Key Points
GD2-specific CAR renders NKT cells cytotoxic against NB cells and results in potent in vivo antitumor activity without graft-versus-host disease.
The 4-1BB-containing CAR constructs induce T helper 1–like polarization in NKT cells.
Abstract
Advances in the design of chimeric antigen receptors (CARs) have improved the antitumor efficacy of redirected T cells. However, functional heterogeneity of CAR T cells limits their therapeutic potential and is associated with toxicity. We proposed that CAR expression in Vα24-invariant natural killer T (NKT) cells can build on the natural antitumor properties of these cells while their restriction by monomorphic CD1d limits toxicity. Primary human NKT cells were engineered to express a CAR against the GD2 ganglioside (CAR.GD2), which is highly expressed by neuroblastoma (NB). We compared CAR.GD2 constructs that encoded the CD3ζ chain alone, with CD28, 4-1BB, or CD28 and 4-1BB costimulatory endodomains. CAR.GD2 expression rendered NKT cells highly cytotoxic against NB cells without affecting their CD1d-dependent reactivity. We observed a striking T helper 1–like polarization of NKT cells by 4-1BB-containing CARs. Importantly, expression of both CD28 and 4-1BB endodomains in the CAR.GD2 enhanced in vivo persistence of NKT cells. These CAR.GD2 NKT cells effectively localized to the tumor site had potent antitumor activity, and repeat injections significantly improved the long-term survival of mice with metastatic NB. Unlike T cells, CAR.GD2 NKT cells did not induce graft-versus-host disease. These results establish the potential of NKT cells to serve as a safe and effective platform for CAR-directed cancer immunotherapy.
Introduction
The engineered expression of chimeric antigen receptors (CARs) on the surface of T cells combines the targeting properties of antibodies with the active trafficking, self-propagation capacity, and potent effector function of T cells.1,2 The currently used CARs typically consist of a single chain variable fragment (scFv) of an antibody for antigen binding, the T-cell receptor (TCR) ζ chain that mimics TCR activation, and 1 or 2 signaling domains derived from CD28 or 4-1BB for costimulation.3-5 Recent clinical trials demonstrated that T cells redirected against the CD19 antigen can induce sustained complete responses in patients with B-cell malignancies, including those with bulky disease.6-9
Clinical results obtained using CAR-redirected immunotherapy in solid tumors have been largely disappointing.10,11 In part, this is attributable to the immunosuppressive tumor microenvironment that impairs T-cell migration, persistence, and effector function.12 Furthermore, the genetic insertion of CAR molecules into polyclonal activated T lymphocytes generates cellular products characterized by high functional heterogeneity that limits their antitumor potential and is associated with increased risk of toxicity.13 Attempts have been made to express CARs in T-cell subsets with more defined biological characteristics. For instance, our group expressed CARs in cytotoxic T lymphocytes (CTLs) specific for viral antigens such as those derived from the Epstein-Barr virus.14 The infusion of CAR-modified CTLs in patients was safe and achieved tumor regression in some patients with refractory/relapsed disease.14,15 However, in vivo persistence and tumor infiltration of these CAR-modified CTLs were limited.
Some lymphocyte subsets, such as natural killer cells, T helper (Th) 17, or γδ T cells, are more efficient than others in cell-mediated cytotoxicity, trafficking, or production of desired cytokines, and these subsets are currently being explored for CAR-redirected immunotherapy.10,13 CD1d-restricted Vα24-invariant (type-I) natural killer T (NKT) cells are of particular interest as a potential CAR carrier because NKT-cell infiltration of primary tumors is associated with better outcomes in diverse tumors such as neuroblastoma (NB) in children and colon cancer in adults.16,17 Moreover, in contrast to the genetic polymorphism and ubiquitous expression of HLA molecules, the CD1d gene is monomorphic and expressed by only a few cell types,18,19 limiting the potential toxicity of NKT cells in the autologous or allogeneic settings.
NKT cells traffic to solid tumors in response to chemokines produced by tumor cells and tumor-associated macrophages (TAMs).16,20 Moreover, NKT cells colocalize with TAMs and can kill or inhibit these growth-promoting cells21 in a CD1d-dependent manner.22 Because adoptive transfer of NKT cells has now become clinically feasible because of the development of reagents allowing robust ex vivo expansion of these cells,20,23 we have proposed that expression of a tumor-specific CAR in NKT cells would enable them to kill both tumor-supportive TAMs and tumor cells themselves, thereby eradicating the tumor. We genetically manipulated ex vivo expanded primary human NKT cells with CARs specific for the GD2 ganglioside (CAR.GD2), an antigen that has already been targeted with CAR.GD2 CTLs in NB patients in a clinical trial that produced promising results.14,15 Our results demonstrate that CAR.GD2 expression renders NKT cells highly cytotoxic against neuroblasts without affecting their ability to kill TAMs. CAR.GD2 NKT cells effectively localized to the tumor site, had potent antitumor activity in a metastatic NB model in humanized NOD/SCID/IL2Rγ(null) (hu-NSG) mice, and, unlike T cells, did not induce graft-versus-host disease (GVHD).
Materials and methods
Human specimens
Cord blood was obtained from a cord blood bank at the MD Anderson Cancer Center under Institutional Review Board (IRB)–approved protocol H-20911. Informed consent was obtained in accordance with IRB policies and procedures and the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) of healthy donors were isolated by gradient centrifugation from buffy coats purchased from Gulf Coast Regional Blood Center.
Cell lines
CHLA-255, CHLA-255/luc NB, and Jurkat J32 T-cell lines were established and maintained as previously described.16,24-26 The 293T cells were purchased from American Tissue Culture Collection and maintained in Iscove modified Dulbecco medium 10% fetal bovine serum (Hyclone) and 2 mM GlutaMAX-I (Gibco-BRL).
Retroviral constructs and retrovirus production
NKT-cell isolation, transduction, and expansion
Monocyte isolation and generation of M2 macrophages
Monocytes were isolated by negative selection from PBMCs using Monocyte Isolation kit (Miltenyi Biotec) and cultured in complete RPMI 1640 with 100 ng/mL macrophage colony-stimulating factor (PeproTech), added every other day for 5 days. The resulting macrophages were examined by fluorescence-activated cell sorter (FACS), and their M2 phenotype was confirmed by FACS as the following: CD33+CD14+CD163highHLA-DRlow.
Multiplex cytokine quantification assay
CAR.GD2 NKT cells were stimulated for 24 hours with irradiated CHLA255 NB cells, Jurkat J32 cells (ratio 1:1), or plate-coated anti-CD3 (OKT3), anti-4-1BB (h41BB-M127), anti-CD28 (NALM) monoclonal antibodies (mAbs). Mouse serum was collected 24 hours after adoptive transfer of NKT cells. Samples were analyzed with Human Cytokine/Chemokine Immunoassay kit (Millipore).
Flow cytometry
The phenotype of NKT cells was assessed using mAbs for CD3 (OKT3), Vα24-Jα18 (6B11), and CD4 (RPA-T4; BD Biosciences). CAR.GD2 expression on transduced NKT cells was detected using the anti-idiotype 1A7 mAb followed by staining with a secondary rat anti-mouse-IgG1-PE mAb (BD Biosciences). Intracellular staining was performed according to BD Phosflow protocol. The expression of GD2 on NB cells was detected using 14G2 amAb (BD Biosciences). BD-suggested fluorochrome- and isotype-matching mAbs were used as negative controls. The analysis was performed on a LSR-II 5-laser flow cytometer (BD Biosciences) using BD FACSDiva software (version 6.0) and FlowJo 7.2.5 (Tree Star).
Cytotoxicity
The cytotoxicity of parental and CAR.GD2 NKT cells or T cells against NB cell lines or M2 macrophages was evaluated using a standard 4-hour 51Cr-release assay as described.29
Immunofluorescent microscopy
The tissues from NB xenografts were frozen in optimum cutting temperature medium, sectioned (7 µm) using Cryostat HM550 (Thermo Scientific), and processed as described.20 Fluorescent images were acquired with Nikon Eclipse Ti Inverted Microscope. The absolute numbers of carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled cells were counted using Nikon Instruments Software (NIS-Elements AR3.2).
In vivo experiments
The metastatic NB model in hu-NSG mice was developed in our laboratory.20 Before injection into the animals, NKT cells were cultured with interleukin (IL) 2 (200 U/mL; National Institutes of Health) for 10 days without TCR or CAR stimulation to achieve resting phase. Tumor growth was assessed by weekly bioluminescent imaging (Small Animal Imaging Core Facility, Texas Children’s Hospital). To determine NKT- or T-cell localization to tumor xenografts, effector cells were labeled with CFSE (Invitrogen) before injection. After 48 hours, mice were euthanized, and parts of their tumors were excised and immediately embedded in optimum cutting temperature medium for immunofluorescent microscopy. For the in vivo persistence experiments, NKT cells were cotransduced with CAR.GD2 and green fluorescent protein/Ffluc constructs, IV injected to tumor-bearing mice, and monitored using bioluminescent imaging. Animal experiments were performed according to Institutional Animal Care and Use Committee approved protocols.
Statistical analyses
For in vitro and in vivo experiments, we used 2-sided, paired Student t test to evaluate continuous variables of 2 groups and 1-way analysis of variance (ANOVA) with posttest Bonferroni to evaluate continuous variables of more than 2 groups. Survival was analyzed by the Kaplan-Meier method and the Gehan-Wilcoxon test to compare pairs of groups. Statistics were computed using GraphPad Prism 5.0 (GraphPad Software) and the R package (R Foundation for Statistical Computing).30 Differences were considered significant when the P value was <.05.
Results
Stable expression of CAR.GD2 in ex vivo expanded NKT cells after retroviral transduction
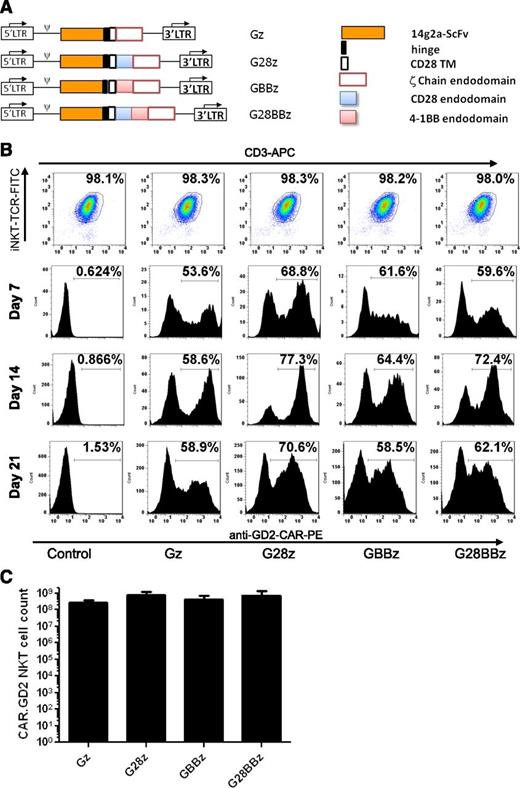
CAR.GD2 constructs in this study were made as previously described27 and shown in Figure 1A. The constructs used an scFv from the GD2-specific antibody 14g2a31 as an antigen-recognizing domain. This scFv connects via a short hinge derived from the IgG1 to the transmembrane domain derived from CD28, followed by 1 of 4 signaling endodomains sequences: (1) TCR ζ chain only (Gz); (2) CD28 endodomain fused with ζ chain (G28z); (3) 4-1BB endodomain fused with ζ chain (GBBz); and (4) CD28, 4-1BB, ζ chain (G28BBz). Primary NKT cells were isolated from the PBMCs of healthy donors, activated with αGalCer-pulsed autologous irradiated PBMCs, and transduced with the indicated CAR.GD2 construct followed by expansion in culture with rIL-2. NKT cells represented at least 95% of cells in the culture by the end of the first week, increasing to >98% after restimulation (Figure 1B). Upon retroviral transduction, 50% to 70% of NKT cells expressed CAR.GD2 on the cell surface, and the expression persisted for at least 3 weeks (Figure 1B). From ∼105 freshly isolated peripheral blood NKT cells, we generated 5.7 × 108 ± 4.3 × 108 CAR.GD2 NKT cells (mean expansion rate 5700-fold, range 842- to 14 300-fold, n = 5) within 21 days of ex vivo culture. There was no significant difference in the rate of expansion between CAR.GD2 NKT cells expressing different CARs (Figure 1C). Therefore, primary human NKT cells can be stably transduced with CAR.GD2 retroviral constructs and expanded ex vivo to clinical scale.
Generation of CAR.GD2 NKT cells. (A) Schematic representation of recombinant retroviral vectors encoding CAR.GD2 constructs. (B) NKT cells were isolated from human PBMCs, stimulated with αGalCer, and transduced with the indicated CAR.GD2 constructs followed by expansion in culture with IL-2. The expanded cells were analyzed by FACS for the frequency of NKT cells using 6B11 and anti-CD3 mAbs (day 21 after transduction, upper panel) and for the frequency of CAR.GD2 NKT cells using anti-idiotype 1A7 mAb on days 7, 14, and 21. Results are from a representative of 5 experiments. (C) Absolute cell count of CAR.GD2-expressing NKT cells at day 21 of expansion. Data are mean ± standard deviation (SD) from 5 donors.
Generation of CAR.GD2 NKT cells. (A) Schematic representation of recombinant retroviral vectors encoding CAR.GD2 constructs. (B) NKT cells were isolated from human PBMCs, stimulated with αGalCer, and transduced with the indicated CAR.GD2 constructs followed by expansion in culture with IL-2. The expanded cells were analyzed by FACS for the frequency of NKT cells using 6B11 and anti-CD3 mAbs (day 21 after transduction, upper panel) and for the frequency of CAR.GD2 NKT cells using anti-idiotype 1A7 mAb on days 7, 14, and 21. Results are from a representative of 5 experiments. (C) Absolute cell count of CAR.GD2-expressing NKT cells at day 21 of expansion. Data are mean ± standard deviation (SD) from 5 donors.
CAR.GD2 NKT cells are cytotoxic against GD2-positive neuroblasts and CD1d-positive M2 macrophages
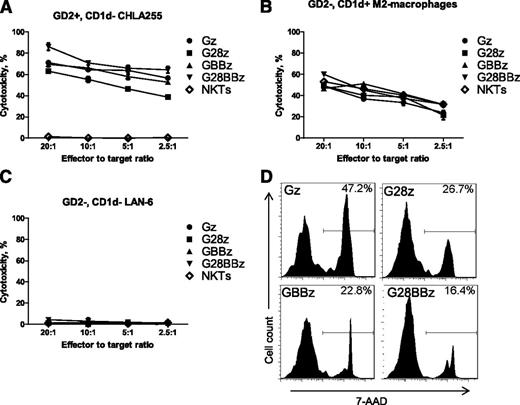
To test whether transgenic expression of CAR.GD2 renders NKT cells cytotoxic against GD2-positive NB cells, we cultured CAR.GD2 NKT cells as effector cells with GD2-positive/CD1d-negative CHLA-255 human NB target cells in a 4-hour 51Cr-release cytotoxicity assay. Figure 2A shows that regardless of the costimulatory moiety, CAR.GD2 NKT cells mediated potent dose-dependent cytotoxicity against CHLA-255 cells, whereas parental NKT cells were not cytotoxic. We also examined whether CAR.GD2 NKT cells retained their native CD1d-restricted reactivity using αGalCer-pulsed M2-polarized macrophages (GD2-negative/CD1d-positive) as target cells. CAR.GD2 NKT cells killed macrophages as effectively as did the parental NKT cells (Figure 2B). To test the specificity of CAR.GD2 NKT-cell cytotoxicity, we used GD2/CD1d double-negative LA-N-6 human NB cell line as a target. Figure 2C demonstrates that LA-N-6 cells could not be killed by either parental or CAR.GD2 NKT cells. Finally, we examined whether recognition of GD2-positive NB cells and the resulting stimulation via transgenic CAR.GD2 affect survival of CAR.GD2 NKT cells. We mixed irradiated CHLA-255 NB cells at 1:1 ratio with 105 parental or CAR.GD2 NKT cells (normalized to 65% CAR expression rate for all constructs) and cultured them for 3 days in the absence of exogenous cytokines. Figure 2D demonstrates that NKT cells expressing Gz, a CAR without costimulation, had the highest rate of cell death compared with NKT cells that expressed CARs with CD28 (G28z), 4-1BB (GBBz), or both (G28BBz) costimulatory endodomains (P < .001, 1-way ANOVA), although no statistically significant difference was observed between constructs with the costimulatory endodomains. Therefore, CAR.GD2 NKT cells have dual-specific cell-mediated cytotoxicity, against GD2- or CD1d-positive cells. Although all CARs mediated equally potent NKT-cell cytotoxicity, the presence of costimulatory signaling capabilities within CARs was associated with improved survival of NKT cells upon target cell recognition.
Dual-specific cytotoxicity of CAR.GD2 NKT cells. The cell-mediated cytotoxicity of parental and CAR.GD2 NKT cells with the indicated constructs was tested using a 4-hour 51Cr-release assay against the following targets: GD2+CD1dneg CHLA255 NB cell line (A); GD2negCD1d+ in vitro polarized M2 macrophages (B); and GD2negCD1dneg LA-N-6 NB cell line (C). (D) 7-AAD staining was performed on day 3 of NKT/NB cell culture. Histograms show percent 7-AAD positive cells after gating on CAR-positive cells with the indicated constructs. Results are from a representative of 3 independent experiments.
Dual-specific cytotoxicity of CAR.GD2 NKT cells. The cell-mediated cytotoxicity of parental and CAR.GD2 NKT cells with the indicated constructs was tested using a 4-hour 51Cr-release assay against the following targets: GD2+CD1dneg CHLA255 NB cell line (A); GD2negCD1d+ in vitro polarized M2 macrophages (B); and GD2negCD1dneg LA-N-6 NB cell line (C). (D) 7-AAD staining was performed on day 3 of NKT/NB cell culture. Histograms show percent 7-AAD positive cells after gating on CAR-positive cells with the indicated constructs. Results are from a representative of 3 independent experiments.
CD28 and 4-1BB CAR endodomains have differential effect on NKT-cell cytokine profile
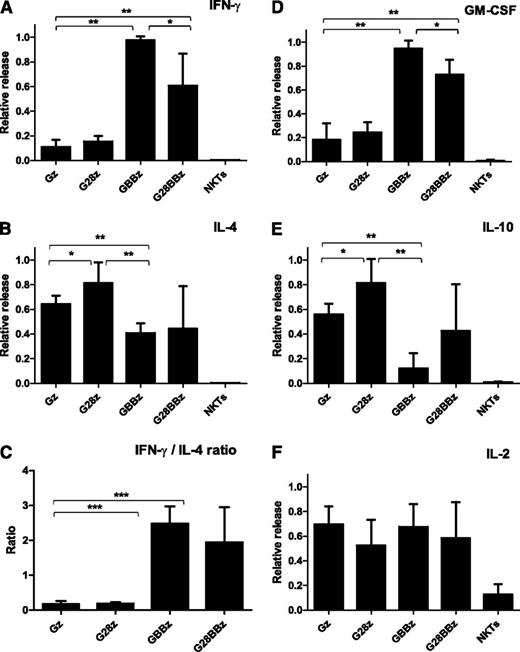
One of the functional hallmarks of NKT cells is the production of large amounts of both Th1 and Th2 cytokines.32,33 To test the cytokine response of CAR.GD2 NKT cells, we cocultured them for 24 hours with irradiated CHLA-255 cells and quantified the concentrations of Th1/Th2 cytokines in the supernatants. Figure 3 demonstrates that while parental NKT cells produced no detectable cytokines, CAR.GD2 NKT cells produced large amounts of cytokines in response to stimulation with GD2-positive cells. Gz skewed the NKT-cell cytokine profile toward Th2 as evidenced by little interferon (IFN) γ and granulocyte macrophage colony-stimulating factor (GM-CSF) and large amounts of IL-4 and IL-10 production. G28z-transduced NKT cells secreted even higher amounts of IL-4 and IL-10 compared with those transduced with Gz, although the IFN-γ to IL-4 ratio was equally low with both constructs. In contrast, we observed a striking Th1 polarizing effect of CAR constructs containing 4-1BB endodomain either alone (GBBz) or with CD28 (G28BBz) compared with Gz. This polarization was highly consistent between individuals and associated with a multifold amplification of IFN-γ and GM-CSF production with concomitant reduction of IL-4 and IL-10. Of note, CAR.GD2 NKT cells, irrespectively of the CAR design, released IL-2 upon stimulation. In accordance with the observed pattern of cytokine production, stimulation of G28z NKT cells induced rapid phosphorylation of signal transducer and activator of transcription 6, signal transducer and activator of transcription 5, and AKT, which are associated with Th2 polarization.34 GBBz NKT cells accumulated phospho-p38 mitogen-activated protein kinase and phospho-p65 nuclear factor κB, which are associated Th1 polarization.34 G28BBz NKT cells exhibited a mixed signaling pattern that included p38 mitogen-activated protein kinase and AKT (supplemental Figure 1).
Cytokine release after CAR.GD2 stimulation. CAR.GD2 NKT cells were stimulated by CHLA255 NB cell line; supernatants were collected after 24 hours, and the concentrations of the indicated cytokines (A-F) were quantified using a Luminex assay. Plots represent combined data from 3 different donors. The maximal cytokine level in each experiment was used as 1, and data were normalized relative to the maximum in each experiment. Data are mean ± SD. *P < .05; **P < .01; ***P < .001, compared with Gz group, 1-way ANOVA with Bonferoni posttest analysis.
Cytokine release after CAR.GD2 stimulation. CAR.GD2 NKT cells were stimulated by CHLA255 NB cell line; supernatants were collected after 24 hours, and the concentrations of the indicated cytokines (A-F) were quantified using a Luminex assay. Plots represent combined data from 3 different donors. The maximal cytokine level in each experiment was used as 1, and data were normalized relative to the maximum in each experiment. Data are mean ± SD. *P < .05; **P < .01; ***P < .001, compared with Gz group, 1-way ANOVA with Bonferoni posttest analysis.
To examine whether CAR expression affects NKT-cell cytokine response to their cognate TCR stimulation, we used αGalCer-pulsed CD1d+GD2neg Jurkat J32 cells.26 Consistent with the reported physical incorporation of CAR elements into the endogenous TCR/CD3 complex,35,36 we found that TCR-stimulated CAR.GD2 NKT cells exhibited a pattern of Th2- or Th1-like polarization that was nearly identical to that elicited by CAR stimulation and was again dependent on the presence of CD28 or 4-1BB endodomains in the CAR constructs (supplemental Figure 2).
Next, we determined whether the observed polarizing effects in CAR-redirected NKT cells are a consequence of the transgenic insertion of the costimulatory endodomains within the CARs or an intrinsic property of NKT cells. Native TCR, CD28, and 4-1BB receptors were stimulated in parental NKT cells with plate-bound agonistic anti-CD3 mAb alone or combined with anti-CD28 or anti-4-1BB mAbs. Under these conditions, the stimulation of the native 4-1BB also resulted in a significant enhancement of IFN-γ and GM-CSF production and a marked reduction of IL-10 release (supplemental Figure 3). Similarly with G28z, stimulation with anti-CD3 and anti-CD28 mAbs had little effect on IFN-γ or GM-CSF production. However, unlike G28z, activation of CD28 receptor did not affect IL-4 and decreased IL-10 production. Thus, the engagement of CARs containing the 4-1BB endodomain allows NKT cells to recapitulate the polarization that occurs physiologically upon activation of the native 4-1BB receptor, whereas activation of the CD28 endodomain within CAR.GD2 does not fully match the function of the native CD28 receptor.
Costimulation influences persistence of transferred CAR.GD2 NKT cells in tumor-bearing mice
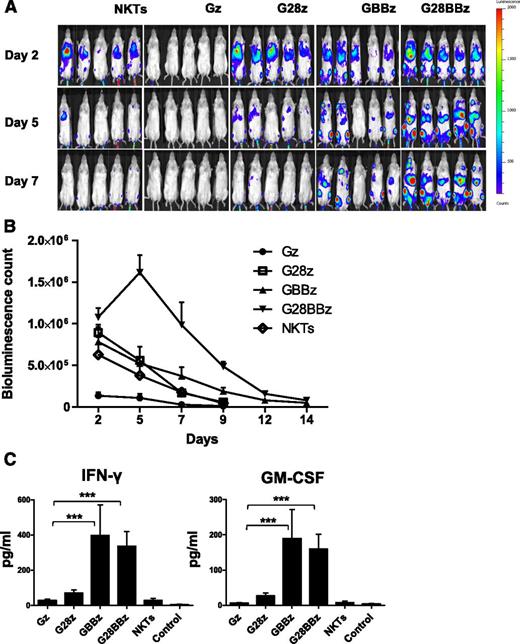
To examine in vivo persistence of adoptively transferred CAR.GD2 NKT cells, we used a metastatic NB xenograft model in NSG mice.20 NKT cells were transduced with a retroviral vector carrying a firefly luciferase complementary DNA alone or cotransduced with a second vector carrying Gz, G28, GBBz, or G28BBz construct. Mice with NB metastases established for 3 weeks were divided into groups to receive parental or CAR.GD2 NKT cells with each construct followed by bioluminescent imaging. Figure 4A-B demonstrates that Gz had a negative effect on cell persistence compared with parental NKT cells (P = .001). Only G28BBz significantly enhanced NKT-cell persistence compared with control until day 12 (P = .05, .001, .001, and .04 on days 2, 5, 7, and 12). In contrast, G28z was ineffective, and GBBz had a marginal effect, achieving a significant difference with control only at day 7 (P = .02). We also measured serum levels of human cytokines in mice at 24 hours after cell therapy. Figure 4C demonstrates that only mice that received NKT cells with 4-1BB-containing CARs had elevated levels of IFN-γ and GM-CSF compared with control animals (P < .001). IL-4, IL-10, and IL-2 were undetectable. Therefore, expression of G28BBz strongly enhanced in vivo persistence of NKT cells and resulted in the systemic elevation of Th1 cytokines in tumor-bearing mice.
In vivo persistence of CAR.GD2 NKT cells in a metastatic NB model. Each mouse received IV injection of 106 CHLA-255 NB cells followed by (day 21) injection of 107 CAR.GD2 NKT cells with the indicated constructs. Cell persistence was monitored by bioluminescent imaging on the indicated days. (A) Bioluminescent images of 5 mice per group are shown from 1 of 2 independent experiments. (B) Bioluminescence photon count on indicated days. Data are mean ± standard error of the mean. Data were analyzed by Student t test at each time point after a logarithmic transformation to stabilize the variance, and relevant P values are described in the text. (C) Serum was collected from mice 24 hours after NKT-cell injection and analyzed by Luminex assay. Data are mean ± SD. *P < .05; **P < .01; ***P < .001, compared with Gz group, 1-way ANOVA with Bonferoni posttest analysis.
In vivo persistence of CAR.GD2 NKT cells in a metastatic NB model. Each mouse received IV injection of 106 CHLA-255 NB cells followed by (day 21) injection of 107 CAR.GD2 NKT cells with the indicated constructs. Cell persistence was monitored by bioluminescent imaging on the indicated days. (A) Bioluminescent images of 5 mice per group are shown from 1 of 2 independent experiments. (B) Bioluminescence photon count on indicated days. Data are mean ± standard error of the mean. Data were analyzed by Student t test at each time point after a logarithmic transformation to stabilize the variance, and relevant P values are described in the text. (C) Serum was collected from mice 24 hours after NKT-cell injection and analyzed by Luminex assay. Data are mean ± SD. *P < .05; **P < .01; ***P < .001, compared with Gz group, 1-way ANOVA with Bonferoni posttest analysis.
CAR.GD2 NKT cells have potent therapeutic activity against metastatic NB in hu-NSG mice
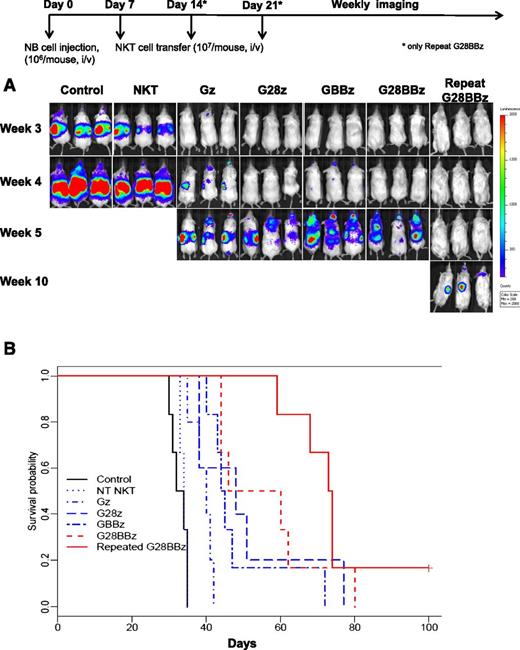
To evaluate the in vivo therapeutic potential of CAR.GD2 NKT cells, we used a metastatic model of NB in hu-NSG mice, in which a human hematopoietic microenvironment is important for supporting the growth of NB xenografts20 and highly relevant to the pathogenesis of the high-risk disease in NB patients.37 Control and CAR-modified NKT cells were adoptively transferred to hu-NSG mice 7 days after IV injection of CHLA-255/luc NB cells. Figure 5A demonstrates that, unlike parental NKT cells, CAR.GD2 NKT cells with any construct delayed the appearance of detectable NB metastases for 1 to 7 weeks and prolonged animal survival compared with controls (P < .01 for Gz). Compared with mice that received Gz, those that received G28BBz (P = .002) and GBBz (P = .013), but not G28z (P = .24), survived significantly longer. However, there was no significant difference in survival between groups treated with G28BBz, GBBz, or G28z (P > .05). Next, we performed FACS to compare the levels of GD2 expression in NB cells derived from tumors of treated and control mice and found no difference (supplemental Figure 4), suggesting that tumors could remain susceptible to repeat injections of CAR.GD2 NKT cells. Indeed, we found that 2 additional weekly injections of G28BBz NKT cells effectively delayed tumor recurrence and prolonged animal survival by more than 5 weeks compared with a single injection of NKT cells with any construct (Figure 5A-B, P < .02). Therefore, CAR.GD2 NKT cells have potent antitumor activity and can be given repeatedly to maximize therapeutic efficacy.
Antitumor activity of CAR.GD2 NKT cells in a metastatic NB model in hu-NSG mice. Three months after human cord blood stem cell transfer, each mouse received IV injection of 106 CHLA-255(ffluc+) NB cells followed by (day 7) injection of 107 CAR.GD2 NKT cells with the indicated constructs. For group “Repeat G28BBz,” additional injections were given on days 14 and 21. (A) Tumor growth was monitored using weekly bioluminescent imaging. Shown are 3 representative of 7 mice per group from 1 of 3 independent experiments. (B) Shown is a representative survival plot from 1 of 3 experiments. Data were analyzed by the Kaplan-Meier method. The differences in survival were then compared using the Gehan-Wilcoxon test.
Antitumor activity of CAR.GD2 NKT cells in a metastatic NB model in hu-NSG mice. Three months after human cord blood stem cell transfer, each mouse received IV injection of 106 CHLA-255(ffluc+) NB cells followed by (day 7) injection of 107 CAR.GD2 NKT cells with the indicated constructs. For group “Repeat G28BBz,” additional injections were given on days 14 and 21. (A) Tumor growth was monitored using weekly bioluminescent imaging. Shown are 3 representative of 7 mice per group from 1 of 3 independent experiments. (B) Shown is a representative survival plot from 1 of 3 experiments. Data were analyzed by the Kaplan-Meier method. The differences in survival were then compared using the Gehan-Wilcoxon test.
Unlike CAR.GD2 T cells, CAR.GD2 NKT cells effectively localize to the tumor site and do not induce GVHD
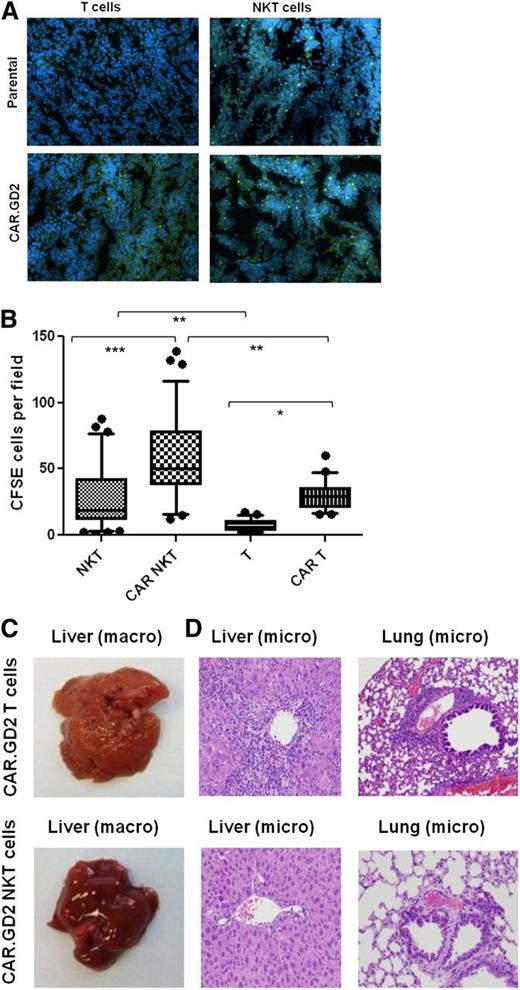
NKT cells are known to traffic to NB tissues in response to chemokines.38 To determine whether CAR.GD2 expression enhances NKT-cell localization to the tumor site, we used fluorescent microscopy to analyze tumor infiltration with CFSE-labeled NKT cells or CAR.GD2 NKT cells 48 hours after adoptive transfer. In parallel, we analyzed tumor infiltration of control T cells or CAR.GD2 T cells that were derived from the same PBMC preparations and transduced with the same CAR.GD2 construct (G28BBz) as the NKT cells. Figure 6A-B demonstrate that CAR.GD2 NKT cells retain their physiological capacity to traffic to NB grafts as observed with control NKT cells. The expression of CAR.GD2 significantly enhanced tumor infiltration both with NKT and T cells. However, the frequency of tumor-infiltrating CAR.GD2 T cells was less than that of CAR.GD2 NKT cells (P < .001, 1-way ANOVA, Figure 6B).
CAR.GD2 NKT cells effectively localize to the tumor site and do not induce GVHD in hu-NSG mice. (A) Tumor xenografts were established after injection of CHLA-255 NB cells under the renal capsule for 3 weeks followed by IV injection of CFSE-labeled parental or G28BBz-transduced NKT or T cells from the same donor. Mice were euthanized after 48 hours, and tumor sections were analyzed by fluorescent microscopy. (B) Multiple random fields of tissue slides were scanned from each tumor, and the absolute numbers of CFSE-labeled cells were counted using the NIS Elements AR3.2 software. Whiskers cover 10 to 90 percentiles. Mean ± SD from 5 to 10 fields per mouse, 5 mice per group from 1 of 2 independent experiments. *P < .05; **P < .01; ***P < .001, 1-way ANOVA with Bonferoni posttest analysis. (C) Animals were euthanized after 4 to 5 weeks of receiving cell therapy. Macroscopic evaluation shows significant swelling and granular transformation of liver in mice treated with CAR.GD2 T cells, whereas CAR.GD2 NKT cells had no effect on liver. (D) Microscopic examination shows lymphocytic infiltrates and necrosis in both liver and lungs of mice treated with CAR.GD2 T cells, whereas mice treated with CAR.GD2 NKT cells have no detectable abnormalities in the same organs. Shown are representative images from 1 of 3 experiments with similar results.
CAR.GD2 NKT cells effectively localize to the tumor site and do not induce GVHD in hu-NSG mice. (A) Tumor xenografts were established after injection of CHLA-255 NB cells under the renal capsule for 3 weeks followed by IV injection of CFSE-labeled parental or G28BBz-transduced NKT or T cells from the same donor. Mice were euthanized after 48 hours, and tumor sections were analyzed by fluorescent microscopy. (B) Multiple random fields of tissue slides were scanned from each tumor, and the absolute numbers of CFSE-labeled cells were counted using the NIS Elements AR3.2 software. Whiskers cover 10 to 90 percentiles. Mean ± SD from 5 to 10 fields per mouse, 5 mice per group from 1 of 2 independent experiments. *P < .05; **P < .01; ***P < .001, 1-way ANOVA with Bonferoni posttest analysis. (C) Animals were euthanized after 4 to 5 weeks of receiving cell therapy. Macroscopic evaluation shows significant swelling and granular transformation of liver in mice treated with CAR.GD2 T cells, whereas CAR.GD2 NKT cells had no effect on liver. (D) Microscopic examination shows lymphocytic infiltrates and necrosis in both liver and lungs of mice treated with CAR.GD2 T cells, whereas mice treated with CAR.GD2 NKT cells have no detectable abnormalities in the same organs. Shown are representative images from 1 of 3 experiments with similar results.
Because of the monomorphic nature of CD1d and its interspecies conservation,18 NKT cells may lack allo- or xenoreactivity, suggesting that CAR.GD2 NKT-cell therapy could be safe in allogeneic settings, and this approach could be tested in a xenogenic host. Hu-NSG mice combine xenogenic mouse tissues with human hematopoietic cells from unrelated stem cell donors. Human T cells are known to cause GVHD in NSG mice,39,40 and we observed a markedly accelerated GVHD in hu-NSG mice. Mice suffered from progressive weight loss and experienced other symptoms of multiorgan toxicity that required euthanasia of animals 4 to 5 weeks after transfer of either T or CAR.GD2 T cells. The macroscopic and microscopic pathological examination of mice that received CAR.GD2 T cells revealed gross abnormalities of liver and lung tissues with massive lymphocytic infiltrates that are consistent with GVHD (Figure 6C-D). In contrast, we observed no clinical symptoms or morphologic signs of GVHD when mice received CAR.GD2 NKT cells (Figure 6C-D), even after repeated injections and up to 3 months after transfer (supplemental Figure 5). Therefore, CAR.GD2 NKT cells more effectively localize to the tumor site than CAR.GD2 T cells, and in contrast to CAR.GD2 T cells, CAR.GD2 NKT cells do not mediate GVHD.
Discussion
In the present study, we demonstrate the potential of NKT cells to serve as a safe and effective platform for CAR-redirected cancer immunotherapy. Results from recent early phase clinical trials indicate that adoptive immunotherapy with CAR-redirected T cells can be highly efficacious as well as toxic in cancer patients.6-9,41 High functional heterogeneity of T cells is a major factor that limits the antitumor potential of CAR-redirected immunotherapy and is associated with increased risk of toxicity. Our results demonstrate that expression of CAR.GD2 in human NKT cells renders them directly cytotoxic against GD2-positive NB cells. These CAR.GD2 NKT cells have potent antitumor activity in a metastatic model of NB in hu-NSG mice. At the same time and in contrast with CAR.GD2 T cells, CAR.GD2 NKT cells do not induce GVHD.
We show that primary human NKT cells can be stably transduced with CAR.GD2 retroviral constructs and expanded ex vivo to clinical scale. Indeed, we were able to consistently obtain >108 CAR.GD2 NKT cells from a small volume of peripheral blood within 3 weeks of ex vivo expansion. Of note, CAR.GD2 expression had no effect on the rate of NKT-cell expansion regardless of the CAR composition, indicating that the efficacy of a ligand-driven NKT-cell in vitro expansion is determined by the cognate TCR stimulation.
CAR.GD2 NKT cells exhibited potent yet specific cytotoxicity against both GD2-positive tumor cells and CD1d-positive M2 macrophages in vitro. This dual-specific cytotoxicity of CAR.GD2 NKT cells gives them a unique antitumor effector function. The presence of TAMs with M2-like phenotype is associated with a recently discovered inflammatory signature in NB that serves as an independent prognostic factor of extremely poor outcome in these patients,37 suggesting CD1d-dependent elimination or inhibition of TAMs by CAR.GD2 NKT cells may sensitize tumor cells to CAR-mediated cytotoxicity and decrease the chances of tumor escape. However, we did not find a difference in TAM frequency between control and NKT-cell–treated mice (data not shown). TAMs in hu-NSG mice expressed low levels of CD1d and might not be able to cross-present endogenous glycolipds to NKT cells. Alternatively, NKT-cell killing of TAMs could be inhibited in the tumor microenvironment.
A number of preclinical studies showed that both CD28 and 4-1BB enhance effector functions of CAR T cells such as cytotoxicity and cytokine production.3,4,27,40,42 The presence of 4-1BB in CAR.19 was associated with enhanced production of IFN-γ.40 NKT cells physiologically produce both Th1 and Th2 cytokines and are known to be resistant to polarization.43 In that respect, it was unexpected to observe Th1-like and Th2-like patterns of polarization when NKT cells expressed 4-1BB- and CD28-containing CARs, respectively. A Th1-like response was also evident in NKT cells that expressed a CAR with a combination of CD28 and 4-1BB endodomains. Importantly, tumor-bearing mice treated with NKT cells with 4-1BB-containing CARs had elevated levels of IFN-γ and GM-CSF, but not IL-4 or IL-10, in the serum. Therefore, CAR expression not only redirects NKT-cell specificity, but also broadly reprograms their effector and regulatory functions.
The prosurvival effect of costimulation in CAR T cells is well documented and extensively reviewed.1,2,27,44,45 Moreover, results from recent clinical trials with 4-1BB- or CD28-containing CD19-specific CARs indicate that persistence of therapeutic CAR T cells correlates with clinical responses in patients with B-cell malignancies.46 Our results with CAR NKT cells show that CD28 and 4-1BB endodomains, alone or combined, were similarly effective in protecting NKT cells from activation-induced cells death in vitro, but only coexpression of CD28 and 4-1BB strongly enhanced NKT-cell persistence in tumor-bearing mice. These results suggest that effective immunotherapy of solid tumors such as NB that do not express CD28 or 4-1BB activating ligands (data not shown) will require a combination of at least 2 costimulatory endodomains in the CAR design.
In contrast to the minimal antitumor activity of Gz-transduced or parental NKT cells, NKT cells expressing costimulatory CARs had potent antitumor activity and significantly prolonged survival of animals in a highly aggressive metastatic model of NB. Although treatment with NKT cells expressing G28BBz did not significantly improve animal survival compared with NKT cells expressing G28z or GBBz, repeated injections of G28BBz NKT cells dramatically improved tumor control and enabled long-term survival. The observed preservation of GD2 expression in the recurrent tumors and their sensitivity to repeated injections of CAR.GD2 NKT cells suggest that the tumor escapes because of limited persistence or inhibition of CAR NKT cells at the tumor site. It remains to be seen whether CAR design or neutralization of immune inhibitory signals in the tumor can further enhance therapeutic efficacy of CAR NKT-cell therapy.
Consistent with the monomorphic nature of CD1d and its interspecies conservation, CAR.GD2 NKT cells did not induce GVHD in hu-NSG mice; whereas CAR.GD2 T cells caused lethal GVHD, which prevented us from evaluating the antitumor activity of the latter. Although less severe than in the humanized mice, the development of xeno-GVHD in NSG mice after adoptive transfer of mature human T cells is a well-established fact,39,40 and, therefore, it is still unclear to what extent the antitumor efficacy of CAR T cells in the previously reported studies is influenced by xenoreactivity. In contrast, NKT cells do not mediate GVHD, even after repeated injections, and may have a unique advantage over T cells for adoptive cancer immunotherapy in the absence of HLA matching.
Although no GVHD has so far been reported in patients infused with autologous CAR-modified T cells, costimulated autologous T cells have been shown to induce severe GVHD syndrome,47 and the risk of such complication increases as new CAR constructs with potent costimulation emerge. In that regard, NKT cells represent a safer alternative for testing such CARs even in autologous settings. Furthermore, a number of studies reported that donor-derived NKT cells may suppress GVHD while maintaining a “graft-versus-tumor or -leukemia” effect.48,49 Consistent with these findings, a recent report demonstrated that reconstitution of NKT cells in peripheral blood is associated with long-term remission of pediatric leukemia patients receiving haploidentical transplantation.50,51 Overall, our results provide the rationale for the development of an NKT-cell–based platform for CAR-directed cancer immunotherapy that could be applied to a broad range of malignancies and used in either autologous or allogeneic settings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Malcolm Brenner (Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX) for helpful discussions.
This work was supported by grants from National Institutes of Health, National Cancer Institute, (RO1 CA116548 [L.S.M.], R01 CA142636 [G.D.], and K12 CA090433 [A.H.]), US Department of Defense (W81XWH-10-10425) (G.D.), Cancer Prevention and Research Institute of Texas (RP1 130588) (L.S.M.), Alex’s Lemonade Stand Foundation for Childhood Cancer (L.S.M.), and Cookies for Kid’s Cancer Foundation (L.S.M.).
Authorship
Contribution: G.D. and E.Y. designed and made the CAR.GD2 constructs; A.H., D.L., G.T., and A.N.C. performed NKT-cell transduction, expansion, and in vitro functional experiments; A.H., J.W., L.G., E.M., and D.L. performed in vivo experiments; X.G. did immunofluorescent microscopy; J.H. performed pathological examination; H.L. and A.H. performed statistical analysis; L.S.M. wrote the manuscript; G.D. and A.H. edited the manuscript; and all authors discussed and interpreted results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.Y. is Department of Stem Cell Transplantation, The University of Texas MD Anderson Cancer Center, Houston, TX.
Correspondence: Leonid S. Metelitsa, Department of Pediatrics, Baylor College of Medicine, 1102 Bates Ave, C.1760.06, Houston, TX; e-mail: lsmeteli@txch.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal