, the OCR in sorted NK1.1+ NK cells, CD4+ CD25+FoxP3+ T cells, and CD11c+ DCs was increased by 78%, 65%, and 45%, respectively. Conversely, CD8+ and CD4+ effector T cells from AAT-treated animals showed a decrease by 20% and 36%, respectively, compared with albumin-treated controls. Furthermore, AAT exposed CD8+ and CD4+ effector cells, but not Tregs, NK cells, and CD11c+ DCs, showed a deficit in respiratory reserve (stimulated by FCCP) as measured by OCR (Figure 6A), and reduced lactate generation as measured by proton production (ECAR) (Figure 6B). CD4+ and CD8+ cells treated with AAT showed lower proton production per cell than albumin-treated controls. Conversely, in NK1.1+, CD4+CD25+FoxP3+ Tregs, and CD11c+ cells, ECAR production was increased, confirming a cell-specific response to AAT (Figure 6A).
Effect of AAT on oxygen consumption, lactate production, mitochondrial superoxide production, and membrane potential. (A) Ex vivo OCR in NK1.1+, CD11c+, CD4+CD25+FoxP3+, CD8+, and CD4+ cells from donors treated with AAT or with albumin (Control). Cells were purified from donor spleens, plated in 24-well plates and exposed to oligomycin, FCCP, and ROT plus AA (see “Materials and methods”). OCR was measured as pmol O2/min/µg. (B) Lactate generation as measured by proton production (ECAR) in CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+, and CD11c+ cells from in wild-type C57/BL6 donors treated with albumin (control) or AAT (n = 5 mice per arm, experiments were done in triplicates from pooled tissue extracts). (C) Staining for mitochondrial superoxide in CD3+, CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+, and CD11c+ DCs (measured by MITOSOX). Bar plots represent the mean ± SD of 5 experiments; P values as displayed. (D) Mitochondrial TMTR in CD3+, CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+ and CD11c+ DCs. Bar plots represent the mean ± SD of 5 experiments (experiments were done in triplicates from pooled tissue extracts). P values as displayed, results were compared using the Student t test.
Effect of AAT on oxygen consumption, lactate production, mitochondrial superoxide production, and membrane potential. (A) Ex vivo OCR in NK1.1+, CD11c+, CD4+CD25+FoxP3+, CD8+, and CD4+ cells from donors treated with AAT or with albumin (Control). Cells were purified from donor spleens, plated in 24-well plates and exposed to oligomycin, FCCP, and ROT plus AA (see “Materials and methods”). OCR was measured as pmol O2/min/µg. (B) Lactate generation as measured by proton production (ECAR) in CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+, and CD11c+ cells from in wild-type C57/BL6 donors treated with albumin (control) or AAT (n = 5 mice per arm, experiments were done in triplicates from pooled tissue extracts). (C) Staining for mitochondrial superoxide in CD3+, CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+, and CD11c+ DCs (measured by MITOSOX). Bar plots represent the mean ± SD of 5 experiments; P values as displayed. (D) Mitochondrial TMTR in CD3+, CD4+, CD8+, NK1.1+, CD4+CD25+FoxP3+ and CD11c+ DCs. Bar plots represent the mean ± SD of 5 experiments (experiments were done in triplicates from pooled tissue extracts). P values as displayed, results were compared using the Student t test.
We predicted, therefore, that the observed mitochondrial responses would be related to increased mitochondrial superoxide production. As illustrated in Figure 6C, staining with MITOSOX revealed that NK1.1+ NK cells, CD4+CD25+ FoxP3+ Tregs, and CD11c+ DCs from AAT-treated donors generated significantly more superoxide than albumin-treated controls (n = 7; P = .0002, P = .047 and P = .0007, respectively). Conversely, AAT-exposed CD4+ and CD8+ cells, generated less superoxide than albumin-treated controls (n = 7, P = .03, and P = .02, respectively). Furthermore, the mitochondrial transmembrane potential (ΔΨm), determined with TMRM, was increased in NK1.1+, CD4+CD25+ FoxP3+ Tregs, and CD11c+ DCs (n = 7, P = .0002, P = .001, and P = .01, respectively).
Therefore, we next investigated the AAT effects transcriptional regulators of mitochondrial oxygen uptake, specifically, the transcriptional activity of NF-E2–related factor (NF-E2) and HO-1. There was significant upregulation of both transcriptional factors in splenocytes derived from AAT pretreated donors in comparison with albumin-treated controls (Figure 2D) (13 log2 for NF-E2, and 6 log2 for HO-1, P = .0001 and P = .003, respectively).
These metabolic changes indicated a high glycolysis–high oxidative phosphorylation profile for NK1.1+ NK cells, CD4+CD25+ FoxP3+ T-regs and CD11c+ DC, but lower glycolysis and oxidative phosphorylation in AAT-exposed CD4+ and CD8+ cells.
Discussion
In allogeneic HCT, interactions of donor and host cells occur in the setting of tissue injury and a dramatically altered cytokine milieu, induced by the conditioning treatment, which contributes to the development of GVHD. Our observations indicate that higher levels of AAT in donor plasma were associated with a reduced GVHD risk in the respective recipients. Along with data in murine models,7-9 these observations suggest that AAT levels might serve as a biomarker with predictive value for GVHD. However, AAT levels vary widely between individuals under steady state conditions, although they rise significantly during inflammation or infections.6,10-13,24,25 In view of the broad range of AAT levels, it would not be surprising to see different effects on donor cells and, as a result, differences in transplant outcome between recipients. In fact, data on rejection of corneal allografts and on the risk of developing immune-mediated type 2 diabetes show correlations with AAT levels.26,27 Although the current study addressed AAT levels only in transplant donors, available data overall suggest a role for AAT in the development of transplant tolerance.
The present results show that AAT treatment of healthy murine donors promotes the expansion of DCs, Tregs, and NK cells and decreases proinflammatory and enhances antiinflammatory cytokines, such as IL-10 and IL-1Ra. Because AAT does not suppress the activity of IL-2, an essential cytokine for the generation of Tregs,7-9 the resulting cytokine profile should favor the development of tolerance.7,28 Our data show a dominant effect of DCs, and the effect on FoxP3+ Tregs appeared to be DC-dependent. The global result of donor AAT treatment was a significant attenuation of GVHD in recipients, comparable or superior to the effects with direct treatment of recipients with AAT,8,9,29 and enhanced GVL activity.
These findings are in agreement with other reports, which show that IL-10 secreting (tolerogenic) CD11c+ DCs promote Treg cell expansion. Tolerogenic DCs and CD4+CD25+FoxP3+ Tregs have the capacity to impact alloimmune and autoimmune responses. In those settings, DCs typically express CD86 and MHC class II and secrete IL-10.30 Manipulation of DC, for example, with IL-10, facilitates tolerance induction in experimental islet cell transplantation.31,32 Retrospective analyses of clinical studies suggest that IL-10 promoter polymorphism that results in reduced IL-10 activity is associated with increased probability of acute GVHD and early mortality after HLA-matched sibling HCT,33,34 a “mirror image” of higher (AAT-induced) levels of functional IL-10 and reduced GVHD.
The mechanism of action of AAT is still incompletely understood. The immune-modulatory properties of AAT are accompanied by inhibition of free radical and nitric oxide generation,35 inhibition of cell apoptosis, regulation of immune cell adhesion, chemotaxis, and phagocytosis.36 The present studies show that AAT modulates immunity in a cell-specific way, favoring the establishment of transplant tolerance via its effects on CD8+CD205+MHC class II+ DCs37 and CD4+CD25+FoxP3+ Tregs.7,38 Importantly, suppression of GVHD did not interfere with GVL; in fact, the GVL effect was enhanced, apparently via expansion of NK1.1+, CD49B+, CD122+, p46NK+ NKG2D-expressing NK cells. Interactions of the NK-cell receptor NKG2D with its ligands, MICA and MICB (H60 and Pan–RAE in mice) are known to activate NK cells and to promote lysis of tumor cells, which express the cognate ligand. We propose, therefore, that the activation of NKG2D in NK cells by AAT was instrumental in the GVL effect observed in our allogeneic transplant model. Although many studies on GVL effects have focused on T cells, the role of NK cells is supported by preclinical and clinical studies, showing potent NK cell–mediated antitumor activity.39-41 In fact, ongoing clinical trials are determining the usefulness of preemptive donor NK-cell infusion in the prevention of post-HCT relapse.42,43
The present experiments provide new insights into the mechanism(s) by which AAT achieves its biological effects. Marrow and spleen cells from AAT-treated donors showed enhanced transcription of IL-10, IL-1Ra, and NF-E2/HO-1, concurrently, there was increased mitochondrial superoxide production, associated with increased aerobic glycolysis in a cell type–specific pattern. Although the OCR was increased in DCs, Tregs, and NK cells, it was decreased in CD4+ and CD8+ effector T lymphocytes, associated with decreased lactate production.44 Similar cell type–specific effects have been described by Yamashita et al who showed that HO-1 induction via cobalt protoporphyrin IX (CoPPIX) promoted the expansion of CD4+CD25+Foxp3+ Tregs, whereas suppressing activation and proliferation of alloreactive T cells, resulting in prolonged graft survival.45,46 In keeping with this concept, HO-1–deficient mice (HO-1−/−) showed higher numbers of activated peripheral CD4+ and CD8+ effector T cells than wild-type mice.45 The pattern of AAT effects in our allogeneic model is also consistent with the therapeutic benefits of AAT in models of autoimmune disease, where pathogenic cells have bioenergetic response, similar to the effector and cytotoxic T lymphocytes described here, that is, increased respiration/superoxide production and depleted antioxidant reserves.47,48 HO-1 is an antioxidant enzyme that opposes cell apoptosis. In the mouse heart, HO-1 activity stimulates the bigenomic mitochondrial biogenesis program via induction of NF-E2 gene expression and nuclear translocation.49 Here we show that both cytoprotective transcription factors are activated via AAT administration.
Mitochondrial metabolism can be pharmacologically manipulated during immune responses to promote the generation of memory cells and protective immunity.44,50 Recent data by Gatza et al show, for example, that the presence of Bz-423, a small-molecule inhibitor of the mitochondrial F(1)F(0) adenosine triphosphate synthase (F(1)F(0)-ATPase), lead to increased superoxide production and apoptosis in alloreactive T cells, generation of memory cells, and protective immunity.44 There are also preliminary data on donor treatment with statins that may reduce GVHD in recipients, possibly via interactions with cyclosporine that involve mitochondrial function.51 The present AAT data support that concept and suggest novel targets for controlling inflammation or adverse immune responses by suppressing oxygen consumption and lactate production; those responses are associated with an increase in superoxide production in donor-derived effector and cytotoxic T lymphocytes, leading to protective immunity in the murine GVHD model.
In conclusion, these experiments show that AAT exerts potent GVHD protection while maintaining or enhancing GVL activity. The functional impact of AAT appears to be dependent upon cell type–specific metabolic effects, leading to expansion of donor DC, Treg, and NK cells. The differential effect of AAT on GVHD and GVL function suggests a promising strategy for the prevention of acute GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ted Gooley, for statistical assistance, and Helen Crawford and Bonnie Larson for help with manuscript preparation.
This work was supported in part by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K08 DK085156 (A.M.M.) and HL036444 (H.J.D.), German Center for Lung Research (DZL), and Hannover Medical School (S.J. and T.W.), National Institutes of Health grants AI 15614 (C.A.D.) and ES020819-03 and P50 CA 138293-03 (D.M. and D.H.). These studies were also supported by an unrestricted grant from Dr Nezih Cereb, Histogenetics, Ossining, NY.
Authorship
Contribution: A.M.M. conceived the study, conducted most of the experiments, and wrote the paper; E.K., M.L., and D.M. carried out experiments; T.W. and D.H. provided critique to the manuscript; C.A.D. and S.J. provided critique and cowrote the paper; and H.J.D. conceived the study, provided critique, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Mario Marcondes, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 198024, Seattle, WA 98109-1024; e-mail: mmarcond@fhcrc.org.



![Figure 3. Effect of AAT on donor cell subpopulations. (A) Increase in the proportion of DCs in spleens of AAT-treated (AAT) and albumin-treated (controls) donors. Shown are spleen cells labeled for CD11c+CD205+CD8+ (left panels), and for MHC class II+CD205+CD86+ (right panels); data on all mice are summarized in the graph. (B) Treatment of CD11c-DTR mice expressing the DT receptor with DT or PBS (control). Bone marrow and spleen cells were collected and stained for CD11c. Following 2 injections of DT, CD11c-expressing cells were 80% to 90% depleted (see “Materials and methods”). (C) Survival of C3H.SW (H 2bc) recipients of marrow plus spleen cells from C57BL/6 [H-2b] donors that had been treated with AAT (AAT) only or treated with AAT and also injected with DT. Recipients of DC-depleted donor cells had greater weight loss and higher GVHD scores (lower panels) than mice transplanted from donors not injected with DT (n = 7, each arm). (D) Treatment of donor mice transgenic for CD11c DTR with DT also significantly reduced the proportion of CD4+CD25+FoxP3+ Tregs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/18/10.1182_blood-2014-04-570440/4/m_2881f3.jpeg?Expires=1769110709&Signature=x8KwWUS5NQa5XsvmlNQ1guirjWf7KU1fthM2pBwJbq2oKaCYjmENn-miMcQYHaPJ-Uty~RO1bXW~1A2S2vj5ypg0jAKv9Kynvx6Jw8CBpEpom3vT6kp9~thbC3zzlYzI1xNpi63rffDRqeXcRx~mGm3qTF0yc7e8hJblIoTKdvVPl59la9pIMOrLYoe4DV-7JCiUpsygDjpubeRQ6PL7IOi9DQQ8CeOb9TCQzYOuqp5vK~hgSvD-23pzM79B5NQPxoOEpTIzCW8UqfipoU5ze-TyVjsilqy27SmAava1Znf2x75hGul8j04LhPM7QF-Qizz6EfenoY3E48bCN3lGyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effect of AAT on NK cells and antitumor activity. (A) Treatment scheme: injection of A20 tumor cells (A20 luc tumor) and transplantation; C57BL/6 (H-2bKb) donors, Balb/c (H-2d Kd) recipients (major mismatch). (B) Either donors (D) or recipients (R) or both were treated with AAT (controls received albumin), resulting in four donor/recipient combinations: transplantation of cells from AAT-treated donors into recipients treated with albumin (AAT/albumin; red); transplantation of cells from albumin-treated donors into AAT-treated recipients (albumin/AAT; black); transplantation of cells from AAT-treated donors into AAT-treated recipients (AAT/AAT; green), and transplantation of cells from albumin-treated donors into albumin-treated recipients (albumin/albumin; purple). Photon reading of luciferase activity; higher readings indicate greater tumor volume. (C) Transplantation with either donors (D) or recipients (R) given AAT while the other partner received albumin: AAT-treated donors (D:AAT; red) into albumin-treated recipients (R:albumin), and albumin-treated donors (D:albumin) into AAT-treated recipients (R:AAT; black). Tumors were imaged 3, 7, and 14 days after donor cell transplantation (ie, 7, 11, and 18 days after tumor cell injection). Tumor presence and size were determined using the luciferase reporter signal. Tumor progression was more delayed with transplantation of cells from AAT-treated donors than with direct recipient treatment. (n = 12, each condition, results presented as the mean of transplantations). (D) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors after treatment of donors and recipients with the various AAT/albumin combinations (P values displayed in the figure). (E) Proportions of spleen-derived NK cells, determined by flow cytometry (FACS) using several immunophenotypic markers, in C57BL/6 donor mice treated with albumin (left, control) or AAT (right), respectively; NK cells were increased in AAT-treated mice (right panel) (horizontal axis, CD49B; vertical axis, NKp46). (F) Cytolytic function of NK cells obtained from the spleens of AAT (AAT) or albumin treated (control) mice (n = 7, each condition), and assayed at effector/target ratios of 1:1; 5:1, 10:1, and 50:1 against A20 cells. Lytic activity (percent of CFSE+, PI+ cells) was measured after 18 hours of incubation of the effector cells. Shown are the mean ± SEM of 5 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/18/10.1182_blood-2014-04-570440/4/m_2881f4.jpeg?Expires=1769110709&Signature=gN5PJkyS2tf9Vgk9i7YkNG5TqrK23U38FSKfhINwHcuqAqhIOF474nRWsaiql0W7HqqRRCif8lFoxKE0c19hs~EvN8J0np7wUo23JgTs7V~8GZGyV3nh~RJ0fiqVWn8IXHiXe1mudVvxthnmKwLA5apltGbY~riDrozCFyoMh-BiBkLpk0znVgxDBdjW32e9Reni-Nf~dM-qv7Lw9tmqG-UOCngpc5DAcvrFvuuhr3AczJaHn6V~VBe8X8soGDlRe5vSosKTA-HOKQ-jPnqG~hT1fUEA~yVoM~s6kmPhgzSyNcQatZP~srNLaVxtMH71a87ccDWEh20t3QKBXjP9CA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. NKG2D is upregulated by AAT and is required for tumor cell kill. (A) NKG2D expression on NK cells from spleens of AAT-treated (blue) or albumin-treated (green) control mice; red = isotype control. Histograms are gated on NK1.1+CD3− populations (n = 5, P = .001). (B) Cytolytic function of NK cells obtained from spleens of AAT-treated donors (n = 7) and assayed at effector to target ratios of 1:1; 5:1, 10:1, and 50:1 against A-20 cells. NK 1.1 cells purified from AAT-treated donors were incubated with isotype control antibodies (Iso IgG2b), with anti-NKG2D antibody or with anti-Rae, the cognate receptor on A-20 cells. Lytic activity was measured after 18 hours of incubation with the effector cells (error bars indicate standard deviation of 5 independent experiments). (C) Recipient mice received 800 cGy TBI and were injected with 104 A-20 cells IV 4 days before transplantation of allogeneic cells from AAT-treated C57BL/6 donors. Tumors were imaged at 6, 10, and 14 days after HCT using the luciferase reporter signal. Tumor growth as determined by bioimaging was more aggressive in mice treated with NKG2D blocking antibody (anti-CD314 [NKG2D] left panel), leading to death or requiring sacrifice. In mice not given the blocking antibody (control, right panel) the tumor regressed by day 14. (n = 12, each condition, transplant experiments were done in triplicates, results presented as the sum of transplantations). (D) Tumor size (luciferase activity) with NKG2D blockade (red), Fc Isotype control IgG2b (black); photon reading of luciferase activity; higher readings indicate greater tumor volume (values represent mean ± SEM of 12 mice, P = .0043). (E) Survival of Balb/c (H-2d Kd) recipients of C57BL/6 [H-2b] donors that had been treated with the above conditions, P values displayed in the figure, (n = 12 per arm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/18/10.1182_blood-2014-04-570440/4/m_2881f5.jpeg?Expires=1769110709&Signature=bi9hnK9YvhskAYiGQWDhyDBEf24Du8evVwS8uA9g7ScuRJYkTSr6VNegFW2b-5Ckr2ZHT9ax41KzA-iXtJX5x2LtQlCQJJtTM3qV1qqMofeSnouGm4GNHtLG47IiNxxAGHvSFvuOylR0vwvaTwyjNNdJB7S1Hwl7D4MlPQ-8sCarkcbI-iyVUUdeJ-ZzVAe2gEo6LXFp1QyNmGPhYHioYmzThaVPXGT95z2UYzmIIM3DHk3n0Cj9UfNGN2t1~DoKg-IKdvfITmpWpFv9C-a5dgLllNjmOw9FI2qJENTESMCbNeCGfQWVwMwLFMmNfEX~9Xpsd2CYMmkKKmJdRj8UXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)