Key Points
Acute myeloid leukemia decreases osteoblast numbers in humans and mice.
Reinstatement of osteoblast number and function in leukemic mice by a pharmacologic approach reduces tumor burden in all sites and prolongs survival.
Abstract
The bone marrow niche is thought to act as a permissive microenvironment required for emergence or progression of hematologic cancers. We hypothesized that osteoblasts, components of the niche involved in hematopoietic stem cell (HSC) function, influence the fate of leukemic blasts. We show that osteoblast numbers decrease by 55% in myelodysplasia and acute myeloid leukemia patients. Further, genetic depletion of osteoblasts in mouse models of acute leukemia increased circulating blasts and tumor engraftment in the marrow and spleen leading to higher tumor burden and shorter survival. Myelopoiesis increased and was coupled with a reduction in B lymphopoiesis and compromised erythropoiesis, suggesting that hematopoietic lineage/progression was altered. Treatment of mice with acute myeloid or lymphoblastic leukemia with a pharmacologic inhibitor of the synthesis of duodenal serotonin, a hormone suppressing osteoblast numbers, inhibited loss of osteoblasts. Maintenance of the osteoblast pool restored normal marrow function, reduced tumor burden, and prolonged survival. Leukemia prevention was attributable to maintenance of osteoblast numbers because inhibition of serotonin receptors alone in leukemic blasts did not affect leukemia progression. These results suggest that osteoblasts play a fundamental role in propagating leukemia in the marrow and may be a therapeutic target to induce hostility of the niche to leukemia blasts.
Introduction
Trabecular bone formation and establishment of hematopoiesis within the marrow cavity are intimately coordinated.1 Osteoblasts, the bone-forming cells, are a regulatory component of the hematopoietic stem cell (HSC) niche influencing the homing and development of neighboring HSCs.2,3 Primitive hematopoietic cells in the bone marrow and implanted lineage-negative HSCs localize adjacent to the endosteal surface where osteoblasts reside.4 Genetic evidence supports the idea that, similar to other stromal cells such as perivascular and endothelial cells, osteoblast progenitors or mesenchymal stem cells with osteoblastic capability are implicated in HSC lineage determination survival and proliferation.5-10 Perturbation of cells of the osteoblast lineage can either stimulate6,11,12 or limit HSC expansion,13,14 promote quiescence and HSC mobilization,15-17 support expansion of the erythroid lineage,11,12 regulate B lymphopoiesis,6,18 and differentially affect progression of myeloid leukemias through parathyroid hormone (PTH)/transforming growth factor β,19 whereas osteocytes expand the myeloid lineage through disruption of Gsα signaling.20 Similarly, osteoblast dysfunction results in pancytopenia via distinct mechanisms. In contrast, osteoclasts, the bone-resorbing cells, appear to be dispensable for the maintenance and mobilization of HSCs.21
Deregulation of hematopoiesis is associated with hematologic malignancies, which may in part be mediated by the microenvironment.22 However, although little is known about the role of osteoblasts in hematologic diseases, the marrow niche has been recently found to determine the fate of lymphoblastic and B-cell malignancies.10,23-25 In addition, mice with defective retinoblastoma (Rb), retinoic acid receptor gamma (RARg), or Notch signaling in hematopoietic and nonhematopoietic cells were shown to develop myeloid disorders, mimicking human myeloproliferative neoplasms, characterized by clonal proliferation of various myeloid lineages, associated with a high frequency of transformation to acute myeloid leukemia (AML).26,27 Cells of the osteoblast lineage were directly implicated in this process when global disruption of gene expression by deletion of Dicer1 in osteoblast progenitors induced myelodysplasia (MDS), another preleukemic disease.28 The fact that perturbation of osteolineage cells can lead to the disorganization of the hematopoietic system, including development of MDS and AML,26,28 suggests that genetic alterations in these cells can initiate a multistep pathway to hematologic malignancies arising in the bone marrow. Indeed, recently constitutive activation of β-catenin signaling specifically in osteoblasts was shown to induce AML in mice through upregulation of Jagged1 expression in osteoblasts and Notch signaling in HSC progenitors.29 That the β-catenin/Notch signaling pathway between osteoblasts and leukemia cells was active in 38% of AML/MDS patients examined indicated its potential implication in human disease.
Recent studies indicated that leukemic blasts in mice compromise the function of osteoblasts without increasing bone resorption.25 We show that MDS and AML patients have a twofold reduction in osteoblast numbers and activity, suggesting that osteoblasts are an important target of leukemic blasts. Collectively, these observations led us to hypothesize that leukemia cells may suppress osteoblast function as a means to permit growth and progression of leukemia, and that osteoblasts may also affect the fate of the leukemic blasts. Using genetic and pharmacologic interventions, we show that depletion of osteoblasts in mice with acute leukemia favors tumor progression and that preservation of osteoblast numbers allows for recovery of normal marrow function, hinders tumor burden, and prolongs survival, suggesting that manipulating osteoblast numbers or function may be a potential means to treat leukemia by creating a hostile niche that will hinder leukemia growth.
Methods
Animals
BALB/c and B6(Cg)-Tyrc-2J (albino C57BL/6) mice were purchased from the Jackson Laboratories. DTAosb mice were maintained on a C57BL/6 background and generated by crossing transgenic mice expressing Cre under the control of 2.3 kb of the proximal promoter of the mouse pro-al(I) collagen gene [α1(I)Collagen-Cre] with mice in which the diphtheria toxin α subunit (DTA) has been introduced into the ubiquitously expressed ROSA26 behind a loxP-flanked STOP cassette.30 The 2.3 kb α1(I)Collagen-Cre transgene is expressed at high levels in osteoblasts and odontoblasts specifically.31 DTAosb mice were heterozygous for the floxed DTA allele, and their littermates carrying the inactive form of DTA were used as wild-type (WT) controls. Experiments were performed in male and female immunocompetent animals and were approved by the Institutional Animal Care and Use Committee of Columbia University. Mice were housed under pathogen-free conditions at animal facilities at Columbia University.
Patient samples
Bone marrow biopsy samples were obtained from patients with AML and MDS diagnosed between 2000 and 2008 under a research exempt waiver approved by the institutional review board (IRB) of Memorial Sloan-Kettering Hospital and Human Biospecimen Utilization Committee. Bone marrow biopsies and serum were obtained from MDS and AML patients after informed consent, stored in an IRB-approved Tissue Repository at Columbia University Medical Center, and used according to protocols approved by the IRB. Bone marrow biopsies from 21 age-matched healthy male controls were randomly selected from a previous study.32 Serum from age-matched healthy controls for osteocalcin measurements was obtained under an IRB-approved study at Columbia University. Research was conducted in accordance with the Declaration of Helsinki. Control subjects had no history of low-trauma fracture, comorbidities, or drug therapy known to affect bone metabolism.
Statistical analysis
Results are given as mean ± standard error of the mean or percentages. Differences in continuous variables were calculated using unpaired 2-tailed Student t test or analysis of variance, as appropriate. Correlations between osteoblast numbers and percentage of blasts in the bone marrow or luminescence counts (nonnormally distributed variables) were assessed by the Spearman correlation test. Time-to-event analysis was used to assess medium survival time to death. Kaplan-Meier curves were generated to illustrate time to death, stratified by group status. Statistical significance of the between-group difference in the median time to end point was assessed by the log-rank test. Statistical analyses were performed using XLSTAT (2012.6.02; Addinsoft), SAS (version 9.2; SAS institute Inc., Cary, NC), and SigmaPlot (version 10.0; Systat Software Inc., San Jose, CA).
Results
Compromised osteoblast numbers in human and murine acute leukemia
A communication between leukemic blasts and bone cells was initially determined by examining the impact of acute leukemia on osteoblasts of MDS and AML patients. Osteoblast number was 55% lower in subjects with AML/MDS, as compared with healthy sex- and age-matched (62.8 ± 2.6 vs 61.7 ± 2.8 years; P = .64) controls (Figure 1A). The majority of patients (81%) had not received treatment of the underlying hematologic disorder at the time of obtaining the bone biopsy (supplemental Table 1). Serum levels of osteocalcin, a marker of osteoblast numbers and function, were also reduced in a subgroup of AML/MDS untreated patients, as compared with sex- and age-matched healthy controls (67.9 ± 1.6 vs 67.8 ± 2.7 years; P = .97) (Figure 1B). Consistent with the observations in humans, mice injected with the myelomonocytic leukemia cell line WEHI-3B or the lymphoblastic EL4 cells33-35 showed rapid decrease in bone volume because of decreased osteoblast numbers without changes in osteoclast numbers or bone resorption (Figure 1C-F). In both models, leukemia cell engraftment was observed in the bone marrow and liver, and blasts were noted in the blood (supplemental Figure 1A-F). WEHI-3B cells also engrafted in the spleen (supplemental Figure 1G). Leukemic blasts localized in close contact with osteoblasts on the endosteal surface (supplemental Figure 1H-J). The decrease in osteoblast numbers in leukemic mice was because of compromised osteoblast differentiation as evidenced by reduced expression of markers of osteoblast formation (supplemental Figure 1K). Consistent with the in vivo observations, treatment of primary osteoblasts with conditioned medium from leukemic blasts suppressed their differentiation (supplemental Figure 1L). The reduction in the osteoblast numbers in mouse models of leukemia is in line with previous findings.25
Acute leukemia decreases osteoblast numbers and function in humans and in mice. (A) Osteoblast number per trabecular bone area (N.Ob/T.Ar) in bone biopsies of 21 male subjects with AML or MDS, and 21 sex- and age-matched healthy controls. (B) Serum osteocalcin levels in a subset of 9 AML/MDS untreated subjects and 9 matched controls. (C-F) Representative vertebral section images from 2-month-old immunocompetent BALB/c or albino C57BL/6 mice injected with the myeloid-monocytic WEHI-3B (n = 8-10 mice per group) (C) or the lymphoblastic EL4 (n = 5-7 mice per group) (D) leukemia cells, respectively. Mineralized bone matrix is stained in black by Von Kossa reagent. Images at ×50. Serum levels of osteocalcin (E) and cross-linked C-telopeptide (CTX) (F) in WT animals and leukemic mice injected with WEHI-3B cells (n = 8-10 mice per group). *P < .05 vs normal control or WT mice. BFR, bone formation rate; BV/TV, bone volume per trabecular volume; N.Ob/T.Ar, number of osteoblasts per trabecular area; Oc.S./B.S., osteoclast surface per bone surface.
Acute leukemia decreases osteoblast numbers and function in humans and in mice. (A) Osteoblast number per trabecular bone area (N.Ob/T.Ar) in bone biopsies of 21 male subjects with AML or MDS, and 21 sex- and age-matched healthy controls. (B) Serum osteocalcin levels in a subset of 9 AML/MDS untreated subjects and 9 matched controls. (C-F) Representative vertebral section images from 2-month-old immunocompetent BALB/c or albino C57BL/6 mice injected with the myeloid-monocytic WEHI-3B (n = 8-10 mice per group) (C) or the lymphoblastic EL4 (n = 5-7 mice per group) (D) leukemia cells, respectively. Mineralized bone matrix is stained in black by Von Kossa reagent. Images at ×50. Serum levels of osteocalcin (E) and cross-linked C-telopeptide (CTX) (F) in WT animals and leukemic mice injected with WEHI-3B cells (n = 8-10 mice per group). *P < .05 vs normal control or WT mice. BFR, bone formation rate; BV/TV, bone volume per trabecular volume; N.Ob/T.Ar, number of osteoblasts per trabecular area; Oc.S./B.S., osteoclast surface per bone surface.
Osteoblast ablation increases leukemia burden
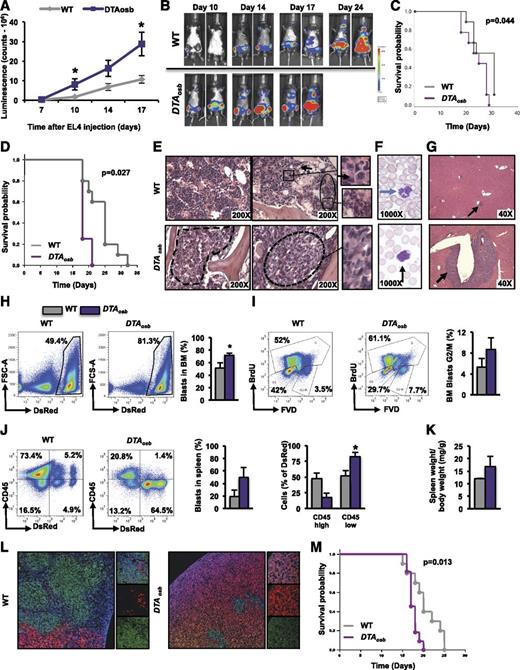
The rapid decrease in osteoblasts raised the possibility that compromised osteoblast function may be required for leukemia progression. We examined this notion in vivo. Mice engineered to lack 50% of their osteoblasts (DTAosb mice) (supplemental Figure 2A), and injected with EL4 cells, showed a threefold increase in tumor burden as compared with WT littermates (Figure 2A-B). Hind limb paralysis incidence, an indicator of aggravated disease progression, was increased in DTAosb mice (supplemental Figure 2B-C). Confirming increased tumor burden, DTAosb mice had significantly shorter overall survival (Figure 2C-D; supplemental Figure 2D).
Increased leukemia blast infiltration in DTAosb mice. (A-C, E-I) Syngeneic 2-month-old C57BL/6 WT or DTAosb mice were injected with EL4-GFP-Luc cells and leukemia progression was assessed with an in vivo imaging system. (A, B) Luminescence intensity was quantified in the whole body at different time points (A) and shown here in representative images (B). (C) Overall survival of male WT and DTAosb mice (A-C, n = 9-11 mice per group). (D) Overall survival of female WT and DTAosb mice (D, n = 4 DTAosb and n = 10 WT mice per group) measured in a separate experiment performed as described for panels A-C. (E) Hematoxylin and eosin (H&E) staining of bone marrow sections. The area depicted by solid line represents normal bone marrow, with evidence of trilineage hematopoiesis, whereas areas delineated by dotted lines indicate blast infiltrates. Right panels show inset magnifications of indicated areas. Black arrows indicate isolated blasts. (F) Wright staining of blood smears. Blasts with fine chromatin and prominent nucleoli are seen in DTAosb mice. Blue arrow indicates normal neutrophil, whereas black arrow indicates a blast. (G) H&E staining of liver sections. Arrows indicate blast infiltrates. (H) Representative flow cytometry plots and percentage of MLL-AF9 cells in the bone marrow. (I) Representative flow cytometry plots and percentage of marrow MLL-AF9 cells in G2/M phase. (J) Representative flow cytometry plots and percentage of MLL-AF9 cells in the spleen. (K) Spleen weight over total body weight in leukemic mice. (L) Immunofluorescence staining of spleen sections showing DsRed-MLL-AF9 cells, CD45-expressing (green) cells, and 4,6-diamidino-2-phenylindole nuclei staining (blue). Left panel (×10); right small panels (×63). (M) Survival probability of male and female WT and DTAosb mice injected with MLL-AF9 cells (n = 11 DTAosb and n = 10 WT mice). (D-F, n = 5-6 mice per group; G-L, n = 3 mice in WT group and n = 4 mice in DTAosb group). *P < .05 vs WT mice.
Increased leukemia blast infiltration in DTAosb mice. (A-C, E-I) Syngeneic 2-month-old C57BL/6 WT or DTAosb mice were injected with EL4-GFP-Luc cells and leukemia progression was assessed with an in vivo imaging system. (A, B) Luminescence intensity was quantified in the whole body at different time points (A) and shown here in representative images (B). (C) Overall survival of male WT and DTAosb mice (A-C, n = 9-11 mice per group). (D) Overall survival of female WT and DTAosb mice (D, n = 4 DTAosb and n = 10 WT mice per group) measured in a separate experiment performed as described for panels A-C. (E) Hematoxylin and eosin (H&E) staining of bone marrow sections. The area depicted by solid line represents normal bone marrow, with evidence of trilineage hematopoiesis, whereas areas delineated by dotted lines indicate blast infiltrates. Right panels show inset magnifications of indicated areas. Black arrows indicate isolated blasts. (F) Wright staining of blood smears. Blasts with fine chromatin and prominent nucleoli are seen in DTAosb mice. Blue arrow indicates normal neutrophil, whereas black arrow indicates a blast. (G) H&E staining of liver sections. Arrows indicate blast infiltrates. (H) Representative flow cytometry plots and percentage of MLL-AF9 cells in the bone marrow. (I) Representative flow cytometry plots and percentage of marrow MLL-AF9 cells in G2/M phase. (J) Representative flow cytometry plots and percentage of MLL-AF9 cells in the spleen. (K) Spleen weight over total body weight in leukemic mice. (L) Immunofluorescence staining of spleen sections showing DsRed-MLL-AF9 cells, CD45-expressing (green) cells, and 4,6-diamidino-2-phenylindole nuclei staining (blue). Left panel (×10); right small panels (×63). (M) Survival probability of male and female WT and DTAosb mice injected with MLL-AF9 cells (n = 11 DTAosb and n = 10 WT mice). (D-F, n = 5-6 mice per group; G-L, n = 3 mice in WT group and n = 4 mice in DTAosb group). *P < .05 vs WT mice.
In a parallel study, tumor burden 15 days following leukemic cell injection was increased in the bone marrow (Figure 2E and supplemental Figure 2F-H), blood (Figure 2F), and liver (Figure 2G and supplemental Figure 2I) of DTAosb mice compared with WT controls, the majority of which presented only sporadic leukemic infiltrates in the bone marrow and liver, with rarely seen circulating blasts.
To examine whether similar effects occur in myeloid leukemias, we used the murine committed granulocyte macrophage progenitors expressing the mixed lineage leukemia (MLL)-AF9 fusion protein encoded by the t(9;11)(p22;q23). This is a mouse AML model that shares several common features with MLL translocation-driven human AML.36 We found that osteoblast ablation did not alter disease distribution but increased leukemia burden in all tissues infiltrated by MLL-AF9 cells, blood, bone marrow, and spleen by increasing the proliferation of MLL-AF9 cells in the bone marrow, but without affecting their quiescence or apoptosis (Figure 2H-M and supplemental Figure 3A-I). MLL-AF9 cells do not infiltrate the liver. Interestingly, osteoblast ablation appeared to alter the phenotype of the MLL-AF9 cells by favoring a decrease in CD45 expression (Figure 2J and supplemental Figure 3C-D). Circulating MLL-AF9 blasts were observed earlier, on day 9 following injection, in the blood of DTAosb mice as compared with WT littermates, and their numbers remained elevated in DTAosb mice on day 14, the day of euthanization (supplemental Figure 3I). In a parallel experiment assessing life span, DTAosb mice transplanted with MLL-AF9 cells had significantly reduced survival as compared with WT littermates (Figure 2M).
Osteoblast ablation alters HSC lineage determination
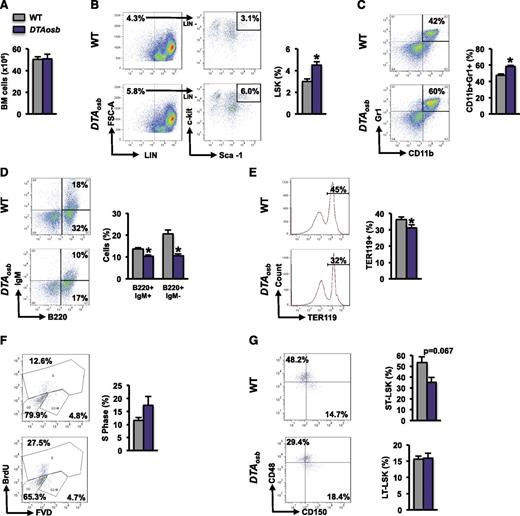
Because osteoblasts have been implicated in hematopoiesis, we examined whether the effects of osteoblast depletion on leukemic blasts were associated with changes in hematopoiesis. At 10 weeks of age, DTAosb mice showed normal marrow cellularity (Figure 3A) but an increase in the marrow hematopoietic stem and progenitor cell pool size, defined by Lin−Sca+c-Kit+ (LSK) cells, compared with WT littermates (Figure 3B). The myeloid/monocytic cell population (CD11b+/Gr1+) increased (Figure 3C), whereas B lymphopoiesis and erythropoiesis were compromised (Figure 3D-E). Spleen size was not altered in 10-week-old mice but increased with increasing age suggesting an age-dependent increase in extramedullary hematopoiesis in DTAosb mice (supplemental Figure 2J-K). Similar results were observed in DTAosb mice that had been injected with MLL-AF9 cells. Osteoblast ablation increased the percentage of LSK progenitors in the S phase without affecting long-term LSK cells but suppressed the percentage of short-term LSK (ST-LSK) progenitors (Figure 3F-G).
Deregulated hematopoiesis in DTAosb mice. (A) Whole bone marrow mononuclear cells collected by crushing 1 femur and tibia of 2-month-old WT and DTAosb mice. (B-E) Flow cytometry analysis of bone marrow cells showing representative images and percentage of LSK cells (B), myeloid cell population (CD11b+/Gr1+) (C), mature (B220+/IgM+) and immature (B220+/IgM−) B-lymphopoietic subsets (D), and erythroid progenitors (Ter119+) in the bone marrow (E). (F-G) Syngeneic 2-month-old C57BL/6 WT or DTAosb mice were injected with MLL-AF9 cells and harvested 14 days after injection. Flow cytometry analysis of bone marrow showing representative plots and percentage of LSK cells in the S-phase (F) and percentage of LT-LSK and ST-LSK cells (G). (A-E = 11-14 mice per group; F-G, n = 3 mice in WT group and n = 4 mice in DTAosb group). *P < .05 vs WT mice. LT-LSK, long-term LSK.
Deregulated hematopoiesis in DTAosb mice. (A) Whole bone marrow mononuclear cells collected by crushing 1 femur and tibia of 2-month-old WT and DTAosb mice. (B-E) Flow cytometry analysis of bone marrow cells showing representative images and percentage of LSK cells (B), myeloid cell population (CD11b+/Gr1+) (C), mature (B220+/IgM+) and immature (B220+/IgM−) B-lymphopoietic subsets (D), and erythroid progenitors (Ter119+) in the bone marrow (E). (F-G) Syngeneic 2-month-old C57BL/6 WT or DTAosb mice were injected with MLL-AF9 cells and harvested 14 days after injection. Flow cytometry analysis of bone marrow showing representative plots and percentage of LSK cells in the S-phase (F) and percentage of LT-LSK and ST-LSK cells (G). (A-E = 11-14 mice per group; F-G, n = 3 mice in WT group and n = 4 mice in DTAosb group). *P < .05 vs WT mice. LT-LSK, long-term LSK.
These results indicate that osteoblast ablation alters lineage determination of HSCs by altering the myeloid and lymphocytic compartments and increases leukemia burden in the marrow cavity. The increase in myeloid populations coupled with a decrease in B lymphopoiesis may predispose subjects to an increase in the burden of leukemia.
Increased osteoblast numbers suppress acute lymphoblastic leukemia
Given the finding that leukemic mice with osteoblast ablation have increased tumor burden and shorter survival, we hypothesized that the course of leukemia would be ameliorated in mice with increased osteoblast numbers. To test this hypothesis, we treated mice with a compound, LP533401, that in rodents increases osteoblast numbers without affecting osteoclasts.37-40 This effect involves inhibition of tryptophan hydroxylase-1 (Tph-1), an enzyme required in gut-derived-serotonin synthesis that suppresses osteoblast numbers.
Adult, healthy mice treated with the Tph-1 inhibitor LP533401 showed 30% decrease in circulating serotonin levels, with a consequent 30% increase in osteoblast numbers (supplemental Figure 4A-B). Administration of LP533401 to mice injected with EL4 cells inhibited the decrement in osteoblast numbers and trabecular bone volume (supplemental Figure 4B), prolonged survival (Figure 4A), and decreased leukemic infiltration (Figure 4B-C). Among 10 leukemic mice treated with LP533401, 2 cleared, 1 developed the disease at a late stage, and 2 never developed leukemia (Figure 4C and supplemental Figure 4C). In leukemic mice, LP533401 treatment decreased the frequency of circulating blasts in peripheral blood (Figure 4D-E) and the incidence of hind limb paralysis (supplemental Figure 4D). LP533401 treatment also tended to ameliorate anemia (supplemental Figure 4E-G) and restore white blood cells, neutrophils, monocytes, and lymphocytes to normal numbers (supplemental Figure 4H-K), although with no statistically significant difference, probably because of the small number of animals per group. Most importantly, osteoblast numbers inversely correlated with leukemia burden (Figure 4F).
Increased osteoblast numbers suppress acute lymphoblastic leukemia. Syngeneic 2-month-old albino C57BL/6 mice were treated orally with LP533401 (200 mg/kg of body weight per day) or vehicle for 15 days and then injected with EL4-GFP-Luc cells. (A) Overall survival of mice treated with LP533401 or vehicle. (B) Whole body luminescence counts assessing leukemia progression. (C) Representative whole body luminescence images. (D) Wright staining of blood smears. Black arrows indicate blasts, blue arrows indicate normal neutrophils, and red arrow indicates lymphocyte. Right panels show inset magnifications of blasts. (E) Percentage of blasts (mean ± standard error of the mean) counted in a total count of 50 cells in peripheral blood smears. (F) Correlation between osteoblast numbers and leukemia tumor burden assessed by luminescence quantification on day 28 (n = 9 mice per group). Overall survival (G) and whole body luminescence counts (H) in syngeneic 2-month-old albino C57BL/6 mice treated orally with LP533401 (200 mg/kg of body weight per day) 1 day following injection with EL4-GFP-Luc cells (n = 10-11 mice per group). *P < .05 vs vehicle-treated mice.
Increased osteoblast numbers suppress acute lymphoblastic leukemia. Syngeneic 2-month-old albino C57BL/6 mice were treated orally with LP533401 (200 mg/kg of body weight per day) or vehicle for 15 days and then injected with EL4-GFP-Luc cells. (A) Overall survival of mice treated with LP533401 or vehicle. (B) Whole body luminescence counts assessing leukemia progression. (C) Representative whole body luminescence images. (D) Wright staining of blood smears. Black arrows indicate blasts, blue arrows indicate normal neutrophils, and red arrow indicates lymphocyte. Right panels show inset magnifications of blasts. (E) Percentage of blasts (mean ± standard error of the mean) counted in a total count of 50 cells in peripheral blood smears. (F) Correlation between osteoblast numbers and leukemia tumor burden assessed by luminescence quantification on day 28 (n = 9 mice per group). Overall survival (G) and whole body luminescence counts (H) in syngeneic 2-month-old albino C57BL/6 mice treated orally with LP533401 (200 mg/kg of body weight per day) 1 day following injection with EL4-GFP-Luc cells (n = 10-11 mice per group). *P < .05 vs vehicle-treated mice.
Direct comparison of leukemia progression in a parallel group of mice, 15 days following EL4 cell injection, showed preservation of osteoblast numbers and decreased tumor burden in LP533401-treated mice (supplemental Figure 5A-C). Histologic analysis of the marrow revealed extended normal areas and sporadic scattered clusters of blasts in LP533401-treated mice, in contrast to untreated animals, which showed heavy infiltrations of blasts (supplemental Figure 6A-C). Whereas 80% of leukemic mice treated with LP533401 had <20% of blasts in the bone marrow, only 40% of vehicle-treated leukemic animals showed similar mild marrow infiltration (supplemental Figure 6D). The remaining 20% of animals from the LP533401-treated group presented with 40% to 60% blasts in their marrow, as compared with 60% of mice in the vehicle group, which had 40% to 80% of blasts. Megakaryocyte numbers were increased in 60% of untreated leukemic mice vs 20% of LP533401-treated leukemic animals (supplemental Figure 6E). This could reflect deregulated hematopoiesis caused by dysfunctional osteoblasts or a leukemia-associated reactive feature. LP533401 treatment decreased circulating blasts (supplemental Figure 6F-G) and the degree of liver infiltration (supplemental Figure 6H) in leukemic mice.
Mirroring the hematopoietic defects observed in DTAosb mice, treatment of healthy mice with LP533401 did not affect total marrow cellularity but decreased LSK and CD11b+/Gr1+ cells (supplemental Figure 7A-E). LP533401 treatment did not affect the B-cell lineage (supplemental Figure 7F-H), but it increased cells of the erythroid lineage in healthy animals (supplemental Figure 7I-J). In leukemic mice, LP533401 did not affect the decrease in marrow cellularity (supplemental Figure 7A) but prevented the increase in LSK and myeloid cells (supplemental Figure 7B-E). The presence of leukemic blasts mildly increased the frequency of the immature (B220+/IgM−) B-lymphopoietic subset (supplemental Figure 7F-G), but the numbers of B cells decreased independent of LP533401 treatment (supplemental Figure 7H). As in healthy animals, LP533401 also increased erythroid progenitor cells in leukemic mice (supplemental Figure 7I-J). Collectively, these observations indicate that preservation of osteoblast numbers in mice with acute lymphoblastic leukemia by inhibition of gut-derived-serotonin synthesis decreases tumor burden, increases survival, and allows recovery of normal marrow function.
Next, we examined the effect of LP533341 after leukemia establishment. Because serotonin levels are reduced 3 days following LP533341 administration (supplemental Figure 8), we administered LP533341 to mice 1 day following their injection with MLL-AF9 cells. Under these conditions, LP53341 tended to suppress leukemia burden, although without a statistically significant difference between treated and untreated leukemic groups, and had a favorable effect in prolonging their survival because 1 mouse from the LP533401-treated group lived as long as 40 days after all untreated leukemic mice had died (Figure 4G-H), and another mouse had very low tumor burden but died of unrelated reasons.
Increased osteoblast numbers suppress AML
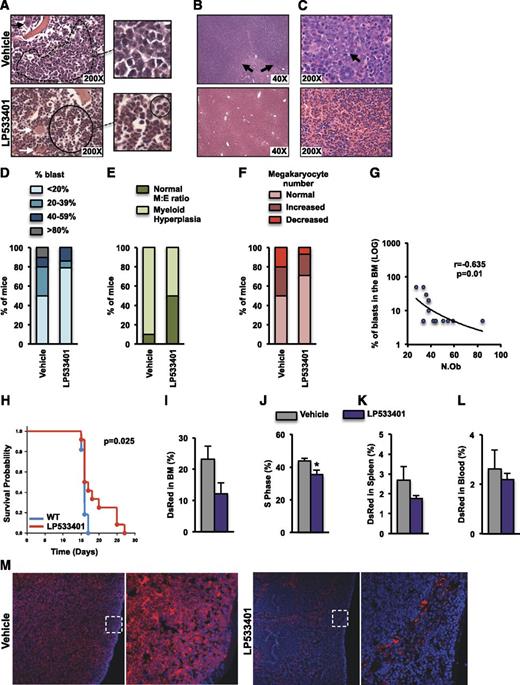
The antileukemic properties of osteoblasts were subsequently evaluated in the WEHI-3B myelomonocytic leukemia model. LP533401 decreased serum serotonin levels by 50% in nonleukemic and leukemic animals, alleviated spleen enlargement, and led to a greater weight gain in leukemic mice, as compared with vehicle-treated animals over the course of the experiment (supplemental Figure 9A-C). More importantly, treatment of WEHI-3B–injected animals with LP533401 reduced leukemic burden (Figure 5A-C and supplemental Figure 9D-G). Half of the animals treated with vehicle, but only 21% treated with LP533401, had more than 20% blasts in their marrow (Figure 5D and supplemental Figure 9H). Among 14 mice treated with LP533401, 11 (71.4%) had <5% blasts compared with 50% of mice treated with vehicle. The myeloid/erythroid ratio was normal in 50% of the LP533401-treated leukemic mice, whereas myeloid hyperplasia was observed in 90% of vehicle-treated animals (Figure 5E). Megakaryocyte numbers also returned to normal in 70% of leukemic mice treated with LP533401 (Figure 5F). LP533401 treatment limited leukemic infiltration of the liver, where only rare collections of blasts were observed (Figure 5B), and the splenic red pulp showed normal maturation of myeloid and erythroid progenitors and normal numbers and appearance of megakaryocytes (Figure 5C and supplemental Figure 9F-G). The outcome of WEHI-3B–induced leukemia appeared to depend on the presence of osteoblasts because osteoblast numbers inversely correlated with the percentage of leukemic blasts in the marrow (Figure 5G). Moreover, LP533401 prevented the decrease in osteoblast numbers caused by AML by inhibiting AML-induced decreases in osteoblast differentiation and proliferation (supplemental Figure 10).
Increased osteoblast numbers suppress AML. (A-G) BALB/c mice at 2 months of age were treated orally, with either vehicle or LP533401 (25 mg/kg of body weight per day), for 4 weeks. WEHI-3B cells were injected on day 14 following the beginning of the treatment. (A-C) H&E staining of sections showing blast infiltration (dotted line) in the marrow of vehicle-treated but normal myeloid and erythroid maturation (continuous line) in the marrow of LP533401-treated mice (inset magnifications of blasts and normal bone marrow are shown in the right panels, and normal megakaryocytes are indicated by white arrows) (A), extensive blast infiltration (black arrows) in the liver of vehicle-treated mice but normal liver morphology in LP533401-treated mice (B), and diffuse blast infiltration of splenic red pulp in vehicle-treated mice (black arrow) but normal red pulp with normal megakaryocytes (white arrow) and myeloid and erythroid colonies in LP533401-treated mice (C). (D-F) Frequency of blast infiltration (D), myeloid hyperplasia (E), and megakaryocyte numbers (F) in the bone marrow of mice treated with vehicle or LP533401 (A-F, n = 10-14 mice per group). (G) Correlation of osteoblast numbers with marrow leukemic blast percentage (n = 7-8 mice per group). (H-M) Syngeneic 2-month-old albino C57BL/6 mice were treated orally with LP533401 (200 mg/kg of body weight per day) or vehicle for 7 days and then injected with DsRed-MLL-AF9 cells and euthanized 12 days following injection. (H) Overall survival of leukemic mice treated with LP533401 or vehicle (n = 12 mice per group). Percentage of MLL-AF9 cells (I) and percentage of MLL-AF9 cells in the S1 phase (J). Percentage of MLL-AF9 cells in the spleen (K) and blood (L). (M) Immunofluorescence staining of spleen sections showing DsRed-MLL-AF9 cells and 4,6-diamidino-2-phenylindole staining (blue) (I-J, n = 5 mice per group). *P < .05 relative to WT vehicle.
Increased osteoblast numbers suppress AML. (A-G) BALB/c mice at 2 months of age were treated orally, with either vehicle or LP533401 (25 mg/kg of body weight per day), for 4 weeks. WEHI-3B cells were injected on day 14 following the beginning of the treatment. (A-C) H&E staining of sections showing blast infiltration (dotted line) in the marrow of vehicle-treated but normal myeloid and erythroid maturation (continuous line) in the marrow of LP533401-treated mice (inset magnifications of blasts and normal bone marrow are shown in the right panels, and normal megakaryocytes are indicated by white arrows) (A), extensive blast infiltration (black arrows) in the liver of vehicle-treated mice but normal liver morphology in LP533401-treated mice (B), and diffuse blast infiltration of splenic red pulp in vehicle-treated mice (black arrow) but normal red pulp with normal megakaryocytes (white arrow) and myeloid and erythroid colonies in LP533401-treated mice (C). (D-F) Frequency of blast infiltration (D), myeloid hyperplasia (E), and megakaryocyte numbers (F) in the bone marrow of mice treated with vehicle or LP533401 (A-F, n = 10-14 mice per group). (G) Correlation of osteoblast numbers with marrow leukemic blast percentage (n = 7-8 mice per group). (H-M) Syngeneic 2-month-old albino C57BL/6 mice were treated orally with LP533401 (200 mg/kg of body weight per day) or vehicle for 7 days and then injected with DsRed-MLL-AF9 cells and euthanized 12 days following injection. (H) Overall survival of leukemic mice treated with LP533401 or vehicle (n = 12 mice per group). Percentage of MLL-AF9 cells (I) and percentage of MLL-AF9 cells in the S1 phase (J). Percentage of MLL-AF9 cells in the spleen (K) and blood (L). (M) Immunofluorescence staining of spleen sections showing DsRed-MLL-AF9 cells and 4,6-diamidino-2-phenylindole staining (blue) (I-J, n = 5 mice per group). *P < .05 relative to WT vehicle.
Treatment of healthy mice with LP533401 did not affect total marrow cellularity (supplemental Figure 11A) but decreased LSK HSC progenitors in the bone marrow (supplemental Figure 11B) and reduced CD11b+/Gr1+ cells without affecting B lymphopoiesis (supplemental Figure 11C-D). Erythroid progenitors tended to increase by LP533401 treatment (supplemental Figure 11E). In leukemic mice, LP533401 reversed the increase in LSK and CD11b+/Gr1+ myeloid cells induced by WEHI-3B injections (supplemental Figure 11B-C), without affecting B lymphopoiesis (supplemental Figure 11D). Erythroid progenitors appeared to increase by 20% in leukemic mice (supplemental Figure 11E), probably because of the fact that 10% of WEHI-3B cells express Ter119 (supplemental Figure 11F).
Similar to the observations with the WEHI-3B cell line model, treatment of mice injected with MLL-AF9 primary leukemia cells with the serotonin inhibitor prolonged survival (Figure 5H). In a parallel study in which all mice were euthanized 12 days after leukemia injection, LP533401 suppressed leukemia cell proliferation in the bone marrow and also tended to suppress disease burden in the bone marrow spleen and blood (Figure 5I-L and supplemental Figure 12E). Quiescence and apoptosis of leukemia cells was not affected by drug treatment (supplemental Figure 12A-D). Similar to our observations with EL4 cells and mirroring the observations in leukemic DTAosb mice, MLL-AF9–induced leukemia increased the percentage of LSK and ST-LSK cells, and treatment with LP533401 reversed both of these effects (supplemental Figure 13A-C). Interestingly, whereas MLL-AF9 leukemia suppressed the percentage of Lin− cells, LP533041 treatment appeared to reverse this effect (supplemental Figure 13D). Blood counts supported the notion that LP533401 tended to normalize the deregulation of hematopoiesis in leukemic mice, although no statistical significance was reached between the groups, probably because of the low number of animals examined for this particular end point (supplemental Figure 13E-K).
Next, to examine the location of leukemia cells relative to bone, we injected WT mice with MLL-AF9 cells and euthanized the leukemic mice at an earlier time point, 6 instead of 12 days following injection. At the time of euthanization, mice had ∼1% of leukemia in the bone marrow, a number low enough to allow us to clearly observe their localization relative to the bone surface. We found that serotonin inhibition tended to reduce the number of leukemic colonies in the marrow but increased the number of leukemic cell colonies on the endosteal bone surface (supplemental Figure 14A-B). In support of this latter observation, distance mapping in bone sections showed that serotonin inhibition increased the proportion of leukemic blasts in proximity to the endosteal bone surface (supplemental Figure 14C). These results indicate a preferential localization of blasts adjacent to bone surfaces and may suggest that osteoblasts recruit leukemic cells to the endosteal location where they suppress their proliferation.
Collectively, these observations suggest that osteoblasts hinder AML by suppressing the proliferation of leukemic blasts and promote normal HSC lineage differentiation, by normalizing erythroid, myeloid, and monocytic populations while restoring lymphopoiesis.
The Tph-1 inhibitor LP533401 hinders leukemia growth by osteoblast-specific, leukemia blast–independent actions
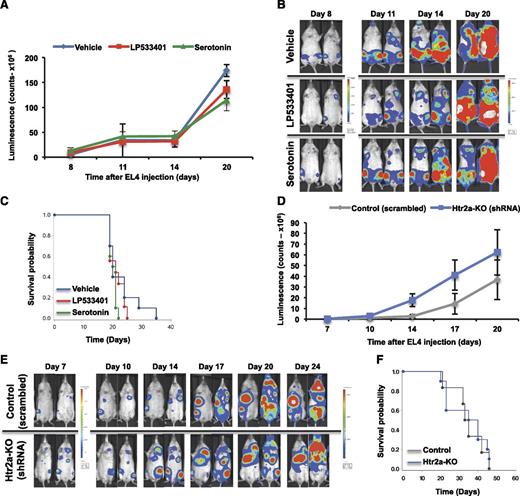
The antileukemic effect of LP533401 was consistently correlated with increased osteoblasts numbers, indicating that the protective effect of LP533401 is attributable to its osteoanabolic actions in osteoblasts. To confirm this hypothesis, we investigated whether additional effects directly targeting leukemic blasts contribute to the antileukemic properties of LP533401. First, we treated EL4 or WEHI-3B cells with LP533401 or serotonin. LP533401 treatments were performed at 0.1- to 1-fold the LP533401 serum concentration in mice treated with this compound. Serotonin was given at concentrations ranging from 25 to 100 μM, doses at which it affects the proliferation of cells expressing serotonin receptors.37,40,41 Neither of these 2 treatments affected leukemic blast proliferation or survival (supplemental Figure 15). To confirm these observations in vivo, EL4 cells were treated with LP533401 or serotonin and subsequently implanted in mice to monitor leukemia engraftment, as commonly described.42 Neither of the 2 treatments altered leukemia burden or lethality (Figure 6A-C).
LP533401 hinders leukemia by osteoblast-specific, leukemic blast–independent actions. (A-C) Albino C57BL/6 mice were injected at 2 months of age with EL4-GFP-Luc cells pretreated with serotonin, LP533401, or vehicle for 24 hours. Luminescence intensity at different time points (A), whole body luminescence representative images (B), and overall survival (C) (A-C, n = 9-10 mice per group). (D-F) EL4 cells were infected with either Htr2a shRNA or scrambled shRNA oligos and injected into albino C57BL/6 mice at 3 months of age. Tumor burden and survival were assessed: Luminescence intensity at different time points (D), whole body luminescence images (E), and overall survival (F) (D-F, n = 6-10 mice per group). shRNA, short hairpin RNA.
LP533401 hinders leukemia by osteoblast-specific, leukemic blast–independent actions. (A-C) Albino C57BL/6 mice were injected at 2 months of age with EL4-GFP-Luc cells pretreated with serotonin, LP533401, or vehicle for 24 hours. Luminescence intensity at different time points (A), whole body luminescence representative images (B), and overall survival (C) (A-C, n = 9-10 mice per group). (D-F) EL4 cells were infected with either Htr2a shRNA or scrambled shRNA oligos and injected into albino C57BL/6 mice at 3 months of age. Tumor burden and survival were assessed: Luminescence intensity at different time points (D), whole body luminescence images (E), and overall survival (F) (D-F, n = 6-10 mice per group). shRNA, short hairpin RNA.
Finally, to confirm that osteoblasts were the cells responsible for suppressing leukemia growth because of inhibition of serotonin signaling by LP533401 treatments, we examined the effects of deleting serotonin receptors in leukemic blasts. We found that, whereas none of the 14 known serotonin receptors are expressed in WEHI-3B cells, Htr2a is highly enriched in EL4 cells (supplemental Figure 16A). Therefore, leukemia outcome was examined in mice injected with Htr2a-silenced cells (supplemental Figure 16B). Inhibition of serotonin signaling in EL4 cells did not affect tumor burden or survival (Figure 6D-F). These results demonstrate that LP533401 suppresses leukemia by directly promoting osteoblast numbers and function.
Osteoclasts do not affect leukemia progression
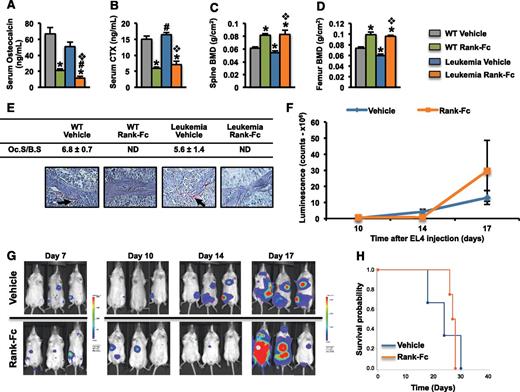
In leukemic mice, despite the decrease in osteoblastogenesis, osteoclast numbers remained unaltered indicating uncoupling between osteoblast-osteoclast functions. As expected, osteoblastic expression of RANKL was increased, whereas expression of osteoprotegerin (OPG) was decreased, leading to a marked increase in the RANKL/OPG ratio in leukemic mice (supplemental Figure 17A). Similar results were obtained when primary osteoblasts were treated with conditioned medium from EL4 or WEHI-3B (supplemental Figure 17B), indicating that either receptor activator of nuclear factor kB ligand (RANKL), or maintenance of osteoclast numbers, could favorably affect leukemia growth. To examine this hypothesis, we evaluated the ability of receptor activator of nuclear factor kB-Fc (RANK-Fc), a selective pharmacologic RANKL inhibitor,43,44 to prevent EL4 leukemic progression. Because EL4 cells do not express RANK, the inhibition of RANKL caused by RANK-Fc would not have a direct effect on these cells (supplemental Figure 17C). RANK-Fc administration to leukemic mice43,44 reduced systemic bone remodeling markers and increased bone mineral density when compared with untreated mice, by eliminating osteoclasts in trabecular bone (Figure 7A-E). However, the RANK-Fc treatment did not alter leukemia burden and survival rates (Figure 7F-H). These results were confirmed in an additional independent experiment (supplemental Figure 18) and suggested that osteoclasts did not affect leukemia progression.
Osteoclasts do not affect leukemia progression. Albino C57BL/6 mice were treated at 2 months of age subcutaneously with 10 mg/kg of murine RANK-muFc or vehicle 3 times per week. In leukemic animals, the treatment started 2 days prior to EL4 cell injection and continued until the time of death. Healthy mice were treated for 4 weeks. Serum levels of osteocalcin (A) and C-telopeptide (CTX) (B), and bone mineral density (BMD) at the spine (C) and femur (D) in WT and leukemic mice treated with either RANK-Fc or vehicle. (E) TRAP+ osteoclasts (black arrows) in spine sections of WT and leukemic mice treated with RANK-Fc or vehicle. (F) Whole body luminescence counts. (G) Representative in vivo images showing leukemia progression. (H) Survival probability in mice injected with EL4 cells and treated with either RANK-Fc or vehicle (n = 4-7 mice per group). *, #,❖P < .05 (*, relative to WT vehicle; #, relative to WT LP533401; and ❖, relative to leukemia vehicle). ND, not detected.
Osteoclasts do not affect leukemia progression. Albino C57BL/6 mice were treated at 2 months of age subcutaneously with 10 mg/kg of murine RANK-muFc or vehicle 3 times per week. In leukemic animals, the treatment started 2 days prior to EL4 cell injection and continued until the time of death. Healthy mice were treated for 4 weeks. Serum levels of osteocalcin (A) and C-telopeptide (CTX) (B), and bone mineral density (BMD) at the spine (C) and femur (D) in WT and leukemic mice treated with either RANK-Fc or vehicle. (E) TRAP+ osteoclasts (black arrows) in spine sections of WT and leukemic mice treated with RANK-Fc or vehicle. (F) Whole body luminescence counts. (G) Representative in vivo images showing leukemia progression. (H) Survival probability in mice injected with EL4 cells and treated with either RANK-Fc or vehicle (n = 4-7 mice per group). *, #,❖P < .05 (*, relative to WT vehicle; #, relative to WT LP533401; and ❖, relative to leukemia vehicle). ND, not detected.
Discussion
We show that increasing the number of osteoblasts in mouse models of acute leukemia (1) decreases blasts in the bone marrow, liver, spleen, and peripheral blood; (2) reestablishes normal hematopoiesis; and (3) prolongs survival. Leukemia is reversed in the presence of a serotonin synthesis inhibitor because of a direct effect on osteoblast numbers, unrelated to serotonin signaling on leukemic blasts. Mirroring these results, mice lacking osteoblasts are more prone to leukemic engraftment and progression and have a shorter survival threshold. In the absence of leukemia, osteoblast-depleted mice develop a hematologic phenotype that favors myeloid but suppresses lymphoid and erythroid expansion. In the presence of leukemia, this phenotype may contribute to marrow failure by allowing the replacement of healthy hematopoietic cells with leukemic blasts.
A potential direct role of serotonin on neoplastic cells in certain hematologic malignancies has been suggested following observations that the serotonin transporter SLC6A4 is present in B-cell clones with diverse origins and in multiple myeloma.45 In vitro studies indicated that some of these neoplastic clones were sensitive to 1 or more serotonergic compounds. In contrast, another study reported serotonin-induced apoptosis in Burkitt lymphoma cells.41 In our studies, serotonin treatment of either the myeloid or the lymphoblastic leukemia cells had no effect on their proliferation or survival. Importantly, neither implantation of serotonin-treated leukemia cells nor silencing of the serotonin receptor expressed in these cells affected leukemia progression. Therefore, our results clearly indicate that at least in 3 syngeneic models of acute leukemia, the decrease in serotonin levels hinders leukemia progression by actions that do not involve direct effects on leukemic blasts.
Our observation that modulation of osteoblasts in the bone marrow niche can alter the course of leukemia is in line with recent studies19 showing that the PTH receptor in osteoblasts signals through transforming growth factor β1 to differentially affect engraftment and survival of leukemia cells in acute vs chronic leukemia mouse models. Moreover, evidence that leukemia stem cells home close to osteoblastic cells in the bone marrow niche supports the idea that osteoblasts influence leukemia stem cell fate.46,47 In addition to osteoblasts, leukemia blasts home to stromal cell-derived factor-1–expressing vascular niches in the bone marrow, creating an inhibitory niche for normal hematopoietic cells.10 Leukemia blast-derived stem cell factor further sequesters normal CD34+ cells in the tumor niche, preventing their mobilization into the peripheral circulation, supporting our findings of disrupted hematopoiesis in mouse models of AML. In addition, our data indicating a lack of involvement of osteoclasts in acute leukemia are in agreement with previous findings showing a small and only transient increase in osteoclast numbers without changes in bone resorption.25
The finding that osteoblast-depleted mice have increased percentage of LSK and myeloid cells in the marrow along with compromised B lymphopoiesis and erythropoiesis is consistent with previous studies by Visnjic et al.48 However, in these studies, although the percentage of LSK cells was increased in osteoblast-depleted mice, the absolute number of LSK cells was 3- to 10-fold reduced. This was explained by a reduction in the total bone marrow cellularity, which was not observed in our studies, probably because of differences in the mouse models used. We have shown a 50% reduction in osteoblast number, whereas Visnjic et al observed a 75% decrease in the number of osteoblasts. Moreover, we used mice engineered to have a depletion of osteoblasts since birth, whereas in the studies by Visnjic et al, the osteoblast ablation was induced after 4 weeks of age.
Our data in humans and mice suggest an inverse correlation between osteoblast function/numbers and leukemia burden, which agrees with rare clinical reports of osteopenia and osteoporosis in newly diagnosed children or adults with acute leukemia.49-53 All these reports correlate osteopenia or osteoporosis with decreased osteoblast function evidenced by low osteocalcin levels with minimal or no changes in resorption markers. Notably, a decrease in disease burden following chemotherapy appeared to correlate with an increase in osteoblast activity and an improvement in bone mass, despite corticosteroid treatment.50,51,54 These reports are consistent with our observations in mice that reinstatement of osteoblast functions reduces disease progression.
In conclusion, our data indicate that osteoblasts hinder leukemia progression, whereas a decrease or compromised osteoblast function promotes leukemia engraftment. This effect likely arises from a combination of actions that directly affect leukemia cells and are also mediated by dysfunctional hematopoiesis that may facilitate leukemia engraftment. We have previously shown that constitutive active β-catenin in osteoblasts upregulates Notch signaling in HSCs and is sufficient to induce AML in mice.29 This pathway seems to be activated in 38% of AML/MDS patients and therefore may be implicated in the pathogenesis of human AML. The current observations on osteoblast-leukemia blast interactions raise 2 important questions. First, are adequate osteoblast numbers sufficient to protect from leukemia progression, or is there a specific, osteoblast-derived signal that can counteract leukemia engraftment? Second, is there a potential role for this cell as a novel target to improve leukemia-associated deregulation of hematopoiesis or to limit disease recurrence? Our findings and emerging data indicating a role of osteoblasts in hematologic malignancies make the osteoblast a potential novel therapeutic target in acute leukemia that can be manipulated to induce hostility of the niche to leukemic blasts.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Michael Moore and Renier Brentjens (Memorial Sloan Kettering Cancer Center, New York) for providing the WEHI-3B murine myelomonocytic leukemia cells and the EL4-GFP-Luc lymphoblastic leukemia cells, respectively, and Dr Lorraine Fitzpatrick for providing the bone biopsies for the human healthy control subjects. The authors also thank Drs Suzanne Lentzsch and Gerard Karsenty for critical reading of the manuscript, Don McMahon for help with statistical analysis, and Elzbieta Dworakowski, Chiu-Yi Liu, Yan Nikhami, Foxwell N. Emmons, and Janine Pichard for technical assistance. The histology and metabolic unit facility of the Diabetes and Endocrinology Research Center (DERC; National Institute of Diabetes and Digestive and Kidney Diseases grant DK063608-07) and the authors thank the Molecular Pathology facility of the Herbert Irving Cancer Center of Columbia University Medical Center for preparation of tissue samples for histopathological analysis.
This work was supported by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01 AR054447 and R01 AR055931, and the National Institute of Aging grant P01 AG032959 (S.K.); the Division of Hematologic Oncology, Memorial Sloan Kettering Cancer Center, New York (E.B.); and the Brazilian National Council of Technological and Scientific Development (B.C.S.).
Authorship
Contribution: B.C.S., E.B., and S.K. initiated the study; M.K., B.C.S., and S.K. designed the experiments; M.K., B.C.S., J.S.M., M.G.-D., G.B., and S.K. analyzed the data; M.K. and B.C.S. carried out most of the experimental work with the help of J.S.M. and M.G.-D. who performed the flow cytometry analysis; M.G.-D. performed immunoflurescence staining; B.C.S., A.K., and D.P. counted osteoblasts in human samples; C.A.Z. performed statistical analyses; G.B. and J.T.-F. performed histolopathological analysis of mouse samples; J.T.-F. and D.P. enumerated osteoblasts in human bone marrow samples; A.R., N.G., J.T.-F., S.M., and E.B. provided human AML and MDS samples and reviewed and discussed human bone biopsy data; D.W.D. provided sections of human bone biopsies from healthy controls; T.L.N. provided serum for osteocalcin measurements from healthy controls; B.G.M. and I.K. performed distance mapping and bone histology; A.N.E. generated the DTA floxed mice; M.K., B.C.S., and S.K. wrote the manuscript; S.K. directed the research; and all authors discussed and commented on the manuscript.
Conflict-of-interest disclosure: W.D. is an employee of Amgen and owns stocks and stock options for Amgen. The remaining authors declare no competing financial interests.
Correspondence: Stavroula Kousteni, The Russ Berrie Medical Sciences Pavilion, 1150 Saint Nicholas Ave, Room 411, New York, NY 10032; e-mail: sk2836@cumc.columbia.edu.
References
Author notes
M.K. and B.C.S. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal