Key Points
XIAP deficiency selectively diminishes BCL10-mediated innate responses and impairs the ability of the host to control specific microbes.
The selective innate immunodeficiency in the XIAP-deficient host leads to the persistent presence of specific pathogens and excess inflammation.
Abstract
Emerging evidence indicates that innate immunodeficiency syndromes are linked to mutations in innate receptors and to specific infections. X-linked lymphoproliferative syndrome type-2 (XLP-2) is associated with deficiency in X-linked inhibitor of apoptosis protein (XIAP), with poorly understood molecular mechanisms. Here we showed that XIAP deficiency selectively impaired B-cell chronic lymphocytic leukemia/lymphoma 10 (BCL10)-mediated innate responses to dectin-1 ligands but did not affect responses to various Toll-like receptor agonists. Consequently, Xiap−/− mice became highly vulnerable on Candida albicans infection. The compromised early innate responses led to the persistent presence of C albicans and inflammatory cytokines in Xiap−/− mice. Furthermore, priming of Xiap−/− mice with the dectin-1 ligand curdlan alone resulted in XLP-2–like syndromes. Restoration of dectin-1–induced Rac1 activation and phagocytosis by resolvin D1, but not up-regulation of nuclear factor-κB, rescued Xiap−/− mice from C albicans lethal infection. Therefore, development of XLP-2 in XIAP-deficient patients could be partly due to sustained inflammation as a consequence of defective BCL10-dependent innate immunity toward specific pathogens. Importantly, our results suggest the potential therapeutic value of resolvin D1 in the treatment of XLP-2 and innate immunodeficiency syndromes.
Introduction
Mutations in innate receptors and their signaling molecules lead to a variety of innate immunodeficiency syndromes.1-4 Patients with primary innate immunodeficiency are characterized by normal leukocyte development and susceptibility to specific infection. For example, interleukin-1 receptor-associated kinase-4 deficiency reveals its indispensable role to control pyrogenic bacteria but not most other pathogens, whereas Toll-like receptor (TLR)-3 deficiency selectively impairs immunity to herpes simplex virus.1,2 Recent findings suggest that Crohn disease also results from a defective innate inflammatory response.5-7
X-linked lymphoproliferative syndrome type-2 (XLP-2) is a lymphoproliferative disease associated with deficiency in the X-linked inhibitor of apoptosis protein (XIAP).8 XLP-2 manifests as sensitivity to Epstein-Barr virus infection, leading to hemophagocytic lymphohistiocytosis, an inflammatory disorder caused by excess cytokine production from overactivated lymphocytes and macrophages.9,10 XLP-2 is also associated with recurrent splenomegaly, fever, and hemorrhagic colitis.10 The molecular mechanism describing how XIAP deficiency leads to XLP-2 remains unknown.9
XIAP is the best-characterized member of the inhibitor of apoptosis protein (IAP) family, and is the only IAP protein that directly inhibits caspase-3, -7, and -9.11,12 However, Xiap−/− mice appear normal and do not exhibit any phenotype reminiscent of XLP-2.13-15 Notably, Xiap−/− mice are sensitive to infection of Listeria monocytogenes, with impaired innate immune responses and defective activation in macrophages.16 XIAP is also known to activate nuclear factor (NF)-κB independent of the inactivation of caspase.12,17-19 The requirement of XIAP in NF-κB activation is stimuli selective, and XIAP is involved in nonobese diabetic (NOD)2-initiated NF-κB activation but is also dispensable in tumor necrosis factor (TNF)-α- and lipopolysaccharide (LPS)-induced NF-κB activation.20-22
Dectin-1 is the receptor recognizing β-glucan and is required for control of fungal infection.23-27 Ligation of dectin-1 activates protein tyrosine kinase Syk, followed by activation of protein kinase C-δ.27 Protein kinase C-δ in turn phosphorylates caspase recruitment domain 9 (CARD9), leading to formation of the CARD9–B-cell chronic lymphocytic leukemia/lymphoma 10 (BCL10)-mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1) complex and activation of IκB kinase and NF-κB.23,24 Dectin-1 signaling also activates extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase (MAPK).28
In the present study, we demonstrate that XIAP is specifically required for dectin-1–induced innate responses. XIAP targeted BCL10, and XIAP deficiency impaired the dectin-1–induced BCL10-mediated activation signals. Consequently, Xiap−/− mice were unable to control infection by Candida albicans. The persistent presence of C albicans resulted in overactivation of macrophages, excess production of inflammatory cytokines, and splenomegaly in Xiap−/− mice. Importantly, restoration of Rac1 activation and phagocytosis, but not NF-κB activation, by resolvin D1 rescued Xiap−/− mice from C albicans lethal infection. Our results provide evidence of an innate immunity-associated mechanism that may contribute to the generation of XLP-2 syndromes in XIAP mutant patients. Furthermore, we suggest the potential therapeutic value of resolvin D1 in treating XLP-2 and, possibly, innate immunodeficiency syndromes.
Methods
Reagents and mice
Zymosan depleted (InvivoGen) is the hot alkali-treated cell wall from Saccharomyces cerevisiae lacking all its endogenous TLR-stimulating activity but retaining dectin-1–activating capacity. The human XIAP constructs were previously described.29 The Xiap−/− mouse14 was a generous gift from Dr David L. Vaux (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia). Mice were maintained in the specific pathogen-free mouse facility of the Institute of Molecular Biology, Academia Sinica. All mouse experiments were conducted with approval from the Institutional Animal Care and Use Committee, Academia Sinica. Detailed reagents are listed in supplemental Methods available on the Blood Web site.
Bone marrow-derived macrophages and human macrophages
Bone marrow cells were collected from tibias and femurs flushed with cold phosphate-buffered saline through a 24-gauge needle and were cultured in complete Dulbecco’s modified Eagle medium containing 20 ng/mL granulocyte macrophage–colony-stimulating factor (R&D) for 8 days to generate bone marrow-derived dendritic cells or in Dulbecco’s modified Eagle medium containing 20% L929 cell-conditioned medium to generate bone marrow-derived macrophages. Human CD14+ monocytes were isolated from peripheral blood mononuclear cells and were differentiated into macrophages in the presence of macrophage–colony-stimulating factor. Rac1V12 or small interfering XIAP was introduced into human macrophages by electroporation in a Nucleofector (Amaxa, Koeln, Germany). Human peripheral blood mononuclear cells were used with approval from the Institutional Review Board of Biomedical Science Research, National Health Research Institutes.
Yeast preparation and quantitation
C albicans (ATCC90028) was cultured on a yeast mold plate at 25°C for 2 days, and a single colony was inoculated into 10 mL YM broth at 30°C for 18 to 24 hours. C albicans were harvested by centrifugation. The yeast pellet was resuspended with phosphate-buffered saline and the OD was determined. For determination of fungal burden in vivo, organs were removed from the infected mice, ground, and resuspended in water. The samples were serially diluted, and the diluents were spread onto YM plates. The number of colonies on plates was counted after incubation at 30°C for 2 days.
Resolvin D1 administration
Aspirin-triggered Resolvin D1 (AT-RvD1; Cayman) (abbreviated as RvD1) was dissolved in phosphate-buffered saline and was administered intravenously (100 ng/mouse)30-32 3 days after C albicans infection. For in vitro experiments, bone marrow-derived macrophages (BMDMs) were treated with RvD1 for 30 minutes before the addition of heat-killed C albicans (HKCA).
Additional methods are reported in supplemental Methods.
Results
XIAP mediates dectin-1–induced responses but is not required for TLR- or TNF-α–triggered activation
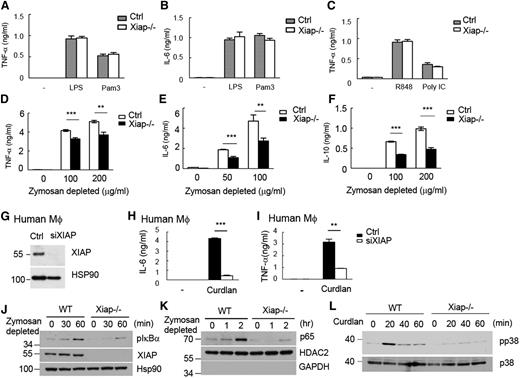
Stimulation of Xiap−/− BMDMs with LPS (for TLR4), Pam3CSK4 (for TLR2), poly (I:C) (for TLR3), or R848 (for TLR7/8) generated comparable amounts of TNF-α and interleukin (IL)-6 as wild-type (WT) BMDMs [Figure 1A-C; data not shown for IL-6 stimulated by poly (I:C) and R848]. We used zymosan depleted to selectively activate dectin-1 in WT and Xiap−/− bone marrow-derived dendritic cells (BMDCs). Dectin-1–induced production of TNF-α, IL-6, and IL-10 was attenuated in Xiap−/− BMDCs (Figure 1D-F). Similarly, XIAP-knockdown human macrophages (Figure 1G) reacted normally to LPS stimulation (supplemental Figure 1A-B) and responded poorly to dectin-1 stimulation during the production of TNF-α and IL-6 (Figure 1H-I). XIAP deficiency did not affect the surface expression of dectin-1 and dectin-2 (supplemental Figure 2A-B). Dectin-1–induced activation of Syk, extracellular signal-regulated kinase, and c-Jun N-terminal kinase was comparable between control and Xiap−/− macrophages (supplemental Figure 2C-D). XIAP deficiency specifically reduced dectin-1–induced IκBα phosphorylation, IκB degradation (supplemental Figure 2E), and p65 nuclear entry, as well as p38 MAPK activation in macrophages (Figure 1J-L). In contrast, comparable NF-κB activation was observed in WT and Xiap−/− macrophages activated by Pam3CSK4 and LPS (supplemental Figure 3A-B) and in WT and Xiap−/− T cells stimulated by TNF-α and LPS (supplemental Figure 3C-D). Therefore, XIAP is required for dectin-1–induced NF-κB activation but is not apparently involved in NF-κB activation stimulated by TNF-α, Pam3CSK4, LPS, poly (I:C), and R848 in T cells and macrophages.
Defective responses of XIAP-deficient macrophages to dectin-1 agonist but not to LPS Pam3CSK4, poly (I:C), or R848. (A-C) Comparable LPS-, Pam3CSK4-, R848-, or poly (I:C)-induced TNF-α and IL-6 production in control and Xiap−/− cells. Control and Xiap−/− BMDMs were activated with LPS (250 ng/mL), Pam3CSK4 (500 ng/mL), R848 (2 μg/mL), or poly (I:C) (50 μg/mL), and the secretion of (A,C) TNF-α and (B) IL-6 was quantitated 18 hours later by enzyme-linked immunosorbent assay (ELISA) using IL-6 DuoSet and TNF-α DuoSet. (D-F) XIAP-knockout reduced dectin-1–induced cytokine secretion. Control and Xiap−/− BMDMs were seeded overnight and treated with zymosan depleted (100 μg/mL) to activate dectin-1. The (D) TNF-α, (E) IL-6, and (F) IL-10 generated were quantitated 16 hours after activation by ELISA. (G) Knockdown of XIAP in human macrophages. Human macrophages were differentiated from peripheral monocytes and were transfected with control and XIAP-siRNA. The expression levels of XIAP were determined 48 hours after transfection. (H-I) Reduced dectin-1–induced TNF-α and IL-6 in XIAP-knockdown human macrophages. Control and XIAP-knockdown human macrophages (48 hours after transfection) were seeded overnight and treated with zymosan depleted (100 μg/mL) to activate dectin-1. The TNF-α and IL-6 generated were quantitated 16 hours after activation by ELISA. **P < .01 and ***P < .001 for paired Student t test. Each data point represents mean of triplicate determinations in a single experiment, and each experiment (A-I) was repeated 3 times with similar results. (J-L) Impaired NF-κB activation and p38 MAPK phosphorylation in Xiap−/− BMDMs stimulated with dectin-1 ligands. (J,L) Total cell lysates and (K) nuclear extracts were prepared from BMDMs stimulated with (J-K) zymosan depleted or (L) curdlan at the indicated time points. (J,L) IκBα and p38 phosphorylation in total cell lysates and (K) the entry of p65 in nuclear extracts were determined by immunoblotting. HSP90 and HDAC2 were the internal controls for the total cell lysate or nuclear extract, respectively. (J-L) were repeated twice with similar results.
Defective responses of XIAP-deficient macrophages to dectin-1 agonist but not to LPS Pam3CSK4, poly (I:C), or R848. (A-C) Comparable LPS-, Pam3CSK4-, R848-, or poly (I:C)-induced TNF-α and IL-6 production in control and Xiap−/− cells. Control and Xiap−/− BMDMs were activated with LPS (250 ng/mL), Pam3CSK4 (500 ng/mL), R848 (2 μg/mL), or poly (I:C) (50 μg/mL), and the secretion of (A,C) TNF-α and (B) IL-6 was quantitated 18 hours later by enzyme-linked immunosorbent assay (ELISA) using IL-6 DuoSet and TNF-α DuoSet. (D-F) XIAP-knockout reduced dectin-1–induced cytokine secretion. Control and Xiap−/− BMDMs were seeded overnight and treated with zymosan depleted (100 μg/mL) to activate dectin-1. The (D) TNF-α, (E) IL-6, and (F) IL-10 generated were quantitated 16 hours after activation by ELISA. (G) Knockdown of XIAP in human macrophages. Human macrophages were differentiated from peripheral monocytes and were transfected with control and XIAP-siRNA. The expression levels of XIAP were determined 48 hours after transfection. (H-I) Reduced dectin-1–induced TNF-α and IL-6 in XIAP-knockdown human macrophages. Control and XIAP-knockdown human macrophages (48 hours after transfection) were seeded overnight and treated with zymosan depleted (100 μg/mL) to activate dectin-1. The TNF-α and IL-6 generated were quantitated 16 hours after activation by ELISA. **P < .01 and ***P < .001 for paired Student t test. Each data point represents mean of triplicate determinations in a single experiment, and each experiment (A-I) was repeated 3 times with similar results. (J-L) Impaired NF-κB activation and p38 MAPK phosphorylation in Xiap−/− BMDMs stimulated with dectin-1 ligands. (J,L) Total cell lysates and (K) nuclear extracts were prepared from BMDMs stimulated with (J-K) zymosan depleted or (L) curdlan at the indicated time points. (J,L) IκBα and p38 phosphorylation in total cell lysates and (K) the entry of p65 in nuclear extracts were determined by immunoblotting. HSP90 and HDAC2 were the internal controls for the total cell lysate or nuclear extract, respectively. (J-L) were repeated twice with similar results.
XIAP interacts with BCL10 and promotes BCL10 K63 ubiquitination
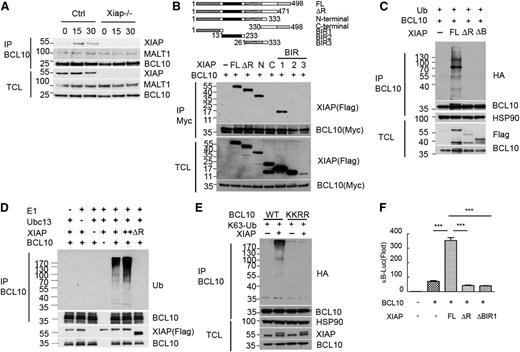
Because XIAP participates in dectin-1–induced, but not TNF-α– and TLR-triggered NF-κB activation, the NF-κB activation stage that would be regulated by XIAP should be upstream of transforming growth factor-beta-activated kinase 1-IκB kinase activation. Dectin-1–induced NF-κB activation differs from TNF-α– and LPS-triggered NF-κB activation in the selective formation of the CARD9-BCL10-MALT1 complex.23-28 A previous study suggested an association of XIAP with BCL10.33 Immunoprecipitation of the overexpressed XIAP-Myc brought down FLAG-BCL10 but not FLAG-MALT1 (supplemental Figure 4). The specific interaction between endogenous XIAP and BCL10 was confirmed in curdlan-stimulated macrophages (Figure 2A). XIAP consists of the N-terminal baculovirus IAP repeat (BIR) 1, BIR2, BIR3, and C-terminal really interesting new gene finger domains.12 Using FLAG-tagged XIAP deletion mutants, we then mapped the domain of XIAP that binds BCL10. The BCL10-binding region was isolated to the BIR1 segment of XIAP (Figure 2B).
XIAP interacts with BCL10 and promotes BCL10 K63 polyubiquitination and BCL10-directed NF-κB activation. (A) Interaction between endogenous XIAP and BCL10 in macrophages. BMDMs were activated with curdlan, and total cell lysates were prepared at the indicated time points. Total cell lysates (1 mg) were immunoprecipitated with anti-BCL10, and the presence of XIAP, MALT1, and BCL10 was determined. (B) BCL10 interacts with the BIR1 domain of XIAP. FLAG-tagged full-length (FL), deleted RING finger (ΔR), N-terminal (N), C-terminal (C), BIR1, BIR2, or BIR3 of XIAP were cotransfected with BCL10-Myc into 293T. Total cell lysates were immunoprecipitated by anti-Myc, and the presence of XIAP and its mutants in immune complexes was detected by anti-FLAG. (C) FL-XIAP, but neither XIAPΔRF nor XIAPΔBIR1, promotes BCL10 polyubiquitination. HA-ubiquitin, BCL10, XIAP, XIAPΔRF, or XIAPΔBIR1 (ΔB) was transfected into 293T cells as indicated. Total cell lysates were prepared and immunoprecipitated by anti-BCL10, and Ub-containing protein was detected by anti-HA. (D) BCL10 K63 ubiquitination was induced by XIAP in vitro. In vitro ubiquitination assays were conducted in reaction mixtures containing human ubiquitin-K63, human E1, E2 (Ubc13), BCL10-FLAG, XIAP, or XIAPΔRF, as indicated. BCL10-FLAG was loaded on protein G magnetic beads through anti-FLAG. Reactions proceeded at 30°C for 1 hour. BCL10-FLAG-protein G was pulled down, separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted with ubiquitin antibody (upper panel). The input is shown in the lower panel. (E) Lys31 and Lys63 on BCL10 were required for XIAP-mediated K63 ubiquitination on BCL10. WT-BCL10 or BCL10-KKRR (K31R, K63R) was cotransfected with XIAP and K63 Ub into 293T cells. Total cell lysates were immunoprecipitated by anti-BCL10. Ubiquitinated BCL10 of the immune complexes was detected by anti-HA antibody. (F) XIAP enhances BCL10-activated κB reporter. κB-Luc was transfected with EGFP, XIAP, XIAPΔRF, XIAPΔBIR1, and BCL10, as indicated, into 293T cells, and luciferase activity was determined 24 hours later. EGFP was used to determine transfection efficiency. ***P < .001 for paired Student t test. (A-F) Each experiment was repeated 3 times with similar results.
XIAP interacts with BCL10 and promotes BCL10 K63 polyubiquitination and BCL10-directed NF-κB activation. (A) Interaction between endogenous XIAP and BCL10 in macrophages. BMDMs were activated with curdlan, and total cell lysates were prepared at the indicated time points. Total cell lysates (1 mg) were immunoprecipitated with anti-BCL10, and the presence of XIAP, MALT1, and BCL10 was determined. (B) BCL10 interacts with the BIR1 domain of XIAP. FLAG-tagged full-length (FL), deleted RING finger (ΔR), N-terminal (N), C-terminal (C), BIR1, BIR2, or BIR3 of XIAP were cotransfected with BCL10-Myc into 293T. Total cell lysates were immunoprecipitated by anti-Myc, and the presence of XIAP and its mutants in immune complexes was detected by anti-FLAG. (C) FL-XIAP, but neither XIAPΔRF nor XIAPΔBIR1, promotes BCL10 polyubiquitination. HA-ubiquitin, BCL10, XIAP, XIAPΔRF, or XIAPΔBIR1 (ΔB) was transfected into 293T cells as indicated. Total cell lysates were prepared and immunoprecipitated by anti-BCL10, and Ub-containing protein was detected by anti-HA. (D) BCL10 K63 ubiquitination was induced by XIAP in vitro. In vitro ubiquitination assays were conducted in reaction mixtures containing human ubiquitin-K63, human E1, E2 (Ubc13), BCL10-FLAG, XIAP, or XIAPΔRF, as indicated. BCL10-FLAG was loaded on protein G magnetic beads through anti-FLAG. Reactions proceeded at 30°C for 1 hour. BCL10-FLAG-protein G was pulled down, separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted with ubiquitin antibody (upper panel). The input is shown in the lower panel. (E) Lys31 and Lys63 on BCL10 were required for XIAP-mediated K63 ubiquitination on BCL10. WT-BCL10 or BCL10-KKRR (K31R, K63R) was cotransfected with XIAP and K63 Ub into 293T cells. Total cell lysates were immunoprecipitated by anti-BCL10. Ubiquitinated BCL10 of the immune complexes was detected by anti-HA antibody. (F) XIAP enhances BCL10-activated κB reporter. κB-Luc was transfected with EGFP, XIAP, XIAPΔRF, XIAPΔBIR1, and BCL10, as indicated, into 293T cells, and luciferase activity was determined 24 hours later. EGFP was used to determine transfection efficiency. ***P < .001 for paired Student t test. (A-F) Each experiment was repeated 3 times with similar results.
We examined whether XIAP promotes BCL10 polyubiquitination. Indeed, coexpression with XIAP increased the polyubiquitination of BCL10 in vivo (Figure 2C). However, deletion of the RING finger domain from XIAP (XIAPΔRF) or the removal of BIR1 eliminated the ability of XIAP to ubiquitinate BCL10 (Figure 2C). Further, the incorporation of K63 Ub into BCL10 was enhanced by XIAP, whereas the addition of K48 Ub to BCL10 was not affected by XIAP in 293T cells (supplemental Figure 4B). We also used in vitro ubiquitination analysis to show that, in reaction mixtures containing ubiquitin, E1, E2, and recombinant BCL10, the addition of recombinant XIAP, but not XIAP∆RF, induced K63 ubiquitination of BCL10 (Figure 2D).
We next examined the possible XIAP-mediated ubiquitination sites on BCL10. Ubiquitination of Lys31 and Lys63 on BCL10 has been functionally linked to the activation of NF-κB.34 Mutation of Lys31 and Lys63 on BCL10 (BCL10-KKRR) prevented XIAP-mediated K63 ubiquitination (Figure 2E), suggesting that K31 and K63 on BCL10 are specifically targeted for ubiquitination by XIAP. In addition, BCL10-stimulated κB-Luc activation35 was profoundly enhanced by the coexpression of WT XIAP but not by XIAPΔBIR1 or XIAPΔRF (Figure 2F). Therefore, the association of XIAP with BCL10 and the ubiquitination of BCL10 modulated the ability of BCL10 to activate NF-κB.
XIAP deficiency impairs BCL10-mediated NF-κB activation stimulated by T-cell receptor, lysophosphatidic acid, and epidermal growth factor receptor
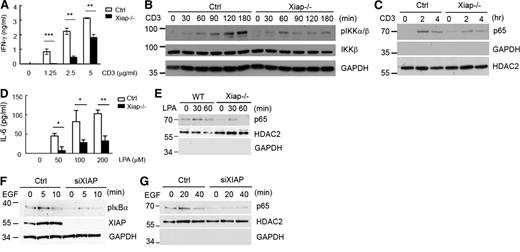
The CARMA1-BCL10 complex is involved in NF-κB activation triggered through the T-cell receptor (TCR) and B-cell receptor.34,36-38 CARMA3 and BCL10 are required for NF-κB activation induced by epidermal growth factor (EGF) and G protein-coupled receptor agonists such as lysophosphatidic acid (LPA).39-43 We therefore examined whether XIAP also participated in NF-κB activation stimulated by these receptors. In the absence of CD28 costimulation, XIAP deficiency led to attenuated CD3-stimulated interferon (IFN)-γ production (Figure 3A), deceased TCR-induced IKKα/β phosphorylation (Figure 3B), and reduced anti–CD3-induced RelA nuclear translocation (Figure 3C). It may be noted that XIAP deficiency did not affect CD3/CD28-induced IKKα/β phosphorylation and IL-2 production (supplemental Figure 5), consistent with the effect previously reported in Xiap−/− T cells13 and CARMA1-knockout T cells.44
XIAP deficiency impairs TCR-, LPA-, and EGF-stimulated cytokine production and NF-κB activation. (A) Diminished IFN-γ production in Xiap−/− T cells. Purified T cells from normal littermate control and Xiap−/− mice were activated with plate-bound anti-CD3 (5 μg/mL), and the IFN-γ secretion was determined 48 hours later. (B-C) Reduced CD3-induced NF-κB activation in Xiap−/− T cells. Control and Xiap−/− T cells were activated with anti-CD3, and (B) total cell lysates and (C) nuclear extracts were prepared at the indicated time points. (B) IKK phosphorylation in total cell lysates and (C) the presence of p65 in nuclear extracts were determined by immunoblotting. HDAC2 was the internal control of the nuclear extract, whereas glyceraldehyde-3-phosphate dehydrogenase was the marker of the cytosolic extract. (D-E) Diminished LPA-stimulated IL-6 production and NF-κB activation in Xiap-deficient macrophages. Peritoneal macrophages were isolated from WT and Xiap−/− mice primed with thioglycolate and were stimulated with LPA (10 μM). (E) Nuclear extracts were prepared at 30 and 60 minutes, and the presence of p65 as determined. (D) IL-6 was quantitated by ELISA 16 hours later. (F-G) XIAP knockdown decreased EGF-induced NF-κB activation. Control siRNA or XIAP-specific siRNA was transfected into HepG2 cells by electroporation. HepG2 cells were plated 16 hours after transfection and treated with EGF (20 ng/mL) after another 24 hours. Total cell lysates and nuclear extracts were prepared at the indicated time points. (F) EGF-induced IκBα phosphorylation and (G) p65 nuclear translocation were measured in total cell lysates and nuclear extracts, respectively. *P < .05, **P < .01, and ***P < .001 for paired Student t test. (A,D) Each data point represents mean of triplicate determinations in a single experiment, and (A-G) each experiment was repeated 3 times with similar results.
XIAP deficiency impairs TCR-, LPA-, and EGF-stimulated cytokine production and NF-κB activation. (A) Diminished IFN-γ production in Xiap−/− T cells. Purified T cells from normal littermate control and Xiap−/− mice were activated with plate-bound anti-CD3 (5 μg/mL), and the IFN-γ secretion was determined 48 hours later. (B-C) Reduced CD3-induced NF-κB activation in Xiap−/− T cells. Control and Xiap−/− T cells were activated with anti-CD3, and (B) total cell lysates and (C) nuclear extracts were prepared at the indicated time points. (B) IKK phosphorylation in total cell lysates and (C) the presence of p65 in nuclear extracts were determined by immunoblotting. HDAC2 was the internal control of the nuclear extract, whereas glyceraldehyde-3-phosphate dehydrogenase was the marker of the cytosolic extract. (D-E) Diminished LPA-stimulated IL-6 production and NF-κB activation in Xiap-deficient macrophages. Peritoneal macrophages were isolated from WT and Xiap−/− mice primed with thioglycolate and were stimulated with LPA (10 μM). (E) Nuclear extracts were prepared at 30 and 60 minutes, and the presence of p65 as determined. (D) IL-6 was quantitated by ELISA 16 hours later. (F-G) XIAP knockdown decreased EGF-induced NF-κB activation. Control siRNA or XIAP-specific siRNA was transfected into HepG2 cells by electroporation. HepG2 cells were plated 16 hours after transfection and treated with EGF (20 ng/mL) after another 24 hours. Total cell lysates and nuclear extracts were prepared at the indicated time points. (F) EGF-induced IκBα phosphorylation and (G) p65 nuclear translocation were measured in total cell lysates and nuclear extracts, respectively. *P < .05, **P < .01, and ***P < .001 for paired Student t test. (A,D) Each data point represents mean of triplicate determinations in a single experiment, and (A-G) each experiment was repeated 3 times with similar results.
We also used Xiap−/− macrophages to confirm that LPA-triggered IL-6 production and p65 nuclear translocation were decreased in Xiap−/− macrophages (Figure 3D-E). In addition, EGF-stimulated IκBα phosphorylation and p65 nuclear entry was reduced in XIAP-knockdown HepG2 cells (Figure 3F-G). Therefore, consistent with the suggestion that XIAP is involved in BCL10-mediated NF-κB signaling, XIAP is required for full NF-κB activation triggered by dectin-1, LPA, epidermal growth factor receptor (EGFR), or TCR alone.
Sensitivity of Xiap−/− mouse to C albicans infection
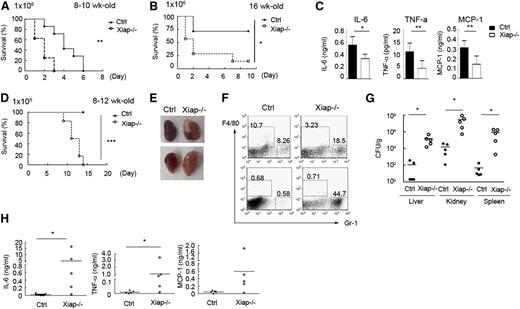
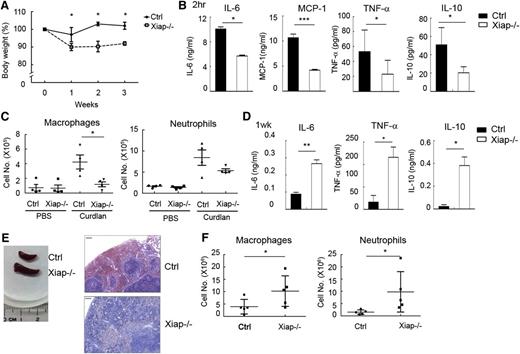
C albicans is recognized by dectin-1 and dectin-2, and the fungal infection is controlled by dectin-induced responses through the CARD9-BCL10-NF-κB pathway.23-27,45,46 We therefore tested whether deficiency in XIAP leads to defective responses toward C albicans. We found that 8- to 10-week-old Xiap−/− mice were highly susceptible to C albicans (1 × 106) infection, with all mice dying from infection by day 3 (Figure 4A). Older Xiap−/− mice (16 weeks old) were modestly more resistant to C albicans (1 × 106; Figure 4B). Immediately after C albicans administration, generation of IL-6, monocyte chemoattractant protein (MCP)-1, and TNF-α was diminished in Xiap−/− mice relative to WT mice (Figure 4C). At a lower dose of C albicans (1 × 105), Xiap+/+ mice were fully resistant, whereas most of the Xiap−/− mice died from infection before day 15 (Figure 4D), accompanied by progressive body weight loss. In these dying Xiap−/− mice, scattered foci of abscesses were observed in the swollen kidneys (Figure 4E), as well as a large fraction of infiltrated neutrophils in kidney (Figure 4F). C albicans titers were also highly elevated in the kidney, liver, and spleen tissues of Xiap−/− mice (Figure 4G). The compromised responses to C albicans in XIAP-deficient mice were comparable to those seen in mice lacking dectin-1, dectin-2, or CARD9.23,28,45,46 Another consequence of XIAP deficiency was found in the accumulation of proinflammatory cytokines following a low dose of C albicans infection. Although the serum contents of IL-6, TNF-α, and MCP-1 were higher in WT mice than Xiap−/− mice 6 hours after a low dose of C albicans infection (data not shown), these cytokines were no longer detectable in the peripheral blood of WT mice 1 week after infection (Figure 4H), consistent with the near clearance of C albicans in these mice (Figure 4G). In contrast, Xiap−/− mice retained a high serum level of IL-6, TNF-α, and MCP-1 at the same time point (Figure 4H), suggesting that the continued presence of C albicans turns the defective innate responses into a proinflammatory status late in infection. A correlation was also found between the extent of the body weight loss and serum levels of IL-6, TNF-α, and MCP-1 in those Xiap−/− mice 1 week after C albicans infection (supplemental Figure 6), illustrating that high levels of proinflammatory cytokines are associated with poor prognosis.
Xiap−/− mice exhibit heightened sensitivity to C albicans infection. (A-B) Poor survival of Xiap−/− mice following C albicans infection. C albicans (1 × 106) was intravenously administered to (A) 8- to 10-week-old and (B) 16-week-old WT and Xiap−/− mice. Survival of mice is presented by a Kaplan-Meier survival curve; n = 7 for A and B. (C) Reduced inflammatory cytokine production in Xiap−/− mice after C albicans infection. Serum from mice in A and B was collected 4 hours after C albicans injection, and the levels of IL-6, MCP-1, and TNF-α were determined; n = 10, 3 mice from A and 7 mice from B. (D) Poor survival of Xiap−/− mice following a low dose of C albicans infection. WT and Xiap−/− mice (8-12 weeks old) were intravenously injected with C albicans (1 × 105), and the survival of mice was monitored; n = 6. (E) Morphology of kidney from WT and Xiap−/− mice infected with low doses of C albicans. WT and Xiap−/− mice were intravenously injected with C albicans (1 × 105). Infected Xiap−/− mice were euthanized on 30% loss of body weight. Kidneys were isolated from Xiap−/− mice and WT mice infected at the same time (days 8-12 after infection). Two pairs of kidney (of 5) are shown. (F) Elevated neutrophil infiltration in C albicans-infected Xiap−/− mice kidney. Total kidney cells were prepared from the kidneys in E and stained with anti-F4/80 and anti-Gr-1, and the fraction of the macrophages (Gr-1−/intF4/80+) and neutrophils (Gr-1+F4/80−) was analyzed by flow cytometry. (G) Highly increased fungal burden in Xiap−/− mice infected with C albicans. Livers, kidneys, and spleens were removed from C albicans-infected WT and Xiap−/− mice in D between days 8 and 12. Colony-forming units of C albicans in each organ was determined. (H) Elevated inflammatory cytokine in Xiap−/− mice 1 week after C albicans infection. Serum from mice in D was collected 7 days after C albicans (1 × 105) injection, and the levels of IL-6, TNF-α, and MCP-1 were determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
Xiap−/− mice exhibit heightened sensitivity to C albicans infection. (A-B) Poor survival of Xiap−/− mice following C albicans infection. C albicans (1 × 106) was intravenously administered to (A) 8- to 10-week-old and (B) 16-week-old WT and Xiap−/− mice. Survival of mice is presented by a Kaplan-Meier survival curve; n = 7 for A and B. (C) Reduced inflammatory cytokine production in Xiap−/− mice after C albicans infection. Serum from mice in A and B was collected 4 hours after C albicans injection, and the levels of IL-6, MCP-1, and TNF-α were determined; n = 10, 3 mice from A and 7 mice from B. (D) Poor survival of Xiap−/− mice following a low dose of C albicans infection. WT and Xiap−/− mice (8-12 weeks old) were intravenously injected with C albicans (1 × 105), and the survival of mice was monitored; n = 6. (E) Morphology of kidney from WT and Xiap−/− mice infected with low doses of C albicans. WT and Xiap−/− mice were intravenously injected with C albicans (1 × 105). Infected Xiap−/− mice were euthanized on 30% loss of body weight. Kidneys were isolated from Xiap−/− mice and WT mice infected at the same time (days 8-12 after infection). Two pairs of kidney (of 5) are shown. (F) Elevated neutrophil infiltration in C albicans-infected Xiap−/− mice kidney. Total kidney cells were prepared from the kidneys in E and stained with anti-F4/80 and anti-Gr-1, and the fraction of the macrophages (Gr-1−/intF4/80+) and neutrophils (Gr-1+F4/80−) was analyzed by flow cytometry. (G) Highly increased fungal burden in Xiap−/− mice infected with C albicans. Livers, kidneys, and spleens were removed from C albicans-infected WT and Xiap−/− mice in D between days 8 and 12. Colony-forming units of C albicans in each organ was determined. (H) Elevated inflammatory cytokine in Xiap−/− mice 1 week after C albicans infection. Serum from mice in D was collected 7 days after C albicans (1 × 105) injection, and the levels of IL-6, TNF-α, and MCP-1 were determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
We also measured T-cell responses to HKCA in WT and Xiap−/− mice. After immunization with HKCA, the production of IFN-γ and IL-4 was comparable between WT and Xiap−/− T cells (supplemental Figure 7A-B). Because T-cell response was not affected as obviously as macrophages in Xiap−/− mice, we focused on the dectin-1–induced innate immunity in Xiap−/− mice during the rest of study.
Dectin-1 ligand curdlan induces XLP-2–like syndromes in Xiap−/− mouse
To further delineate the effect of XIAP in the BCL10-dependent innate response, we used a model dectin-1 ligand, curdlan. Although WT mice did not display any abnormalities following weekly curdlan intraperitoneal immunization, Xiap−/− mice exhibited body mass loss 1 week after curdlan priming (Figure 5A) and did not recover until the fourth week (data not shown). Serum levels of IL-6, IL-10, MCP-1, and TNF-α were decreased in Xiap−/− mice after administration of curdlan (Figure 5B). Curdlan-induced immediate peritoneal infiltration of macrophages and neutrophils was reduced in Xiap−/− mice (Figure 5C). The compromised early innate responses to curdlan in Xiap−/− mice were reversed a week later. The generation of IL-6, IL-10, TNF-α, and MCP-1 were much higher in Xiap−/− mice than the WT control (Figure 5D). Splenomegaly was found in Xiap−/− mice 1 week after curdlan priming, with profound infiltration of mononuclear cells (Figure 5E). Also, the peritoneal presence of macrophages and neutrophils became higher in Xiap−/− mice than in control mice 1 week after curdlan administration (Figure 5F).
Xiap-deficient mice were unable to resolve the presence of curdlan. (A) Loss of body weight in Xiap−/− mice with weekly treatment of curdlan. Female mice (8-12 weeks old) were primed with intraperitoneal administration of curdlan (5 mg/20 g body weight) every week, and body weight was determined; n = 13. (B) Attenuated inflammatory cytokine production in the Xiap−/− mouse immediately after curdlan immunization. Serum was collected 2 hours after curdlan priming, and the quantity of IL-6, IL-12, MCP-1, and TNF-α was determined; n = 5. (C) Reduced recruitment of macrophages and neutrophils in the Xiap−/− mouse. Peritoneal cavity infiltrates were collected 4 hours after curdlan priming, cell numbers were counted, and the population of macrophages and neutrophils was analyzed by flow cytometry. (D) Enhanced inflammatory cytokines secretion in Xiap−/− mice 1 week after curdlan stimulation. Serum was collected from mice 1 week after immunization of curdlan, and the contents of cytokines were quantitated; n = 5. (E) Curdlan treatment leads to splenomegaly in Xiap−/− mice. Spleens were isolated from control and Xiap−/− mice 7 days after curdlan immunization for comparison. Sections of spleen were stained with hematoxylin and eosin. One representative pair (of 5 pairs) is shown. Bar indicates 50 μm. (F) Increased macrophage and neutrophil infiltration in Xiap−/− mice 1 week after curdlan stimulation. Cells were harvested from the peritoneal cavity in mice from D, and the quantities of macrophages and neutrophils were determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
Xiap-deficient mice were unable to resolve the presence of curdlan. (A) Loss of body weight in Xiap−/− mice with weekly treatment of curdlan. Female mice (8-12 weeks old) were primed with intraperitoneal administration of curdlan (5 mg/20 g body weight) every week, and body weight was determined; n = 13. (B) Attenuated inflammatory cytokine production in the Xiap−/− mouse immediately after curdlan immunization. Serum was collected 2 hours after curdlan priming, and the quantity of IL-6, IL-12, MCP-1, and TNF-α was determined; n = 5. (C) Reduced recruitment of macrophages and neutrophils in the Xiap−/− mouse. Peritoneal cavity infiltrates were collected 4 hours after curdlan priming, cell numbers were counted, and the population of macrophages and neutrophils was analyzed by flow cytometry. (D) Enhanced inflammatory cytokines secretion in Xiap−/− mice 1 week after curdlan stimulation. Serum was collected from mice 1 week after immunization of curdlan, and the contents of cytokines were quantitated; n = 5. (E) Curdlan treatment leads to splenomegaly in Xiap−/− mice. Spleens were isolated from control and Xiap−/− mice 7 days after curdlan immunization for comparison. Sections of spleen were stained with hematoxylin and eosin. One representative pair (of 5 pairs) is shown. Bar indicates 50 μm. (F) Increased macrophage and neutrophil infiltration in Xiap−/− mice 1 week after curdlan stimulation. Cells were harvested from the peritoneal cavity in mice from D, and the quantities of macrophages and neutrophils were determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
As a control, immunization of Pam3CSK4 did not generate any differential response between control and Xiap−/− mice (supplemental Figure 8). Repetitive administration of Pam3CSK4 did not produce detrimental effects in Xiap−/− mice (data not shown). Because Xiap−/− mice reacted normally to Pam3CSK4 in NF-κB activation and inflammatory cytokine production (Figure 1A-B; supplemental Figure 3A), we examined whether priming of Pam3CSK4 increased the resistance of Xiap−/− mice to C albicans infection. Prior administration of Pam3CSK4 did not rescue Xiap−/− mice from C albicans (1 × 105) infection, despite an increase in the proinflammatory cytokine levels (supplemental Figure 9A-C). Instead, Pam3CSK4 accelerated C albicans-mediated mortality in both control and Xiap−/− mice (supplemental Figure 9D-E), indicating that restoration of BCL10-dependent NF-κB activation and inflammatory cytokine expression in Xiap−/− mice failed to compensate for the defective innate response to C albicans.
Impaired dectin-1–mediated Rac1 activation and phagocytosis in XIAP-deficient macrophages
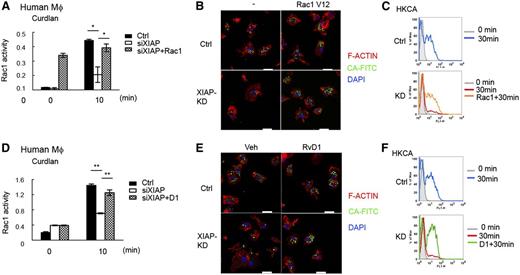
BCL10 was recently shown to participate in Rac1 activation, F-actin remodeling, and phagosome closure that are independent of NF-κB activation.47 We found that curdlan-induced Rac1 activation was attenuated in Xiap−/− BMDMs (supplemental Figure 10A). Similarly, dectin-1–induced Rac1 activation was impaired in XIAP-knockdown human macrophages (Figure 6A). For phagocytosis, the uptake of plain latex beads or Pam3CSK4-coated latex in Xiap−/− BMDMs was not affected, as measured by confocal microscopy or flow cytometry (supplemental Figure 10B-C). In contrast, the phagocytosis of HKCA was impaired in Xiap−/− BMDMs (supplemental Figure 10D). Similar to Xiap−/− BMDMs, XIAP-knockdown human macrophages were defective in the phagocytosis of HKCA (Figure 6B). Quantitation by flow cytometry further confirmed the impaired uptake of C albicans in XIAP-deficient human macrophages (Figure 6C). We also introduced active Rac1 (Rac1-V12) into XIAP-knockdown human macrophages, shown by the constitutive Rac1 activity (Figure 6A). The defective uptake of C albicans was reversed by the expression of Rac1-V12 in XIAP-knockdown human macrophages (Figure 6B-C), suggesting that impaired Rac1 activation accounts for the ineffective uptake of HKCA in XIAP-deficient macrophages.
Defective dectin-1–mediated Rac1 activation and phagocytosis in XIAP-deficient human macrophages are corrected by resolvin D1. (A) Impaired Rac1 activity in XIAP-knockout human macrophages. Control (Ctrl) and XIAP-knockdown (small interfering XIAP) human macrophages, with or without active Rac1 (Rac1V12) transfected, were incubated with curdlan (100 μg/mL) and harvested at 0 and 10 minutes. The activation of Rac1 was measured by the G-LISA Rac1 activation assay kit. (B-C) Defective phagocytosis of C albicans in XIAP-knockdown human macrophages and restoration by Rac1V12 expression. Control and XIAP-knockdown human macrophages, with or without Rac1V12 transfected, were incubated with fluorescein isothiocyanate-conjugated heat killed C albicans (FITC-HKCA) for 30 minutes. Cells were collected at 0 and 30 minutes and analyzed by (B) confocal microscopy and (C) flow cytometry. (B) Cells were fixed, permeabilized, and stained with Alexa Fluor 594-Phalloidin (for F-actin) and 4′,6 diamidino-2-phenylindole for confocal microscopy. Bar indicates 20 μm. (C) Cells were also treated with trypan blue to quench the extracellular associated FITC, and the internalized FITC-conjugated bead was determined by flow cytometry. Gray shadow, fluorescence-activated cell sorting (FACS) profile at 0 minutes; blue and red curves, uptake of FITC-HKCA at 30 minutes; orange curve, internalization of FITC-HKCA at 30 minutes by Rac1V12-transfected macrophages. (D) Resolvin D1 restores Rac1 activity in XIAP-deficient human macrophages. Control and XIAP-knockdown human macrophages were incubated with curdlan (100 μg/mL) with or without AT-resolvin D1 (RvD1, 100 nM) pretreatment. Cells were harvested at 0 and 10 minutes, and the activation of Rac1 was measured. (E-F) Resolvin D1 restores phagocytosis of C albicans in XIAP-knockdown human macrophages. Control and XIAP-knockdown human macrophages, pretreated with vehicle or RvD1 (100 nM), were incubated with FITC-HKCA for 30 minutes. Cells were collected at 0 and 30 minutes and analyzed by (E) confocal microscopy and (F) flow cytometry. Gray shadowed curve, FACS profile at 0 minutes; blue and red curves, internalization of FITC-HKCA at 30 minutes; green curve, internalization of FITC-HKCA at 30 minutes by RvD1-treated macrophages. *P < .05 and **P < .01 for paired Student t test. Confocal images were obtained a Zeiss LSM 780 confocal microscope (Jena, Germany) with an objective lens of plan-Apochromat 63×/1.4 Oil DIC M27 at room temperature. 4′,6 Diamidino-2-phenylindole fluorescence was excited at 405 nm and collected at 420 to 470 nm. FITC fluorescence was excited at 488 nm and collected at 500 to 550 nm. Cy3 fluorescence (transfection indicator, not shown in figure) was excited at 561 nm and collected at 568 to 629 nm. Alexa Fluor 594 fluorescence was excited at 633 nm and collected at 641 to 758 nm. The pinhole was at 61.7 μm, with an 11.4-μm section. Images were taken by an AxioCam digital microscope camera (Zeiss) using Carl Zeiss software Zen 2010B SP1, version 6.0.
Defective dectin-1–mediated Rac1 activation and phagocytosis in XIAP-deficient human macrophages are corrected by resolvin D1. (A) Impaired Rac1 activity in XIAP-knockout human macrophages. Control (Ctrl) and XIAP-knockdown (small interfering XIAP) human macrophages, with or without active Rac1 (Rac1V12) transfected, were incubated with curdlan (100 μg/mL) and harvested at 0 and 10 minutes. The activation of Rac1 was measured by the G-LISA Rac1 activation assay kit. (B-C) Defective phagocytosis of C albicans in XIAP-knockdown human macrophages and restoration by Rac1V12 expression. Control and XIAP-knockdown human macrophages, with or without Rac1V12 transfected, were incubated with fluorescein isothiocyanate-conjugated heat killed C albicans (FITC-HKCA) for 30 minutes. Cells were collected at 0 and 30 minutes and analyzed by (B) confocal microscopy and (C) flow cytometry. (B) Cells were fixed, permeabilized, and stained with Alexa Fluor 594-Phalloidin (for F-actin) and 4′,6 diamidino-2-phenylindole for confocal microscopy. Bar indicates 20 μm. (C) Cells were also treated with trypan blue to quench the extracellular associated FITC, and the internalized FITC-conjugated bead was determined by flow cytometry. Gray shadow, fluorescence-activated cell sorting (FACS) profile at 0 minutes; blue and red curves, uptake of FITC-HKCA at 30 minutes; orange curve, internalization of FITC-HKCA at 30 minutes by Rac1V12-transfected macrophages. (D) Resolvin D1 restores Rac1 activity in XIAP-deficient human macrophages. Control and XIAP-knockdown human macrophages were incubated with curdlan (100 μg/mL) with or without AT-resolvin D1 (RvD1, 100 nM) pretreatment. Cells were harvested at 0 and 10 minutes, and the activation of Rac1 was measured. (E-F) Resolvin D1 restores phagocytosis of C albicans in XIAP-knockdown human macrophages. Control and XIAP-knockdown human macrophages, pretreated with vehicle or RvD1 (100 nM), were incubated with FITC-HKCA for 30 minutes. Cells were collected at 0 and 30 minutes and analyzed by (E) confocal microscopy and (F) flow cytometry. Gray shadowed curve, FACS profile at 0 minutes; blue and red curves, internalization of FITC-HKCA at 30 minutes; green curve, internalization of FITC-HKCA at 30 minutes by RvD1-treated macrophages. *P < .05 and **P < .01 for paired Student t test. Confocal images were obtained a Zeiss LSM 780 confocal microscope (Jena, Germany) with an objective lens of plan-Apochromat 63×/1.4 Oil DIC M27 at room temperature. 4′,6 Diamidino-2-phenylindole fluorescence was excited at 405 nm and collected at 420 to 470 nm. FITC fluorescence was excited at 488 nm and collected at 500 to 550 nm. Cy3 fluorescence (transfection indicator, not shown in figure) was excited at 561 nm and collected at 568 to 629 nm. Alexa Fluor 594 fluorescence was excited at 633 nm and collected at 641 to 758 nm. The pinhole was at 61.7 μm, with an 11.4-μm section. Images were taken by an AxioCam digital microscope camera (Zeiss) using Carl Zeiss software Zen 2010B SP1, version 6.0.
We then looked for reagents that may correct the defect of Xiap−/− macrophages. Resolvin D1 is an anti-inflammatory lipid mediator biosynthesized from ω-3 fatty acid docosahexaenoic acid.30,31 Resolvin D1 displays a specific activity to enhance phagocytosis in macrophages.32,48-50 We found that aspirin-triggered resolvin D1 (AT-RvD1) restored dectin-1–induced Rac1 activation in Xiap−/− BMDMs (supplemental Figure 11A). RvD1 did not further increase the phagocytosis of HKCA in control BMDMs but significantly increased the uptake of HKCA in Xiap−/− BMDMs (supplemental Figure 11B-C). A similar effect of RvD1 was found in XIAP-knockdown human macrophages. The addition of RvD1 corrected the defective dectin-1–induced Rac1 activation (Figure 6D) and restored the phagocytosis of HKCA in XIAP-deficient human macrophages (Figure 6E-F). Notably, RvD1 did not increase dectin-1–mediated p38 MAPK phosphorylation or NF-κB activation in Xiap−/− BMDMs (supplemental Figure 11D), indicating that the effect of RvD1 is independent of p38 and NF-κB.
Resolution of inflammation rescues Xiap−/− mouse from C albicans infection
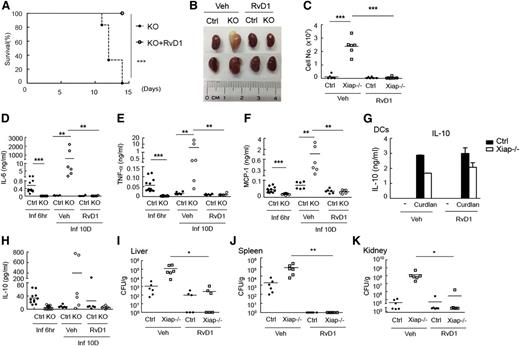
We then examined the in vivo effect of RvD1 in Xiap−/− mice. Administration of RvD1 3 days after C albicans (1 × 105) infection fully rescued Xiap−/− mice from C albicans-mediated mortality (Figure 7A). Development of the pathological morphology in the kidneys of Xiap−/− mice after C albicans infection was completely prevented by RvD1 (Figure 7B). For reasons that are unclear, kidneys from 4 of the 6 RvD1-treated Xiap−/− mice appeared to be smaller than those from RvD1-treated WT mice. Neutrophil infiltration in the kidney from C albicans-infected Xiap−/− mice was abrogated by RvD1 administration (Figure 7C). The high levels of IL-6, TNF-α, and MCP-1 in Xiap−/− mice 10 days after C albicans infection was no longer detectable after RvD1 administration (Figure 7D-F). The fungal loads in liver, spleen, and kidney were effectively reduced by RvD1 treatment (Figure 7I-K). RvD1 also increases the production of IL-10,49,51 known to participate in the resolution of inflammation. We found that curdlan-triggered IL-10 generation in Xiap−/− BMDCs was not altered by the presence of AT-RvD1 (Figure 7G). Application of RvD1 did not increase serum levels of IL-10 in Xiap−/− mice with prior C albicans infection (Figure 7H). Together, these results suggest that increases in dectin-1–induced Rac1 activation and phagocytosis by RvD1 helped Xiap−/− mice resolve inflammation and survive C albicans infection.
Resolvin D1 effectively rescues Xiap−/− mice from C albicans infection. (A) Resolvin D1 rescues Xiap−/− mice from C albicans infection. C albicans (1 × 105) was intravenously administered to Xiap−/− mice (8-12 weeks old), with or without intravenous injection of RvD1 (100 ng)30-32 3 days later. The survival of mice was monitored and is presented as a Kaplan-Meier survival curve; n = 6. (B) Resolvin D1 restores the normal morphology of kidney from C albicans-infected Xiap−/− mice. WT (Ctrl) and Xiap−/− mice were treated as in A. Infected Xiap−/− mice were euthanized on 30% loss of body weight. Kidneys were isolated from Xiap−/− mice and WT mice infected at the same time. Kidneys shown were representative of 6 mice. (C) Resolvin D1 alleviates kidney neutrophil infiltration in C albicans-infected Xiap−/− mice. Total kidney cells were prepared from kidneys isolated in B, and the fraction of neutrophils was determined. (D-F,H) Resolvin D1 resolves the persistent inflammatory cytokine levels in Xiap−/− mice after C albicans infection. Serum from C albicans-infected mice was collected 6 hours and 10 days after infection, and the levels of (D) IL-6, (E) TNF-α, (F) MCP-1, and (H) IL-10 were determined. (G) Resolvin D1 does not increase IL-10 production. WT and Xiap−/− BMDCs were pretreated with vehicle or resolvin D1 and stimulated with curdlan. Secreted IL-10 was determined 24 hours after activation. (I-K) Resolvin D1 eliminates the fungal burden in Xiap−/− mice infected with C albicans. (I) Livers, (J) kidneys, and (K) spleens were removed form C albicans-infected WT and Xiap−/− mice in day 10. Colony-forming units of C. albicans in each organ was determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
Resolvin D1 effectively rescues Xiap−/− mice from C albicans infection. (A) Resolvin D1 rescues Xiap−/− mice from C albicans infection. C albicans (1 × 105) was intravenously administered to Xiap−/− mice (8-12 weeks old), with or without intravenous injection of RvD1 (100 ng)30-32 3 days later. The survival of mice was monitored and is presented as a Kaplan-Meier survival curve; n = 6. (B) Resolvin D1 restores the normal morphology of kidney from C albicans-infected Xiap−/− mice. WT (Ctrl) and Xiap−/− mice were treated as in A. Infected Xiap−/− mice were euthanized on 30% loss of body weight. Kidneys were isolated from Xiap−/− mice and WT mice infected at the same time. Kidneys shown were representative of 6 mice. (C) Resolvin D1 alleviates kidney neutrophil infiltration in C albicans-infected Xiap−/− mice. Total kidney cells were prepared from kidneys isolated in B, and the fraction of neutrophils was determined. (D-F,H) Resolvin D1 resolves the persistent inflammatory cytokine levels in Xiap−/− mice after C albicans infection. Serum from C albicans-infected mice was collected 6 hours and 10 days after infection, and the levels of (D) IL-6, (E) TNF-α, (F) MCP-1, and (H) IL-10 were determined. (G) Resolvin D1 does not increase IL-10 production. WT and Xiap−/− BMDCs were pretreated with vehicle or resolvin D1 and stimulated with curdlan. Secreted IL-10 was determined 24 hours after activation. (I-K) Resolvin D1 eliminates the fungal burden in Xiap−/− mice infected with C albicans. (I) Livers, (J) kidneys, and (K) spleens were removed form C albicans-infected WT and Xiap−/− mice in day 10. Colony-forming units of C. albicans in each organ was determined. *P < .05, **P < .01, and ***P < .001 for paired Student t test.
Discussion
XIAP is a caspase-binding antiapoptotic protein critical for cell survival and has been shown to activate NF-κB. XIAP deficiency in humans leads to XLP-2, although the mechanisms remain unclear. However XIAP-knockout mice appear normal.13-15 In the present study, we identified the involvement of XIAP in selective NF-κB activation. We showed that TNF-α/IL-6 production induced by TLR2, TLR3, TLR4, or TLR7/8 was normal in Xiap−/− BMDMs, and XIAP deficiency did not affect TLR-induced NF-κB activation in T cells and macrophages, consistent with several recent reports.16,20-22 XIAP deficiency impaired only NF-κB activating processes that are mediated by BCL10, such as those stimulated by dectin-1, TCR, EGF, or LPA (Figures 1 and 3). Our data also agree with the observations that TNF-α–triggered NF-κB activation is normal in BCL10-knockout cells40 and that LPS-induced NF-κB activation is not affected by deficiency in CARD9,23 the BCL10-binding partner. We demonstrated that XIAP promoted BCL10 K63 ubiquitination, but further work is required to delineate the exact role of XIAP-BCL10 in CARMA1-BCL10-MALT1–mediated NF-κB activation. TCR-stimulated NF-κB in Xiap−/− T cells was indistinguishable from control T cells when stimulated through CD3/CD28 (supplemental Figure 5). A defect in T-cell activation was observed in Xiap−/− T cells only when activated by anti-CD3 alone (Figure 3A). We also found that immunization with HKCA generated comparable T-cell responses in WT and Xiap−/− mice (supplemental Figure 7). Despite the fact that XIAP targets to BCL10, there was a different sensitivity to TCR and dectin-1 stimulation in Xiap−/− T cells and macrophages. Further work will be needed to delineate this discrepancy.
We demonstrated that Xiap−/− mice died from C albicans infection. XLP-2 is known for its association with Epstein-Barr virus, and no C albicans infection has been reported in XLP-2, likely due to the rarity of XLP-2 patients. Because Xiap−/− T-cell responses are nearly normal, XLP-2 may be attributed to abnormal innate immunity in XIAP-deficient hosts. We further illustrated that the defective BCL10-dependent uptake of C albicans plays a major role in the vulnerability of Xiap−/− mice to C albicans infection. BCL10 participates in Rac1 activation, F-actin remodeling, and phagosome formation that are NF-κB independent.47 XIAP-deficient macrophages displayed no defects in the phagocytosis of plain latex beads and Pam3CSK4-coated latex beads but were ineffective in the uptake of C albicans, indicating a selective deficiency in dectin-1–induced phagocytosis. Introduction of active Rac1 restored the phagocytosis of C albicans in XIAP-deficient macrophages (Figure 6A-C). Up-regulation in NF-κB activation and proinflammatory cytokine production by Pam3CSK4 failed to rescue Xiap−/− mice from C albicans-mediated inflammation and lethality (supplemental Figure 9). Instead, restoration in the Rac1 activation and uptake of C albicans effectively led to clearance of infection (Figure 7). The inability of Xiap−/− mice to respond to C albicans infection thus could be attributed mostly to the impaired BCL10-dependent Rac1 activation and phagocytosis necessary for yeast clearance.
Importantly, we demonstrated that XIAP-deficient human macrophages recapitulated the specific defects in Xiap−/− mouse macrophages (Figures 1 and 6). XIAP-knockout human macrophages reacted indistinguishably from control macrophages to LPS treatment (supplemental Figure 1) but were unable to respond to dectin-1 stimulation (Figure 1H-I). Similar to Xiap−/− macrophages, XIAP-deficient human macrophages were compromised in dectin-1–stimulated Rac1 activation and were deficient in the uptake of C albicans. The present study likely represents the first identification of the physiological defect in XLP-2 macrophages. XLP-2 patients are characterized by hemophagocytic lymphohistiocytosis.9,10 Whether impaired Rac1 activation and defective phagocytosis lead to visible remains of erythrocytes and leukocytes in XLP-2 macrophages remains to be determined.
We further used the dectin-1 ligand curdlan to demonstrate that the reduced innate responses in Xiap−/− mice lead to development of selected XLP-2 syndromes. The initial inability of Xiap−/− mice to mount effective innate immunity to curdlan (Figure 5B) resulted in the persistent presence of curdlan, and its continued stimulation was accompanied by accumulation of the proinflammatory cytokines, leading to elevated accumulation of macrophages and neutrophils in the peritoneal cavity and splenomegaly (Figure 5D-F). Therefore, the inability of Xiap−/− mice to generate effective immune responses to curdlan and C albicans results in some inflammatory syndromes seen in XLP-2 patients.
It has been shown that moderate T-cell immunodeficiency, but not severe T-cell immunodeficiency, generates immune dysregulation and autoimmunity.52 We propose that XIAP deficiency is an example that partial innate immunodeficiency generates uncontrolled inflammatory consequences. In an SPF environment without pathogenic challenge, Xiap−/− mice are indistinguishable from WT mice. However, for microbes or pathogen-associated molecular patterns that activate BCL10-dependent pattern recognition receptors, Xiap−/− mice are unable to mount effective innate immune responses to resolve infection or eliminate pathogen-associated molecular patterns, and their continued stimulation leads to inflammation and XLP-2–like syndromes including persistent activation of macrophages, excess production of inflammatory cytokines, and splenomegaly. Our results suggest that defective BCL10/NOD2-directed innate activation represents one of the mechanisms that contribute to the pathogenesis of XLP-2 in XIAP-deficient patients. Because XIAP deficiency impairs the innate reactivity of myeloid cells, our results may explain why myeloablative conditioning regimens lead to poor survival in allogeneic hematopoietic cell transplantation on XIAP-mutated patients,53 as myeloid cell depletion further aggravates pathogenesis caused by innate immunodeficiency.
Innate immunodeficiency is characterized by sensitivity to a narrow spectrum of infection.1-3 Increasing numbers of inflammatory diseases, including cystic fibrosis, are attributed to innate immunodeficiency.54 In the present study, we used Xiap−/− mice to suggest a molecular basis of such selectivity. An interesting analogy is that Crohn disease-associated NOD2 variants are loss-of-function mutants.55 How the loss of function of a proinflammatory signal molecule mechanistically contributes to an inflammatory disorder has been subjected to extensive investigations.5-7 It has been suggested that patients with NOD2 mutants are unable to resolve infection by specific pathogens due to innate immunodeficiency.5,7 It is likely that the persistence of microbes leads to continued inflammation and adaptive immunity activation, similar to what we observed in this study in Xiap−/− mice.
We also demonstrated that RvD1 restored Rac1 activation and phagocytosis in XIAP-deficient macrophages, resulting in effective resolution of inflammation, elimination of C albicans, and full survival of the infected Xiap−/− mice. Interestingly, RvD1 did not rescue the activation of NF-κB and p38 in Xiap−/− macrophages. These results suggest that, for innate immunodeficiency-induced inflammatory syndromes such as XLP-2, after the initial failure of host responses to clear pathogen, resolution of the latter-stage inflammation is likely the key for the success in the therapy. Furthermore, our results suggest that increased uptake of pathogens and resolution of the inflammation by RvD1 are potential therapeutic interventions for inflammatory diseases caused by primary innate immunodeficiency. Further work will determine the feasibility of such an approach.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David L. Vaux for the Xiap−/− mouse and Yamin Lin and the FACS Core, Sue-Ping Lee, and the Confocal Core of the Institute of Molecular Biology, Academia Sinica, for cell sorting and confocal microscopy. Dr AndreAna Peña, English editor of Institute of Molecular Biology, Academia Sinica, provided editorial assistance to the authors during preparation of this manuscript.
This work was supported by National Science Council grant 102-2321-B-001-045 and an Academia Sinica Investigator Award from Academia Sinica, Taiwan, Republic of China.
Authorship
Contribution: W.-C.H., Y.-T.C., and I.-H.C. performed experiments; S.-C.H. and S.-C.M. contributed to generation of key materials and constructs; and M.-Z.L. conceived of, designed, and supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming-Zong Lai, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, Republic of China; e-mail: mblai@gate.sinica.edu.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal