Key Points
This is the first study of carfilzomib-cyclophosphamide-dexamethasone in elderly patients with newly diagnosed multiple myeloma.
Carfilzomib-cyclophosphamide-dexamethasone induced high complete response rates and was associated with low toxicity.
Abstract
This multicenter, open-label phase 2 trial determined the safety and efficacy of carfilzomib, a novel and irreversible proteasome inhibitor, in combination with cyclophosphamide and dexamethasone (CCyd) in patients with newly diagnosed multiple myeloma (NDMM) ≥65 years of age or who were ineligible for autologous stem cell transplantation. Patients (N = 58) received CCyd for up to 9 28-day cycles, followed by maintenance with carfilzomib until progression or intolerance. After a median of 9 CCyd induction cycles (range 1-9), 95% of patients achieved at least a partial response, 71% achieved at least a very good partial response, 49% achieved at least a near complete response, and 20% achieved stringent complete response. After a median follow-up of 18 months, the 2-year progression-free survival and overall survival rates were 76% and 87%, respectively. The most frequent grade 3 to 5 toxicities were neutropenia (20%), anemia (11%), and cardiopulmonary adverse events (7%). Peripheral neuropathy was limited to grades 1 and 2 (9%). Fourteen percent of patients discontinued treatment because of adverse events, and 21% of patients required carfilzomib dose reductions. In summary, results showed high complete response rates and a good safety profile. This trial was registered at clinicaltrials.gov as #NCT01346787.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy.1 In Europe, 33 000 new cases were estimated to be diagnosed in 2013, and MM was estimated to result in ∼20 300 deaths.2 The incidence of MM progressively increases with age; the median age at diagnosis is 70 years.1,3
In the last decade, the increased use of novel agents as initial therapy significantly improved overall survival (OS) in patients ineligible for autologous transplantation.3 The 5-year survival improved from 31% (2001-2005) to 56% (2006-2010) (P < .001).4
Although bortezomib-melphalan-prednisone (VMP) and melphalan-prednisone-thalidomide combinations are routinely used in elderly patients,5,6 dose-limiting hematologic toxicity from melphalan and peripheral neuropathy (PN) from bortezomib or thalidomide limit their optimal use.7,8 Better tolerated alkylating agents, such as cyclophosphamide, which lack the cumulative hematologic toxicity of melphalan, have been used successfully in combination with dexamethasone and either thalidomide9 or bortezomib10 in elderly newly diagnosed MM (NDMM) patients.
Carfilzomib, a novel and selective proteasome inhibitor, has demonstrated higher rates of response and lower rates of PN relative to bortezomib or thalidomide.11 Carfilzomib has been approved in the United States for use as a single agent in the treatment of patients with relapsed and refractory MM, based on the results from the phase 2 PX-171-003-A1 trial.12,13 Among 257 efficacy-evaluable relapsed and/or refractory patients, 23.7% achieved at least a partial response (PR) with a median survival of 15.6 months. The most common grades 3 and 4 adverse effects (AEs) were thrombocytopenia (29%), anemia (24%), and lymphopenia (20%). PN incidence rates were 12% for any grade and 1% for grade 3.
Given the improved hematologic safety profile of cyclophosphamide and the encouraging efficacy and safety profile of carfilzomib, we initiated a phase 2 trial of carfilzomib-cyclophosphamide-dexamethasone (CCyd) in elderly NDMM patients. We report the safety and efficacy results of the trial herein.
Methods
Patients
Patients with symptomatic NDMM who were ≥65 years of age or who were ineligible for autologous stem cell transplantation were included in the study. Further eligibility criteria included measurable disease, a Karnofsky performance status ≥60%, creatinine clearance ≥15 mL/minute, platelet count ≥50 × 109/L (≥30 × 109/L if myeloma involvement in the bone marrow was >50%), and an absolute neutrophil count ≥1 × 109/L without the use of growth factors. Patients were excluded from the study if they had nonsecretory MM (unless serum-free light chains were present, and the ratio was abnormal), PN higher than grade 2, active viral infection, myocardial infarction or unstable angina for ≤4 months, or other clinically significant heart disease.
All patients gave written informed consent to participate in the study, which had been approved by the institutional ethics committee. The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice.
Study design and treatment
This multicenter, nonrandomized, open-label, phase 2 study determined the safety and efficacy of CCyd in NDMM patients. A 2-stage study design according to Bryant and Day was used.14 During stage 1, primary end points included evaluation of toxicity and response to obtain the optimal dose of carfilzomib, defined as the dose needed to obtain PR in ≥35% of patients with <45% dose-limiting toxicities (DLTs) at the end of cycle 3. DLTs included any of the following treatment-related events occurring during the first 3 cycles: grade 3 or higher nonhematologic toxicity or grade 4 hematologic toxicity, excluding anemia (grade 4 neutropenia must last >3 days, and grade 4 thrombocytopenia must last >7 days). Nineteen patients were to be enrolled in stage 1. Once the optimal dose was established, 34 additional patients were to be enrolled in stage 2. The dose of cyclophosphamide (900 mg/m2 per cycle) was chosen based on preliminary experiences with the combination of bortezomib-cyclophosphamide-dexamethasone,10,15 with the total dose split into 3 separate doses of 300 mg/m2 to reduce the risk of toxicity in this elderly population. The dose of dexamethasone chosen was the standard low-dose dexamethasone described previously.16 Nine induction cycles are considered the standard number of induction cycles in patients who are not eligible for autologous stem cell transplantation.5,17
Primary end points included evaluation of toxicity and efficacy (PR) at the end of cycle 3. Secondary end points included response rates, progression-free survival (PFS), time to progression, duration of response (DOR), OS, time to next therapy, rates of PN, subgroup analyses of prognostic factors, the evaluation of the effect of maintenance on PFS and OS, and the relationship between responses and PFS in responding and nonresponding patients.
All patients received oral cyclophosphamide 300 mg/m2 on days 1, 8, and 15; oral dexamethasone 40 mg on days 1, 8, 15, and 22; and carfilzomib intravenously over 30 minutes on days 1, 2, 8, 9, 15, and 16 (20 mg/m2 on days 1 and 2 of cycle 1 and 36 mg/m2 thereafter) (supplemental Figure 1, available on the Blood Web site). Treatment was given every 28 days for 9 cycles. Patients then received maintenance therapy with 36 mg/m2 carfilzomib on days 1, 2, 15, and 16 every 28 days until progression or intolerance. Intolerance was defined as any grade 4 neutropenia or febrile neutropenia, grade 4 lymphopenia persisting for >14 days, grade 4 thrombocytopenia with active bleeding, and treatment-related nonhematologic toxicity grade 3 or higher requiring treatment discontinuation. CCyd dosing could be held for up to 2 weeks to resolve toxicity and then restarted at the same dose or at a reduced dose, depending on the type of toxicity.
Assessment
For all patients receiving ≥1 dose of any study drug, toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria, version 4.0.18 Response was assessed according to the International Myeloma Working Group criteria; assessments were undertaken at the beginning of each treatment cycle (Figure 1) during induction and every 3 cycles during maintenance. Fluorescence in situ hybridization was used for t(4:14), t(11:14), t(14;16), del13, and del17p.
Kaplan-Meier analysis of time to response, showing proportion of responding patients achieving their best response over time.
Kaplan-Meier analysis of time to response, showing proportion of responding patients achieving their best response over time.
Statistical analysis
This phase 2 trial sought to consider treatment efficacy and safety by examining error rates.14 The upper-bound limit for the probability of erroneously accepting treatment when the response rate was inadequate or the toxicity rate was high was set at 0.10. The upper-bound limit for the probability of erroneously failing to accept treatment when the response rate was favorable or the toxicity rate was low was set at 0.20 (80% power). The unacceptable and acceptable probabilities for treatment response were fixed at 0.35 and 0.60, whereas those for toxicity rates were fixed at 0.45 and 0.30, respectively. According to these parameters, 19 patients were required for stage 1. Progression from stage 1 to stage 2, where an additional 34 patients were to be enrolled (total N = 53), was allowed if there were >6 patients with PR and <9 toxicities at the end of cycle 3. Stage 2 results were to be considered positive if there were ≥23 patients with PR and ≤20 drug-related toxicities.
Response rates and safety were analyzed in patients who received ≥1 dose of study treatment. Time-to-event end points were determined using the intent-to-treat population, with a censor date of October 31, 2013. The Kaplan-Meier product limit method was used to estimate survivorship functions for time-to-event end points. Cox proportional hazards regression was used to assess the effects of demographic and prognostic variables on relative treatment differences. Continuous and categorical data were summarized using descriptive statistics. SAS System, version 8.2 system (SAS Institute, Cary, NC) was used.
Role of the funding source
The study was sponsored by the Hemato Oncology Foundation for Adults in The Netherlands (HOVON) and was cosponsored by Fondazione Neoplasie Sangue Onlus and supported by funding from Onyx Pharmaceuticals Inc., an Amgen subsidiary, and Stichting Hemato-Oncologie voor Volwassenen Nederland. The HOVON Foundation was part of the steering committee of this study and participated in the study design. The sponsor and cosponsor had no role in the collection, analysis, or interpretation of data. Onyx Pharmaceuticals critically reviewed the manuscript for scientific accuracy. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Patients
Patients were enrolled from June 21, 2011 to September 15, 2012, in 10 centers in Italy. Fifty-eight patients were enrolled in the study. The median age was 71 years; 31% of patients had an unfavorable chromosomal profile, defined as the presence of t(4;14), del17p, or t(14;16), and 40% were International Staging System (ISS) stage III (Table 1). Fifty-five patients were evaluable for response; 2 were not evaluable because they did not start treatment and 1 because of a missing assessment following cycle 1 (supplemental Figure 2). Fifty-six patients received ≥1 dose of study drugs and were evaluable for safety (supplemental Figure 2). The median duration of induction treatment was 9 cycles (range, 1-9 cycles). At the time of analysis, 43 patients have proceeded to maintenance therapy; 35 could be assessed for response (supplemental Figure 2). The median duration of maintenance treatment was 9 months (range, 2-19 months).
Patient characteristics at baseline
| Characteristic . | N = 58 . |
|---|---|
| Male, n (%) | 27 (47) |
| Age | |
| Median (IQR), years | 71 (68-75) |
| ≥75 years, n (%) | 17 (29) |
| ISS stage, n (%) | |
| I | 16 (28) |
| II | 19 (33) |
| III | 23 (40) |
| Creatinine clearance, mL/min, n (%) | |
| <30 | 2 (3) |
| 30-60 | 29 (50) |
| >60 | 27 (47) |
| Chromosomal abnormalities, n (%) | |
| t(4;14) | 9 (16) |
| t(14;16) | 1 (2) |
| Del 17 | 8 (14) |
| Unfavorable profile* | 18 (31) |
| Data missing | 7 (12) |
| Characteristic . | N = 58 . |
|---|---|
| Male, n (%) | 27 (47) |
| Age | |
| Median (IQR), years | 71 (68-75) |
| ≥75 years, n (%) | 17 (29) |
| ISS stage, n (%) | |
| I | 16 (28) |
| II | 19 (33) |
| III | 23 (40) |
| Creatinine clearance, mL/min, n (%) | |
| <30 | 2 (3) |
| 30-60 | 29 (50) |
| >60 | 27 (47) |
| Chromosomal abnormalities, n (%) | |
| t(4;14) | 9 (16) |
| t(14;16) | 1 (2) |
| Del 17 | 8 (14) |
| Unfavorable profile* | 18 (31) |
| Data missing | 7 (12) |
Unfavorable profile was defined as the presence of t(4;14) or t(14;16) or deletion of chromosome 17.
Stage 1
After 3 cycles of CCyd treatment, the first 19 patients were evaluated for response and side effects. Seventeen patients (89%) achieved at least a PR, including 12 (63%) with very good PR (VGPR) and 5 (26%) with near CR (nCR). Five patients (26%) experienced DLTs: 1 grade 4 neutropenia for >3 days, 1 grade 3 cardiac event, 2 grade 3 infections, and 1 grade 3 renal event. These data allowed the trial to progress to stage 2.
Efficacy
Overall, 52 of 55 (95%) patients had at least a PR, 39 of 55 (71%) patients had at least a VGPR, 27 of 55 (49%) patients had an nCR or CR, and 11 of 55 (20%) patients had a stringent CR (sCR; Table 2). All sCRs were confirmed by multiparametric flow cytometry. The depth of response increased in patients receiving more treatment cycles. At the end of 4 cycles, 41 of 46 (89%) patients achieved at least a PR, including 11 of 46 (24%) patients with an nCR/CR and 1 of 46 (2%) patients with an sCR. Among patients who completed 9 cycles of treatment, 43 of 43 (100%) had at least a PR and 26 of 43 (60%) had an nCR/CR, including 10 of 43 (23%) with an sCR (Table 2). Six of 43 patients (14%) showed further improvement in response during the first 9 months of maintenance with carfilzomib (Figure 1): 2 patients during the first 3 months, 3 between the fourth and sixth months, and 1 thereafter. The median time to achieve PR was 1 month, and 94% of patients with CR achieved CR during induction (Figure 1). The median DOR was 14.0 months (interquartile range [IQR], 11.7-19.2 months). The DOR was related to the quality of response. At 2 years, the proportion of patients alive and in remission was 100% in patients who achieved sCR, 74% in those who achieved CR, and 67% in those who achieved PR. Response rates were generally similar across patient groups according to age, ISS stage, and chromosomal profile (Table 2).
Response to treatment and by patient characteristics
| Patient subgroup . | n . | Response category, n (%) . | ||||
|---|---|---|---|---|---|---|
| ≥PR . | ≥VGPR . | ≥nCR . | ≥CR . | sCR . | ||
| Overall | 55 | 52 (95) | 39 (71) | 27 (49) | 18 (33) | 11 (20) |
| Age | ||||||
| <75 years | 41 | 38 (93) | 29 (71) | 22 (54) | 13 (32) | 8 (20) |
| ≥75 years | 14 | 14 (100) | 10 (71) | 5 (36) | 5 (36) | 3 (21) |
| ISS stage | ||||||
| I | 16 | 15 (94) | 10 (63) | 8 (50) | 5 (31) | 3 (19) |
| II | 18 | 18 (100) | 14 (78) | 12 (67) | 8 (44) | 6 (33) |
| III | 21 | 20 (95) | 15 (71) | 7 (33) | 5 (24) | 2 (10) |
| Chromosomal abnormalities | ||||||
| Normal/favorable | 31 | 29 (94) | 23 (74) | 17 (55) | 10 (32) | 6 (19) |
| Unfavorable* | 17 | 16 (94) | 12 (71) | 7 (41) | 5 (29) | 3 (18) |
| Treatment duration | ||||||
| Second cycle | 53 | 40 (75) | 16 (30) | 3 (6) | — | — |
| Fourth cycle | 46 | 41 (89) | 26 (57) | 11 (24) | — | 1 (2) |
| Sixth cycle | 43 | 43 (100) | 34 (79) | 13 (30) | — | 4 (9) |
| Ninth cycle | 43 | 43 (100) | 33 (77) | 20 (47) | — | 10 (23) |
| Patient subgroup . | n . | Response category, n (%) . | ||||
|---|---|---|---|---|---|---|
| ≥PR . | ≥VGPR . | ≥nCR . | ≥CR . | sCR . | ||
| Overall | 55 | 52 (95) | 39 (71) | 27 (49) | 18 (33) | 11 (20) |
| Age | ||||||
| <75 years | 41 | 38 (93) | 29 (71) | 22 (54) | 13 (32) | 8 (20) |
| ≥75 years | 14 | 14 (100) | 10 (71) | 5 (36) | 5 (36) | 3 (21) |
| ISS stage | ||||||
| I | 16 | 15 (94) | 10 (63) | 8 (50) | 5 (31) | 3 (19) |
| II | 18 | 18 (100) | 14 (78) | 12 (67) | 8 (44) | 6 (33) |
| III | 21 | 20 (95) | 15 (71) | 7 (33) | 5 (24) | 2 (10) |
| Chromosomal abnormalities | ||||||
| Normal/favorable | 31 | 29 (94) | 23 (74) | 17 (55) | 10 (32) | 6 (19) |
| Unfavorable* | 17 | 16 (94) | 12 (71) | 7 (41) | 5 (29) | 3 (18) |
| Treatment duration | ||||||
| Second cycle | 53 | 40 (75) | 16 (30) | 3 (6) | — | — |
| Fourth cycle | 46 | 41 (89) | 26 (57) | 11 (24) | — | 1 (2) |
| Sixth cycle | 43 | 43 (100) | 34 (79) | 13 (30) | — | 4 (9) |
| Ninth cycle | 43 | 43 (100) | 33 (77) | 20 (47) | — | 10 (23) |
Presence of t(4;14) or t(14;16) or deletion chromosome 17.
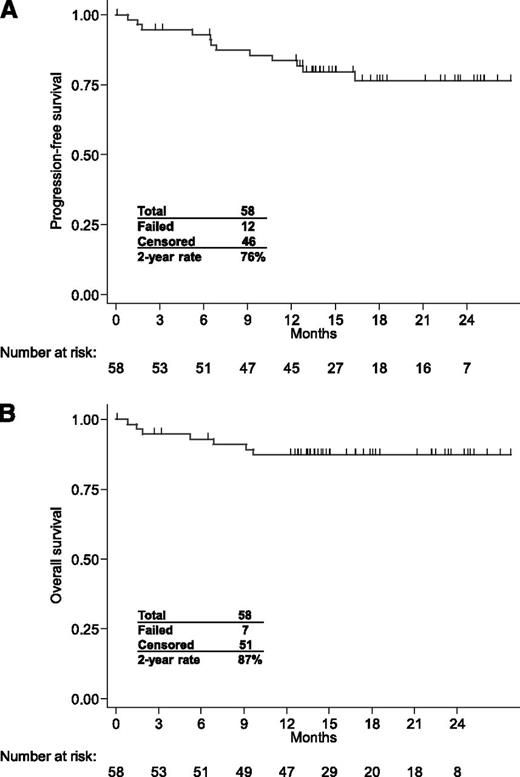
After a median follow-up of 18 months (IQR, 14-23 months), the 2-year PFS and OS rates were 76% and 87%, respectively (Figure 2). The risk of progression was slightly higher in patients with ISS stage III (hazard ratio, 2.81; 95% confidence interval, 0.58-13.56) and with high-risk chromosomal abnormalities (hazard ratio, 1.85; 95% confidence interval, 0.59-5.85).
Safety
During induction, the most common toxicities of any grade were anemia (70%), thrombocytopenia (38%), neutropenia (36%), nausea/vomiting (18%), fever (25%), fatigue (20%), and cardiac events (16%). Hematologic grade 3 to 5 toxicities included neutropenia (20%), anemia (11%), and thrombocytopenia (4%). The most common grade 3 to 5 nonhematologic AEs were metabolic events (9%), infections (5%), cardiac events (7%), and renal events (4%) (Table 3). PN was experienced by 9% of patients and was limited in severity to grades 1 and 2. Treatment-emergent serious AEs occurred during induction in 12 (21%) patients and included 4 cardiac events (heart failure, arrhythmia, myocardial infarction, and hypertension; each n = 1), increase in creatinine (n = 2), and 1 event each of infection (pneumonia), fever, intestinal perforation, stroke, acute pulmonary edema, and pulmonary thromboembolism. A limited number of patients required dose modification during induction: 8 patients (14%) discontinued treatment because of AEs, and 12 patients (21%) required carfilzomib dose reductions. The cumulative dose intensity was >90% (supplemental Table 1). The safety profile was generally similar in the 15 patients >75 years of age who received ≥1 dose of study treatment. Hematologic grade 3 to 5 toxicities included neutropenia only (27%). The most common grade 3 to 5 nonhematologic AEs were infections, cardiac events, and vascular events (n = 1 [7%] each). Five patients (33%) required carfilzomib dose reductions and 3 patients (20%) discontinued treatment because of AEs.
Treatment-related adverse events during induction
| Events, n (%) . | N = 56 . | |
|---|---|---|
| Any grade . | Grades 3 to 5 . | |
| Hematologic | ||
| ≥1 event | 44 (79) | 15 (27) |
| Neutropenia | 20 (36) | 11 (20) |
| Thrombocytopenia | 21 (38) | 2 (4) |
| Anemia | 39 (70) | 6 (11) |
| Nonhematologic | ||
| ≥1 event | 40 (71) | 16 (29) |
| Cardiac events | 9 (16) | 4 (7) |
| Arrhythmia | 2 (4) | 1 (2) |
| Myocardial infarction | 1 (2) | 1 (2) |
| Heart failure | 1 (2) | 1 (2) |
| Hypertension | 5 (9) | 1 (2) |
| Vascular events | 3 (5) | 1 (2) |
| Pulmonary thromboembolism | 1 (2) | 1 (2) |
| Phlebitis | 2 (4) | — |
| Constitutional events | 34 (61) | 2 (4) |
| Edema | 7 (13) | — |
| Fever | 14 (25) | 1 (2) |
| Fatigue | 11 (20) | 1 (2) |
| Dermatologic events | 5 (9) | — |
| Gastrointestinal events | 25 (45) | 1 (2) |
| Constipation | 3 (5) | — |
| Diarrhea | 8 (14) | — |
| Nausea/vomiting | 11 (20) | — |
| Intestinal perforation | 1 (2) | 1 (2) |
| Other | 2 (4) | — |
| Infection events | 10 (18) | 3 (5) |
| Upper respiratory tract | 6 (11) | 1 (2) |
| Pneumonia | 2 (4) | 2 (4) |
| Febrile neutropenia | 1 (2) | 1 (2) |
| Genitourinary tract | 1 (2) | — |
| Neurological events | 15 (27) | 2 (4) |
| Sensitive PN | 4 (7) | — |
| Motor PN | 1 (2) | — |
| Mood alteration | 1 (2) | 1 (2) |
| Stroke | 1 (2) | 1 (2) |
| Other | 8 (14) | — |
| Metabolic events | 19 (34) | 5 (9) |
| AST/ALT increase | 4 (7) | — |
| Hyperglycemia | 7 (13) | 1 (2) |
| Hypoglycemia | 1 (2) | — |
| Lymphopenia | 5 (9) | 4 (7) |
| Other | 2 (4) | — |
| Renal events | 3 (5) | 2 (4) |
| Respiratory events | 9 (16) | 1 (2) |
| Dyspnea | 4 (7) | — |
| Respiratory failure | 1 (2) | — |
| Acute pulmonary edema | 1 (2) | 1 (2) |
| Other | 3 (5) | — |
| Other events | 6 (11) | — |
| Events, n (%) . | N = 56 . | |
|---|---|---|
| Any grade . | Grades 3 to 5 . | |
| Hematologic | ||
| ≥1 event | 44 (79) | 15 (27) |
| Neutropenia | 20 (36) | 11 (20) |
| Thrombocytopenia | 21 (38) | 2 (4) |
| Anemia | 39 (70) | 6 (11) |
| Nonhematologic | ||
| ≥1 event | 40 (71) | 16 (29) |
| Cardiac events | 9 (16) | 4 (7) |
| Arrhythmia | 2 (4) | 1 (2) |
| Myocardial infarction | 1 (2) | 1 (2) |
| Heart failure | 1 (2) | 1 (2) |
| Hypertension | 5 (9) | 1 (2) |
| Vascular events | 3 (5) | 1 (2) |
| Pulmonary thromboembolism | 1 (2) | 1 (2) |
| Phlebitis | 2 (4) | — |
| Constitutional events | 34 (61) | 2 (4) |
| Edema | 7 (13) | — |
| Fever | 14 (25) | 1 (2) |
| Fatigue | 11 (20) | 1 (2) |
| Dermatologic events | 5 (9) | — |
| Gastrointestinal events | 25 (45) | 1 (2) |
| Constipation | 3 (5) | — |
| Diarrhea | 8 (14) | — |
| Nausea/vomiting | 11 (20) | — |
| Intestinal perforation | 1 (2) | 1 (2) |
| Other | 2 (4) | — |
| Infection events | 10 (18) | 3 (5) |
| Upper respiratory tract | 6 (11) | 1 (2) |
| Pneumonia | 2 (4) | 2 (4) |
| Febrile neutropenia | 1 (2) | 1 (2) |
| Genitourinary tract | 1 (2) | — |
| Neurological events | 15 (27) | 2 (4) |
| Sensitive PN | 4 (7) | — |
| Motor PN | 1 (2) | — |
| Mood alteration | 1 (2) | 1 (2) |
| Stroke | 1 (2) | 1 (2) |
| Other | 8 (14) | — |
| Metabolic events | 19 (34) | 5 (9) |
| AST/ALT increase | 4 (7) | — |
| Hyperglycemia | 7 (13) | 1 (2) |
| Hypoglycemia | 1 (2) | — |
| Lymphopenia | 5 (9) | 4 (7) |
| Other | 2 (4) | — |
| Renal events | 3 (5) | 2 (4) |
| Respiratory events | 9 (16) | 1 (2) |
| Dyspnea | 4 (7) | — |
| Respiratory failure | 1 (2) | — |
| Acute pulmonary edema | 1 (2) | 1 (2) |
| Other | 3 (5) | — |
| Other events | 6 (11) | — |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
During maintenance, the most common toxicities of any grade were anemia (21%), thrombocytopenia (5%), neutropenia (2%), fever (23%), and nausea/vomiting (9%). Hematologic grade 3 to 5 toxicities included neutropenia (2%), anemia (2%), and thrombocytopenia (2%). Grade 3 to 5 nonhematologic AEs were rare and occurred in <5% of patients. Ten patients (23%) experienced grade 1 and 2 fever not associated with chills, rigors, dyspnea, creatinine increase, and/or symptoms of infection. All cases were resolved by administering 4 mg oral or intravenous dexamethasone prior to all subsequent carfilzomib doses as prophylaxis. PN remained limited in severity to grades 1 and 2 (supplemental Table 2). Treatment-emergent serious AEs occurred during maintenance in 3 patients (7%) and included 1 fever, 1 diarrhea, and 1 acute diverticulitis with intestinal perforation. A limited number of patients required dose modification during maintenance: 1 patient (2%) discontinued treatment, and 2 patients (5%) required carfilzomib dose reductions due to AEs (supplemental Table 2). The cumulative dose intensity was 100% (supplemental Table 1).
Seven patients died while on the study: 2 due to disease progression, 1 due to intestinal perforation (considered related to carfilzomib), 1 due to heart failure (considered related to carfilzomib), 1 due to atrial fibrillation (not considered related to carfilzomib), 1 due to pneumonia (not considered related to carfilzomib), and 1 due to an unknown cause.
Discussion
This phase 2 study demonstrated that treatment with CCyd was highly effective and well tolerated in elderly NDMM patients. Responses were rapid and deep and showed improvement over time. Forty-nine percent of patients achieved at least an nCR, and 20% of patients achieved an sCR. After a median follow-up of 18 months, the 2-year PFS rate was 76%. Severe hematologic AEs occurred in <20% of patients, and nonhematologic AEs occurred in <10% of patients, with a low (18%) rate of discontinuation.
The achievement of CR has been associated with prolonged PFS and OS, including in elderly patients.19 Maintenance therapy also improves outcome, and its role has been extensively investigated.17,20,21 Additionally, drug discontinuation due to AEs has been associated with lower cumulative-delivered dose and shorter OS.22 The ideal treatment should combine high response rates and continuous therapy to prolong PFS with an optimal safety profile to reduce the rate of treatment discontinuation.
Despite the limitations of cross-trial comparisons, CCyd treatment compares favorably with the current standard treatments for elderly patients. The combination melphalan-prednisone-thalidomide showed a high CR/nCR rate (27%), but a high treatment discontinuation rate (35%) translated to a median PFS of 20.3 months.8,23 The combination VMP induced a CR rate of 30%, but a discontinuation rate of 33% and the absence of planned maintenance translated to a median PFS of 21 months.5 Melphalan-prednisone-lenalidomide showed a lower CR/nCR rate (19%), but a drug discontinuation rate of 24%. The continuous lenalidomide treatment translated to a median PFS of 31 months.17 The combination of lenalidomide and low-dose dexamethasone was associated with a CR/nCR rate of 14%, a drug discontinuation rate of 19%, and a median PFS of 21 months.16 In a phase 1/2 dose-escalation study, melphalan-prednisone-carfilzomib showed that 91% of patients had at least a PR, including 55% achieving at least a VGPR, and a median event-free survival of 21.8 months.24 In another phase 1/2 dose escalation study, lenalidomide-dexamethasone-carfilzomib resulted in 64% of patients reaching at least a CR, including 55% achieving an sCR, as well as a 3-year PFS rate of 79% and a 3-year OS rate of 96%.25,26 The higher CR rate observed in these patients was probably attributable to the combination of a proteasome inhibitor and an immunomodulatory agent and particularly to the enrollment of younger patients. Indeed, in that study, the median age was 59 years—with 57% of patients <65 years of age and thus potentially transplant eligible—whereas in the previous studies, the median age was ∼70 years.
In our study, the CCyd regimen was found to be well tolerated. The most frequently reported AEs were hematologic and mainly grades 1 to 2. Grade 3 to 5 neutropenia occurred in 20% of patients. The myelosuppressive effect of this regimen was lower than that reported with other frontline regimens with melphalan, such as VMP, where grade 3 to 4 neutropenia was reported in 40% of patients.5 Grade 3 to 5 thrombocytopenia occurred in 4% of patients, a markedly lower incidence than that reported in the Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) trial in patients treated with bortezomib (40%).5 Cyclophosphamide may therefore represent a valid, less toxic alternative to melphalan for elderly patients with NDMM.
No grade 3 to 4 PN was reported, and only 9% of patients experienced grade 1 to 2 PN. Historically, grade 3 to 4 PN has been reported in 6% of patients receiving thalidomide,8 in 14% of patients receiving twice-weekly bortezomib,5 and in 6% to 8% of patients receiving once-weekly bortezomib or subcutaneous administration.27,28 A recent study found that carfilzomib and bortezomib have different effects on neurodegeneration, with bortezomib inhibiting several nonproteasomal targets within neurons.29 This may, in part, explain the lower rates of PN reported with carfilzomib.
In our study, rates of grade 3 to 5 nonhematologic AEs were low. Severe cardiac events, occurring in 4 patients (7%), were heterogeneous and included congestive heart failure, hypertension, and irregular heart rhythm. One patient had controlled hypertension, whereas the 3 other patients had no preexisting cardiac comorbidities. For elderly patients involved in future trials with carfilzomib, a full cardiac workup is suggested to detect cardiac abnormalities that may be exacerbated during treatment.
The results of this study are limited by the relatively small sample size, the single-arm, nonrandomized design, the lack of independent review of response, and the short follow-up. Future studies are needed to determine the most effective strategies for the use of CCyd in the frontline setting. Given the challenges of long-term, twice-weekly infusions of carfilzomib as reported here, more convenient dosing schedules, as well as higher doses of carfilzomib, may be needed.
Our study showed that, in elderly patients who are not eligible for transplant, CCyd was highly effective, with excellent CR rates (including sCR), and was well tolerated with a low rate of treatment discontinuation. A longer follow-up is needed to draw more definitive conclusions on long-term outcomes and safety.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Federica Leotta and Giorgio Schirripa from the coordinating site in Torino, Italy, for assistance with the study. Critical review of the manuscript for scientific accuracy was undertaken by Thomas Renau (Onyx Pharmaceuticals). Medical writing and editing services were provided by Penny Baron and Christopher M. Brown, of BlueMomentum, a division of KnowledgePoint360 Group, San Bruno, CA, and supported by funding from Onyx Pharmaceuticals.
This study was sponsored by the HOVON Foundation, cosponsored by Fondazione Neoplasie Sangue Onlus, and supported by funding from Onyx Pharmaceuticals and Stichting Hemato-Oncologie voor Volwassenen Nederland.
Authorship
Contribution: All authors participated in the interpretation of data and reviewed and approved of all drafts of the manuscript, including the decision to submit for publication; S.B., M.B., G.C., P.S., and A.P. contributed to the study design; S.B., L.B., G.C., and A.P. conducted the data analyses; S.B. and A.P. wrote the first draft of the manuscript; and M.T.P., A.L., C.C., D.R., V.M., P.M., M.O., P.O., F.G., G.B., M.G., V.M., S.O., T.C., and P.T. provided patients and/or study materials.
Conflict-of-interest disclosure: S.B. has received honoraria from Celgene, Janssen-Cilag, and Novartis and has served on advisory committees for Merck Sharp & Dohme; M.T.P. has received honoraria from Celgene and Janssen-Cilag and has served on the advisory committee for Bristol-Myers Squibb; A.L. has received honoraria from Celgene and Janssen-Cilag; M.O. has received honoraria from Celgene and Janssen-Cilag; S.O. has received honoraria from Celgene; M.B. declares research support, consultancy, and scientific advisory board participation from Celgene and Janssen-Cilag; P.S. has received research support from Onyx, Janssen, Celgene, and Millennium and has participated on advisory boards for Onyx, Janssen, Celgene, and Millennium; and A.P. has received consultancy fees and honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Antonio Palumbo, Myeloma Unit, Division of Hematology, University of Turin, Via Genova 3, 10126 Turin, Italy; e-mail: appalumbo@yahoo.com.