Abstract
Our understanding of the pathophysiology of aplastic anemia is undergoing significant revision, with implications for diagnosis and treatment. Constitutional and acquired disease is poorly delineated, as lesions in some genetic pathways cause stereotypical childhood syndromes and also act as risk factors for clinical manifestations in adult life. Telomere diseases are a prominent example of this relationship. Accelerated telomere attrition is the result of mutations in telomere repair genes and genes encoding components of the shelterin complex and related proteins. Genotype-phenotype correlations show genes responsible for X-linked (DKC1) and severe recessive childhood dyskeratosis congenita, typically with associated mucocutaneous features, and others (TERC and TERT) for more subtle presentation as telomeropathy in adults, in which multiorgan failure may be prominent. Telomerase mutations also are etiologic in familial pulmonary fibrosis and cryptic liver disease. Detection of a telomere disease requires awareness in the clinic, appropriate laboratory testing of telomere content, and genetic sequencing. In treatment decisions, genetic screening of related donors for hematopoietic stem cell transplantation is critical, and androgen therapy may be helpful. Telomeres shorten normally with aging, as well as under environmental circumstances, with regenerative stress and oxidative damage. Telomere biology is complexly related to oncogenesis: telomere attrition is protective by enforcing senescence or apoptosis in cells with a long mitotic history, but telomere loss also can destabilize the genome by chromosome rearrangement and aneuploidy.
Introduction
Aplastic anemia (AA) is the exemplar of human bone marrow failure syndromes and has itself undergone paradigmatic shifts in our understanding of its pathophysiology—with profound implications for the treatment of patients with a fatal blood disease.1 First was the appreciation of the failure of hematopoiesis, inferred from the pathologic examinations of the empty bone marrow, by Ehrlich in Berlin at the end of the 19th century and Vaques in Paris a few years later. Pathological characterization of the disease allowed recognition in the clinic, the collection of case series, and epidemiology. In the second major era, AA was diagnosed in benzene workers and appeared as a prominent toxicity of chemotherapy in the cancer clinic and was described as the major manifestation of inherited disease by Fanconi. Thus, both environmental and intrinsic factors appeared to cause marrow failure. Next followed important inferences from treatment strategies: stem cell loss was suggested by early colony culture studies and confirmed by the success of bone marrow transplant, and autologous reconstitution of aplastic marrow after failed bone marrow transplant was the first clue to an immunologic pathophysiology of stem cell destruction. Environmental triggers, like seronegative hepatitis, have been identified in a few cases but remain elusive in most, as they do in most human immune-mediated illness. We are now in the era of molecular genetics and genomics applied to bone marrow failure diseases.
This review focuses on telomere biology, genetics, and physiology, especially in bone marrow failure but also in other organ systems. We concisely discuss telomere structure and genetics, telomere maintenance and repair, and diagnostic and treatment strategies for the telomeropathies, and we speculate on possible links between telomere biology as it relates to bone marrow failure and malignancy, especially myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
There is no consensus for the naming of telomere diseases (telomeropathies, telomere syndromes, impaired telomere maintenance spectrum disorder, dyskeratosis congenita), and the term “dyskeratosis congenita” is used by pediatricians and internists. For purposes of this review, we restrict the use of dyskeratosis congenita to the well-defined childhood syndrome, and we use telomere disease, telomeropathies, and telomere syndromes interchangeably.
Telomeres and telomerase biology
Telomeres: disease, aging, cancer
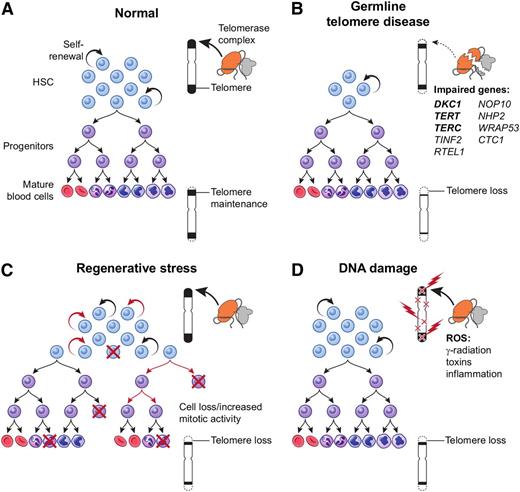
Telomere biology was first linked to medicine by bone marrow failure: measurement of short telomeres in the leukocytes of acquired AA patients,2,3 followed by the discovery of telomerase genes mutant in an inherited AA, dyskeratosis congenita.4-7 Accelerated telomere attrition can be genetic in origin, a physiologic result of regenerative stress, or secondary to DNA damage (Figure 1).
Mechanisms of telomere attrition. (A) Physiological telomere attrition in normal individual hematopoietic cells is the result of balance between telomerase mediated telomere elongation and telomere loss with each cell division. Gradual decrease in mean telomere content occurs with age. (B) Germ-line mutations in a telomere repair gene lead to a reduced stem cell pool and severe telomere loss during replication. (C) Regenerative replicative stress during bone marrow failure, recovery after chemotherapy, or hematopoietic transplant also leads to telomere loss due to increased mitotic activity in stem cells and/or progenitors. (D) Reactive oxygen species (generated from radiation, toxins, and inflammation) cause DNA damage and telomere loss as a direct effect on the telomere. HSC, hematopoietic stem cell.
Mechanisms of telomere attrition. (A) Physiological telomere attrition in normal individual hematopoietic cells is the result of balance between telomerase mediated telomere elongation and telomere loss with each cell division. Gradual decrease in mean telomere content occurs with age. (B) Germ-line mutations in a telomere repair gene lead to a reduced stem cell pool and severe telomere loss during replication. (C) Regenerative replicative stress during bone marrow failure, recovery after chemotherapy, or hematopoietic transplant also leads to telomere loss due to increased mitotic activity in stem cells and/or progenitors. (D) Reactive oxygen species (generated from radiation, toxins, and inflammation) cause DNA damage and telomere loss as a direct effect on the telomere. HSC, hematopoietic stem cell.
Telomere biology is intimately related to diseases of organ failure and also to cancer and normal aging. However, extrapolation of cell biology to organ homeostasis is cautioned (organs are a collection of heterogeneous cell populations, and organisms are organized by organs exposed to diverse environmental conditions), and mouse models poorly reflect human telomere disease.8 Because telomeres protect chromosomes and stabilize the genome as a whole, telomere preservation has been assumed to be a benefit to the cell and presumably to the organism. However, telomerase up-regulation or activation of the alternative pathway of telomere repair9 is a common feature of malignant cells. Physiologically, telomere loss eliminates cells with a long proliferative history and at risk for replication-dependent adverse genetic events.10 Telomere attrition occurs normally, and aging has been blamed on telomere loss (and the telomeropathies, described below, considered “progeric”), despite the overlap between the shortest telomere length of young children and the longest of the very aged.11 There is some support from population studies for telomere content of white blood cells as predictive of overall mortality and cancer prevalence and deaths.12,13 These associations need not be causative, as telomeres, intrinsic to the chromosome, are an easily assayed representation of overall DNA damage, from exposure to reactive oxygen species to environmental toxins and irradiation (Figure 1).14,15
Molecular biology of telomeres and telomerase
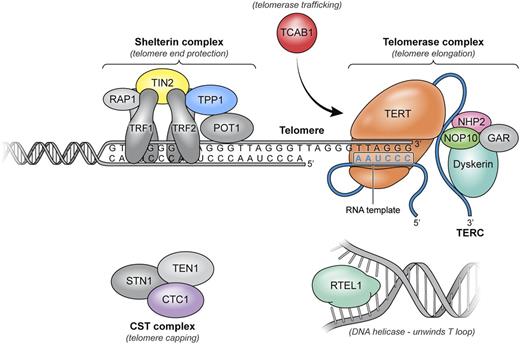
Telomere, the ends of the chromosomes of vertebrate species, and telomerase, the molecular machinery of telomere repair, solved 2 basic problems in cell biology: without telomeres, chromosome ends might be recognized as fragments or viral DNA; without telomerase, DNA replication would lead to erosion of genetic material at each cell division.16,17 Telomeres are hexanucleotide (TTAGGG) tandem repeats of DNA at chromosome termini, with associated proteins collectively termed shelterin,18 and a secondary structure termed the T loop. Telomerase is a ribonucleoprotein enzyme complex that synthesizes telomeres; the complex includes a reverse transcriptase, the telomerase enzyme (encoded by the gene TERT), an RNA template (encoded by TERC), and associated proteins that affect assembly, trafficking, recruitment of telomerase to telomeres, and stability of telomerase, such as dyskerin (Figure 2).
Components of telomere maintenance implicated in human disease. Telomeres are composed of thousands of TTAGGG repeats localized at the ends of linear chromosomes. Telomeres are coated by shelterin, a 6-protein complex (TRF1, TRF2, TIN2, POT1, TPP1, and RAP1) with multiple roles in maintaining telomere length homeostasis by forming the T loops, preventing DNA damage response activation, and recruiting the telomerase complex and modulating its activity. The 3′ end of the telomeric leading strand terminates as a single-stranded overhang, which folds back and invades the double-stranded telomeric helix, forming the T loop. Unwinding of the T loop, needed for telomere elongation, is done by a DNA helicase, RTEL1. Telomerase, the enzyme responsible for telomere elongation, has a 4-protein scaffold (dyskerin, NOP10, NHP2, and GAR) and an RNA template (TERC) and reverse transcriptase TERT. TCAB1 ensures trafficking of the telomerase complex to the telomeric ends. The CST complex has multiple proposed roles through its 3 members (CTC1, STN1, and TEN1); CTC1 inhibits telomerase activity and also promotes lagging strand synthesis by binding single stranded DNA and interacting with α polymerase primase. Components in gray have not been implicated in human disease.
Components of telomere maintenance implicated in human disease. Telomeres are composed of thousands of TTAGGG repeats localized at the ends of linear chromosomes. Telomeres are coated by shelterin, a 6-protein complex (TRF1, TRF2, TIN2, POT1, TPP1, and RAP1) with multiple roles in maintaining telomere length homeostasis by forming the T loops, preventing DNA damage response activation, and recruiting the telomerase complex and modulating its activity. The 3′ end of the telomeric leading strand terminates as a single-stranded overhang, which folds back and invades the double-stranded telomeric helix, forming the T loop. Unwinding of the T loop, needed for telomere elongation, is done by a DNA helicase, RTEL1. Telomerase, the enzyme responsible for telomere elongation, has a 4-protein scaffold (dyskerin, NOP10, NHP2, and GAR) and an RNA template (TERC) and reverse transcriptase TERT. TCAB1 ensures trafficking of the telomerase complex to the telomeric ends. The CST complex has multiple proposed roles through its 3 members (CTC1, STN1, and TEN1); CTC1 inhibits telomerase activity and also promotes lagging strand synthesis by binding single stranded DNA and interacting with α polymerase primase. Components in gray have not been implicated in human disease.
However, the repair process is not complete, and telomere attrition at ∼50 to 150 bp/cell division is normal.19 The cell is sensitive to critically short telomeres on any chromosome, which trigger DNA damage responses and lead to cell senescence or apoptosis (and occasionally other consequences; see below). Telomere length (actually telomere content in most assays) is thus a “mitotic clock,” a record of the cell’s proliferative history. Telomere loss is the explanation for the failure of fibroblasts to replicate indefinitely in tissue culture—the Hayflick phenomenon.20-22 Once telomeres become critically short, chromosome ends are recognized as DNA double-strand breaks, engaging DNA damage response pathways and p53 activation and leading to apoptosis/senescence. Fibroblasts do not repair their telomeres, but telomerase normally is activated in some proliferating cell types, including hematopoietic stem cells and lymphocytes. In tissue culture, telomerase deficiency may limit proliferation by “off-target” effects not directly related to telomere length.23 In mice that inherit short telomeres from telomerase-mutated parents, coinheritance of normal telomerase activity suffices to restore telomere content and prevent disease.24
Genotype and phenotype in telomere disease
Dyskeratosis congenita
In this stereotypical childhood syndrome disease, features are present early in life, with high penetrance, severity of clinical manifestations, and specific organ involvement. DKC1 mutations cause X-linked dyskeratosis. Affected boys frequently show the classic dermatologic triad of dystrophic nails, leukoplakia, and hypopigmented skin.4 Loss of dyskerin functionally destabilizes the telomerase repair complex, an apparently severe molecular lesion. (Dyskerin also functions in other cellular processes, particularly as small nucleolar ribonucleoprotein complexes that contribute to ribosome processing, linking the genotype to ribosomopathies such as Diamond-Blackfan anemia.25 ) Hoyeraal Hreidarsson syndrome is severe dyskeratosis congenita, with cerebellar hypoplasia; families have been reported with X chromosome mutations in DKC15 or compound mutations in RTEL1, which encodes a helicase that unwinds the T loop.26,27 Revesz syndrome, dyskeratosis with retinal pathology, has been linked to dominant mutations in TINF2, which encodes a component of the shelterin complex.28 Another dyskeratosis congenita variant is cerebroretinal microangiopathy with calcifications and cysts, which resembles Revesz but cerebellar and hematologic manifestations are less prominent or lacking; cerebroretinal microangiopathy with calcifications and cysts is due to compound heterozygous mutations in CTC1.29 Mutations in NOP10, NHP2, and WRAP53 (encoding TCAB1), all very rarely cause autosomal recessive dyskeratosis congenita.30-32 These mutations are not responsible for bone marrow failure in adults because of the requirement for homozygous loss of gene function or perhaps due to anticipation, worsening of clinical manifestations, and earlier presentation in successive generations.33,34
Telomere disease in adults
TERC and TERT gene mutations cause telomeropathies in both children and adults.7,35,36 Loss of function of 1 of the 2 autosomal chromosome genes is sufficient to reduce telomerase activity and to accelerate telomere attrition. The degree of functional inactivity is variable among mutations.37 In vitro assays may not detect subtle alterations of function, such as telomerase processivity38 or gene sequencing of exomes may miss mutations in regulatory regions like promoters.39 Disease manifestations may be more severe with TERC mutations compared with TERT mutations, perhaps due to more profound effects on telomerase activity, at least as measured in vitro.40 Patients with mutations in either gene can present in and beyond middle age. Symptoms and signs are often milder than in children, and mucocutaneous findings and other physical anomalies are infrequent. The pattern of affected organs and penetrance of phenotype are extremely variable among affected individuals in a pedigree. Indeed, a family history of blood count abnormalities or hematologic disease is often lacking.
A recent publication reports a novel mutation in the ACD gene, that encodes for a shelterin complex protein, TPP1. This novel mutation was identified in a family with variable severity of bone marrow failure in both adults and children. Of note, all affected family members lacked the classic dyskeratosis congenita triad.41
Organ failure in the telomeropathies
The main organs subject to failure of function and fatty or fibrotic replacement are the marrow, the lung, and the liver. All 3 may be affected in an individual or a pedigree, with variable severity.
Bone marrow
The marrow in dyskeratosis congenita and in adults with telomere gene mutations is hypocellular and aplastic when there are significant cytopenias. With severe pancytopenia, the bone marrow is indistinguishable from acquired AA. From pedigrees in whom mutant but apparently healthy family members have been examined, marrow hypocellularity appears consequent to the mutations, but hematopoiesis is adequate to support blood counts that are normal or near normal. Macrocytosis with or without anemia is common in the telomeropathies (but does not distinguish them from other inherited forms of AA or from acquired bone marrow failure). Adults may present with typical acute AA or have chronic anemia and/or thrombocytopenia, which may be initially mild or severe and progressive or stable over time. Some base changes, such as the TERT A1062T variant, have functional effects that might be dependent on the genetic or environmental context; this variant has been identified in a rare number of healthy controls, but at higher frequencies in cohorts of AA and hematologic malignancies.36,42 TERT mutations have been found in our clinic in patients diagnosed with large granular lymphocytosis, pure red cell aplasia, and idiopathic neutropenia. We have seen typical telomere disease with mutations in TERT accompanied by small clones of paroxysmal nocturnal hemoglobinuria cells. Of some importance, mutations do not exclude an immune pathophysiology, as patients with TERT lesions can respond to standard immunosuppressive therapy (unpublished data) apparently at expected rates, and despite the presumed quantitative deficit in stem cell numbers and qualitative repair defect.
Lung
Idiopathic pulmonary fibrosis (histologically, interstitial pneumonitis) is the classical lung disease of the telomeropathies and accounts for 65% of the telomere-mediated lung pathology.43 As many as one-sixth of familial pulmonary fibroses are blamed on TERT and TERC mutations, and short telomeres are a risk factor for the development of sporadic pulmonary fibrosis. Pulmonary fibrosis due to telomere dysfunction presents in adulthood, usually appearing well into middle age.44 Smoking is a likely cofactor, but siblings with the same mutation may show very different progression rates.45 The diagnosis of pulmonary fibrosis is made by demonstration of patchy, basal, and peripheral reticular opacity, ground glass opacity, and honeycombing on high-resolution computerized tomography, avoiding the need for lung biopsy. Both high-resolution computerized tomography and pulmonary function tests are used for serial assessment. In addition to pulmonary fibrosis, a range of pulmonary pathologies is reported, including bronchiolitis obliterans organizing pneumonia, chronic hypersensitivity pneumonitis, emphysema alone, or combined pulmonary fibrosis and emphysema. Lung pathology can be modeled in telomerase-deficient mice.46 The cooccurrence of pulmonary fibrosis and bone marrow failure is highly specific for the presence of an underlying telomere disorder.47
The liver is also affected in the telomere diseases. Establishing hepatic involvement often requires biopsy but can be suggested by imaging suggestive of cirrhosis, portal hypertension, or steatosis.48 Subclinical or cryptogenic liver disease is typical. There may be a personal or family history of liver failure at a young age blamed on ethanol, but the putative “alcoholism” may disappear with uncovering of a genetic basis. Liver histology is heterogeneous: inflammation, iron accumulation in the absence of transfusional or hereditary hemochromatosis, hepatocyte necrosis, bridging fibrosis, or nodular regenerative hyperplasia can be seen.48
Other organs
Esophageal and lacrimal duct stenosis, enteropathy, enterocolitis, osteoporosis, avascular necrosis, and immunodeficiency have been described.49-52 Overt disease is notably absent in other mitotically active organs, such as skeletal muscle, uterus, mammary gland, and the epidermis is only modestly affected.53 Primary myelofibrosis might be expected to be a telomeropathy, but there is no evidence of telomerase gene mutations.
Diagnosis and treatment of telomere disease
Treating a disease with the potential for multiorgan compromise has practical consequences for patients and physicians. As examples, individuals may be susceptible to increased organ toxicity from chemotherapy; screening of potential family donors prior to bone marrow transplantation is critical; and certain drugs may be helpful in maintaining blood counts and even reversing telomere loss.54 Preventive measures may be instituted, due to the possible roles of environmental factors like smoking and alcohol in organ stress. Routine surveillance of lung and liver may be indicated, as they influence therapeutic decisions. Extrapolations from patients with acquired AA suggest worse long-term outcomes following immunosuppressive therapy are likely in patients with telomere diseases. Patients with acquired AA with telomere lengths in the low-normal range subsequently treated with antithymocyte globulin-based regimens suffer more frequent relapses, clonal evolution to myelodysplastic syndrome, and worse survival.55
History and physical examination
The diagnosis can be made in the clinic examining room, based on a good personal and family history and even a cursory physical examination. Patients may make their own diagnosis using the Internet—better than subspecialists who do not query outside their organ system of interest. Nail dystrophy and leukoplakia of the tongue in children and prematurely gray hair in adults are diagnostic clues (the historical incrimination of hair dyes and AA may be an example of confusion between cause and effect—the dye being used to mask the mutation’s cosmetic phenotype).
A telomeropathy should be considered in all patients with AA or hypoplastic MDS, and testing should be performed when treatment decisions might be affected (Figure 3). Testing should also be performed on patients who do not satisfy standard criteria for AA but have long-standing cytopenias, unexplained thrombocytopenia (chronic immune thrombocytopenic purpura) or macrocytic anemia, or simply an unexplained hypocellular bone marrow. Patients with MDS or AML with a suspicious personal or family history should also be considered, although the presence of blasts confounds the telomere length assay. Telomere disease is suggested when a stem cell transplant donor fails to mobilize, and if there is a suspicious personal or family history, and of course for potential family donors in confirmed cases of telomeropathy.56
Diagnostic algorithm for germline telomere diseases. Mean telomere content should be done in all patients with suspected bone marrow failure from peripheral blood after Fanconi anemia was ruled out in patients under the age of 40 years. Mean telomere content normalized to age-matched controls can be suggestive of telomere disease if below the first percentile or if associated with a strong family history, warranting genetic testing for inherited mutations in telomere maintenance genes. Leukocytes’ telomere content should be interpreted with caution in patients with leukemia (blasts have short telomeres), MDS, and in individuals who have received chemotherapy. If flow-FISH testing is used, the lymphocyte subsets are more accurate than granulocytes. BMF, bone marrow failure; BMT, bone marrow transplant; DEB, deopxybutane; MMC, mitomycin C; PB, peripheral blood.
Diagnostic algorithm for germline telomere diseases. Mean telomere content should be done in all patients with suspected bone marrow failure from peripheral blood after Fanconi anemia was ruled out in patients under the age of 40 years. Mean telomere content normalized to age-matched controls can be suggestive of telomere disease if below the first percentile or if associated with a strong family history, warranting genetic testing for inherited mutations in telomere maintenance genes. Leukocytes’ telomere content should be interpreted with caution in patients with leukemia (blasts have short telomeres), MDS, and in individuals who have received chemotherapy. If flow-FISH testing is used, the lymphocyte subsets are more accurate than granulocytes. BMF, bone marrow failure; BMT, bone marrow transplant; DEB, deopxybutane; MMC, mitomycin C; PB, peripheral blood.
Laboratory testing
Assays that reflect the biology of telomere disease are available commercially; interpretation can be problematic (Figure 3). The measurement of average telomere lengths of chromosomes within peripheral blood leukocytes is a useful and functionally meaningful assay for disease screening. The standard test to measure average chromosome telomere lengths is the terminal restriction fragments (TRFs) length analysis by Southern blot, but this method is cumbersome, difficult to quantitate, and impractical for high-throughput screening.57 The quick, relatively inexpensive, and standardized fluorescence in situ hybridization of telomere repeats measured by flow cytometry (flow-FISH) and quantitative polymerase chain reaction (qPCR) methods were developed for clinical samples, and low telomere content is determined by comparison with age-matched healthy controls, because telomere length normally shortens with age. Cautious interpretation is advised when comparing with controls, because telomere length varies with ethnicity.58 These techniques are sufficient at measuring overall telomere content but insensitive to detecting individual chromosome telomere lengths that are more relevant to pathogenesis because the shortest telomeres are most important at triggering senescence and are subject to end-to-end fusions.59-61 Single telomere length assay is a PCR-based method that measures chromosome specific telomere length62 from very small amounts of DNA (as few as 50 cells) with the caveat of low throughput and has been used in research laboratories.
Measurement of mean telomere content by flow-FISH in peripheral blood lymphocytes less than the first percentile compared with age-matched controls accurately identifies individuals with telomerase gene mutations if they have classic dyskeratosis congenita (granulocytes are less specific).63,64 In cohorts of children and adults lacking typical features of dyskeratosis congenita, the sensitivity and specificity are less established. Only 1 of the 7 patients with adult-onset AA originally identified as having a mutation in TERT had telomere lengths measured below the 1st percentile (most were between the 1st and 10th percentile).36 In 194 patients with identified gene mutations (DKC1, TINF2, TERT, and TERC), there were dramatically different telomere lengths between subtypes as measured by qPCR and TRF.40 Telomere length may be normal, despite a strong suspicion based on the clinical findings.40 As yet unidentified genes that affect telomerase expression might mildly impair telomere repair or have off-target pathologic effects. The sensitivity of the assay may be lower in older patients because telomere length differences compared with normal over age 40 years is less pronounced.11
Short leukocyte telomere length may also be a biomarker of a diminished hematopoietic stem cell reserve or reflective of DNA exposure to oxidative damage65 via inflammation or other environmental insults (Figure 2). Possibly for these reasons short telomeres can be observed in other inherited bone marrow failure diseases, like that of Fanconi anemia, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome.11,66-68 When leukocyte telomere length is measured following immunosuppressive therapy or chemotherapy, shorter telomeres may be evidence of a history of replicative stress of hematopoietic stem cells rather than an underlying constitutional defect.2,69
Treatment decisions
Historically, Fanconi anemia was clearly delineated from acquired AA; telomere disease is more of a spectrum. For example, immunosuppression was not believed to be appropriate for telomere disease,36 but we have observed that adult patients with TERT and TERC mutations showed blood count improvement after therapy—consistent with the genetic mutations acting as risk factors in stem cell susceptibility and immune attack acting as an environmental stress. In Fanconi anemia, modification of transplant conditioning is critical to avoid disastrous multiorgan failure due to deficient DNA damage repair, but the sensitivity of telomeropathy patients to chemotherapy and radiation has not been established. Increased pulmonary and liver toxicity has been reported after transplant in dyskeratosis congenita due to DKC1 mutations and other pediatric telomeropathies.70,71 In a small series of cases, reduced intensity regimens appear to ameliorate these complications.72,73
Androgens have been used to treat bone marrow failure since the mid-20th century, with hematologic improvement rates of 50% or better in a few case series.74-77 Patients with telomeropathies may be particularly responsive to male hormones.78,79 In a retrospective analysis of 16 patients on androgen therapy, mostly children with dyskeratosis congenita, 11 achieved clinically significant hematologic responses (red cell or platelet transfusion independence and, in 2, trilineage improvement).80 The mechanism of action of male hormones is likely direct modulation of TERT gene expression to increase telomerase, as inferred from tissue culture experiments and mouse models.81,82 Male hormones might help to slow the rate of telomere attrition, enhance cell regeneration, and improve not only hematopoiesis but also other organ dysfunction resulting from telomere attrition. In 1 case report, telomere elongation was concordant with hematologic response in a man with anemia and thrombocytopenia secondary to a TERT mutation.83 In a prospective research trial at the National Institutes of Health that has enrolled >24 patients (clinicaltrials.gov identifier: #NCT0144137), danazol appears effective in improving blood counts and reversing telomere attrition.
Cancer and telomere attrition
Telomere biology and chromosome instability
The cellular and molecular biology that links telomere loss to malignancy is complex. Physiologically, telomere attrition can be viewed as protective. Accumulation of critically short and dysfunctional telomeres, which are recognized by the cell as double-stranded breaks, triggers DNA damage response pathways (particularly ataxia telangiectasia mediated and γ-H2AX), which halt cell proliferation and induce growth arrest or apoptosis.21,84 Human cells appear to tolerate up to 5 critically short telomeres without catastrophic sequelae of permanent senescence or massive chromosome instability and death. Cells bearing dysfunctional telomeres, as a result of deficient or defective shelterin proteins or DNA damage response proteins, have a variety of end-to-end chromosome fusions and nonreciprocal translocations. In radiation exposure experiments, telomeres and subtelomeric regions appear especially sensitive to oxidative damage from ionizing radiation and nonionizing UV irradiation.85 Telomeres have been proposed as potentially unstable chromosome regions that perpetuate genotoxic damage from radiation across many generations of progeny, ultimately allowing conversion of transient effects to fixed chromosome abnormalities by a variety of molecular mechanisms, including break-fusion-break amplification cycles. In manipulated cell lines and in animal models, inhibition of p53 or cell cycle checkpoint function is often needed to reveal a malignant phenotype in cells and animals with critically short telomeres.85 In telomerase-deficient mice, no cancers develop for many generations, perhaps due to the very long telomeres of intensively inbred laboratory strains, but mice closer to wild animals and with shorter telomeres exhibit a telomerase-deficient phenotype earlier.86 Cross-breeding of mice that are telomerase deficient with those genetically defective in p53 elicits malignant disease, with phenotypes more typical of human cancer than murine tumors and associated with chromosome rearrangements and aneuploidy.87 In tissue culture experiments, up-regulation or correction of a telomerase defect can rescue cells from chromosome instability. Up-regulation of telomerase or induction of the alternative pathway of telomere repair (which is based on chromatid exchange rather than reverse transcription) is present in most human cancers, an apparent requirement for a malignant phenotype, and suggestive of the crucial importance of overcoming the consequences of telomere attrition for oncogenesis.88
Mechanisms of telomere attrition
When there are underlying genetic mutations affecting telomere repair (repair complex genes such as DKC1, TERC, or TERT) or telomere maintenance (shelterin genes such as TINF2 or the helicase gene RTEL1), telomere attrition is accelerated under physiologic conditions (Figure 1). Under circumstances of regenerative stress, telomere attrition should be increased by the demand for cell divisions, depending on the recuperative strength of the telomerase response. In humans, the most relevant example is the decline during the first year after allogeneic stem cell transplant in telomere length of donor leukocytes.89-91 Telomere content of white cells is also low following autologous transplantation and after chemotherapy,69,92 in approximate correlation with its intensity, and has been correlated with secondary myelodysplasia and acute leukemia.93
Telomere loss is appealing as a general mechanism to link regeneration, especially in response to infection, inflammation, and altered immunity, and cancer predisposition. In Barrett’s esophagitis and esophageal adenocarcinomas and in inflammatory bowel disease and colon cancer, both telomere length of the affected tissue and short telomeres of leukocytes have predicted cancer.94-97 Genome-wide analyses frequently have linked single nucleotide polymorphisms in TERT to a wide range of cancer types.98-100 In population-based studies, shorter telomeres of leukocytes have correlated with increased risk for all cancer morbidity.12 There are confounders to a simple view of telomere loss due to excessive mitotic activity as a simple mechanism. Telomere loss might be a marker of generalized DNA damage, as from reactive oxygen species generated by exposure to radiation, chemical toxicity, or inflammation; viral effects (herpesviruses can integrate into telomeres101 and contain telomeric sequences); and subtle unknown genetic determinants that may influence telomerase expression, activity, regulation, and response and affect shelterin and other relevant proteins’ diverse functions.
Cancer predisposition in dyskeratosis congenita, telomeropathy, and in acquired AA
Children with dyskeratosis congenita have a much-increased risk of AML, estimated at 100-fold higher in 1 survey, and an even higher risk for squamous carcinomas of the tongue.102 A general cancer predisposition is less obvious for TERT and TERC mutations. One clue to telomere disease is the presence in a kindred of individuals with MDS and AML, but even this finding is occasional and not regular, and pedigrees do not typically show an abundance of cancer cases. The difference between telomere lesions in adults and children may be due to their milder functional effects, and a less severe hematopoietic presentation occurs in midlife rather than in early childhood.
In AA in general, there is the central problem of “clonal evolution,” the development of MDS and/or AML late in the disease course in ∼15% of cases over a decade, sometimes after successful treatment with immunosuppression.103 Clonal evolution is marked almost always by the appearance of a new marrow cytogenetic abnormality, usually monosomy 7 but also trisomy 8, and a variety of chromosome translocations. The clinical consequences of monosomy 7 are almost invariably profound progressive pancytopenia and/or the development of leukemia. As a biological event, clonal evolution offers a unique opportunity to test the role of telomere attrition in hematologic malignancy, due to the availability of multiple serial samples of marrow and of blood that reflects the marrow origin of the process. We examined the impact of telomere content on outcomes in a large cohort of almost 200 patients with severe AA who had received antithymocyte globulin and cyclosporine on National Institutes of Health protocols.55 Telomere content in the lowest quartile was a major risk factor for clonal evolution in general and for monosomy 7 in particular, with estimated rates 5 to 6 times higher than in patients with higher telomere content. Marrow cells from patients collected years earlier, when they were thawed and cultured in vitro, showed both telomere loss and chromosomal aberrations many months or years prior to clinical evolution to MDS/AML.104 Short leukocyte telomere content has also been identified in a cohort of 65 patients with low to intermediate-1 risk of MDS compared with age-matched controls.105 In recent work, telomere attrition has been compared with mutations as drivers of early oncogenesis. In a group of 13 patients who had evolved to monosomy 7 and in whom adequate serial specimens were available, average annual telomere attrition was about 420 bp/year, which is much higher than the normal rate in patients with stable AA of ∼65 bp/year. Single telomere length analysis showed that accelerated attrition was due to acquisition of low-molecular-weight telomere fragments. In contrast, candidate genes, identified in patients with MDS and AML, were not mutated in most of these cases.106 Telomere loss, and its consequent chromosome instability, would follow from the limited stem and progenitor cell pool and the replicative stress imposed on the few remaining primitive hematopoietic cells. Telomere driven genomic instability would lead to chromosome loss and haploinsufficiency of genes on chromosome 7 relevant to leukemia.
Prediction and prevention of cancer due to telomere loss
Monosomy 7 is difficult to treat, stem cell transplant is the only effective modality, and monitoring of telomere content might be useful to predict onset of aneuploidy in the clinic, directing patients to replacement therapies earlier. As discussed above, androgens are effective in treating the cytopenias of the telomeropathies. In retrospect, it is remarkable that historic reports of male hormone therapy in AA do not cite significant rates of clonal evolution to MDS and AML. Sex hormone-mediated up-regulation of TERT gene activity might be an attractive modality for telomere repair and potentially protect against chromosome instability—not only in bone marrow failure diseases but under other circumstances of regenerative stress such as chemotherapy, irradiation, and stem cell transplant.
Conclusions
Telomere biology is important in human bone marrow failure disease. Children with dyskeratosis congenita and adults with telomeropathies have AA as a result of mutations in genes of the telomerase complex or of proteins in the shelterin complex. Accelerated telomere attrition of genetic origin can lead to bone marrow failure and lung and liver fibrosis, and the full clinical spectrum has not yet been completely defined. Telomere loss may result from physiologic responses to regenerative stress, absent abnormal telomere, and telomerase genes. Telomere attrition has been linked in the laboratory to genomic instability and in the clinic to myelodysplasia and leukemia. Telomere biology may represent a long sought link between inflammation, tissue regeneration, and cancer.
Authorship
Contribution: D.M.T., B.D., and N.S.Y. wrote the review article.
Conflict-of-interest disclosure: All authors received research funding from GlaxoSmithKline for clinical trials in aplastic anemia using eltrombopag.
Correspondence: Danielle M. Townsley, National Heart, Lung and Blood Institute, National Institutes of Health, 10 Center Dr, CRC 3-5216, Bethesda, MD 20892; e-mail: townsleydm@nhlbi.nih.gov.