Abstract
This phase 1/2 study in patients with newly diagnosed multiple myeloma (N = 53) assessed CRd—carfilzomib (20, 27, or 36 mg/m2, days 1, 2, 8, 9, 15, 16 and 1, 2, 15, 16 after cycle 8), lenalidomide (25 mg/d, days 1-21), and weekly dexamethasone (40/20 mg cycles 1-4/5+)—in 28-day cycles. After cycle 4, transplantation-eligible candidates underwent stem cell collection (SCC) then continued CRd with the option of transplantation. The maximum planned dose level (carfilzomib 36 mg/m2) was expanded in phase 2 (n = 36). Thirty-five patients underwent SCC, 7 proceeded to transplantation, and the remainder resumed CRd. Grade 3/4 toxicities included hypophosphatemia (25%), hyperglycemia (23%), anemia (21%), thrombocytopenia (17%), and neutropenia (17%); peripheral neuropathy was limited to grade 1/2 (23%). Most patients did not require dose modifications. After a median of 12 cycles (range, 1-25), 62% (N = 53) achieved at least near-complete response (CR) and 42% stringent CR. Responses were rapid and improved during treatment. In 36 patients completing 8 or more cycles, 78% reached at least near CR and 61% stringent CR. With median follow-up of 13 months (range, 4-25 months), 24-month progression-free survival estimate was 92%. CRd was well tolerated with exceptional response rates. This study is registered at http://www.clinicaltrials.gov as NCT01029054.
Introduction
Although multiple myeloma (MM) remains incurable, the introduction of targeted therapy with proteasome inhibitors (bortezomib [V]) and immunomodulatory drugs (thalidomide [T] or lenalidomide [R]) has advanced the goals of treatment, with significant improvements in long-term outcomes. In initial studies, investigators reported good clinical activity when these agents were used either alone or in combination with corticosteroids.1-6 As the development of these agents progressed, in subsequent studies investigators demonstrated that combination therapies with an immunomodulator, a proteasome inhibitor, and a corticosteroid (eg, low- or high-dose dexamethasone [d or D]) provided rapid, deep, and more durable responses compared with standard treatment approaches and with acceptable tolerability because of nonoverlapping toxicity.7-11
Triple-agent regimens that use bortezomib, lenalidomide, and/or thalidomide have emerged as a preferred frontline strategy in patients with newly diagnosed MM.12 The results of 2 recent randomized phase 3 trials, in which investigators compared induction with 3-drug with 2-drug novel regimens (VTD vs TD and vtD vs VD) in transplantation-eligible patients, appear to support this approach.10,11 Nonetheless, maintaining dose levels over the long-term can be limited by emerging toxicities. After only a short course of 3 cycles of induction with VTD, grade 3/4 peripheral neuropathy was present in 10% of patients with a rate of 34% for all grades.10 Treatment with RVD also has been shown to be highly active in the frontline setting and appears to be better tolerated than VTD, but during prolonged treatment (median of 10 cycles), sensory neuropathy developed in 80% of patients, although high-grade events were infrequent (2% for grade 3).9
Studies in patients with newly diagnosed MM also have shown that the depth of response (eg, complete response [CR] or very good partial response [VGPR]) with frontline combination regimens is associated with improved control of the disease and survival.13-18 A retrospective analysis of 3 phase 3 trials evaluating proteasome inhibitors and immunomodulators in transplantation-ineligible patients with newly diagnosed MM revealed that CR compared with VGPR was associated with improved progression-free survival (PFS) at 3 years (67% vs 27%; P < .001) and overall survival (OS; 91% vs 70%; P < .001).15 On the basis of these and similar observations,19,20 recent investigations have focused on development of novel frontline combinations with a goal to further improve the depth and duration of response compared with established treatment approaches, with improved tolerability and a minimum impact on stem cell collection (SCC).
Carfilzomib is a next-generation proteasome inhibitor that selectively and irreversibly binds to the proteasome, targeting chymotrypsin-like activity. Carfilzomib provides sustained proteasome inhibition without off-target effects and inhibits proliferation and induces apoptosis in myeloma models.21-23 In clinical studies, investigators have shown that single-agent carfilzomib provides durable anticancer activity in patients with relapsed and/or refractory MM with an acceptable tolerability profile, including limited neuropathy after prolonged treatment.24-27 In a phase 2 study of single-agent carfilzomib (N = 266), 23.7% of evaluable patients with relapsed and refractory MM achieved at least a partial response (PR) with a median duration of response of 7.8 months.27 In an integrated analysis of 3 phase 2 studies with single-agent carfilzomib in patients with relapsed and refractory MM (N = 526), the most common grade 3/4 adverse events (AEs) were thrombocytopenia (23%), anemia (22%), and lymphopenia (18%); peripheral neuropathy was 14% for any grade and 1.3% for grade 3 with no grade 4 events.26 Investigators also have assessed carfilzomib as part of combination regimens. In an interim analysis of a phase 2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone (CRd) for relapsed MM (N = 52), 78% of evaluable patients achieved at least a PR, 40% at least a VGPR, and 18% a CR or stringent CR (sCR) with good tolerability.28
Herein, we report results from the first prospective phase 1/2 study of the CRd combination in patients with newly diagnosed MM. Our primary objectives were to determine the maximum tolerated dose (MTD) of carfilzomib when added to Rd during phase 1 and to assess the safety and activity of CRd in a combined phase 1/2 patient population.
Methods
Patients
Both transplantation-eligible and -ineligible patients with newly diagnosed MM could be enrolled, but the disease had to be symptomatic and measurable per International Myeloma Working Group (IMWG) Criteria.29,30 Patients were required to have an Eastern Cooperative Oncology Group Performance Status of 0-2. Patients were ineligible if they had grade 3/4 neuropathy, a calculated creatinine clearance < 50 mL/min or serum creatinine ≥ 2 g/dL, absolute neutrophil count (ANC) < 1.0 × 109/L, hemoglobin < 8.0 g/dL, platelet count < 75 × 109/L, serious comorbidities, or other plasma cell dyscrasias such as POEMS syndrome (ie, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes), plasma cell leukemia, or Waldenström macroglobulinemia.
The study was conducted in accordance with US Food and Drug Administration and International Conference on Harmonisation Guidelines for Good Clinical Practice, the Declaration of Helsinki, and applicable local health authority, institutional review board, or Independent Ethics Committee requirements. The study protocol was approved by the institutional review board of participating institutions, and all patients provided written informed consent. The study is registered at http://www.clinicaltrials.gov as NCT01029054.
Study design and treatment
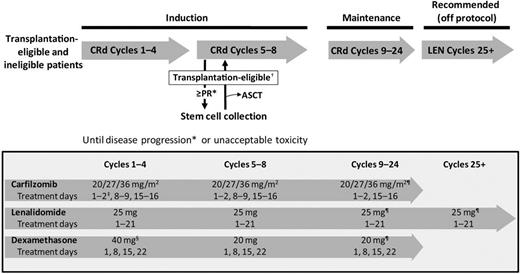
This multicenter, open-label, phase 1/2 study was conducted at 4 US centers. Patients received CRd induction therapy in 28-day cycles for up to 8 cycles or until disease progression or unacceptable toxicity (Figure 1). Per protocol design, transplantation-eligible patients achieving at least a PR could proceed to SCC any time after cycle 4 but then were to resume CRd treatment with an option to proceed to autologous stem cell transplantation (ASCT). The acceptance of deferred transplantation was included in eligibility criteria with concept and clinical data thoroughly discussed with patients before enrollment and informed consent. However, proceeding to transplantation was not mandated, with the decision left to the patient and treating physician. After 8 cycles, patients received maintenance CRd. Per initial design, CRd maintenance was planned for an indefinite period of time, but in view of limited progression events and no discontinuation of maintenance because of toxicity, the study was amended to 24 total cycles of CRd. After completion of 24 cycles, single-agent lenalidomide was recommended off protocol.
Study design and treatment schema. *Assessment on days 1 and 15 of cycle 1 and day 1 of each subsequent cycle using modified IMWG Uniform Criteria with the addition of nCR. †Patients achieving a PR after cycle 4 underwent SCC and then continued CRd with the option to proceed to ASCT. ‡Initial dose of 20 mg/m2 during cycle 1 days 1-2 for all patients regardless of dose cohort. §At the discretion of the investigator, patients could receive 4 mg of dexamethasone orally or intravenously on days 2, 9, and 16 (cycles 1 and 2) before the infusion of carfilzomib if signs of tumor flare-up were present. ¶Or the last tolerated dose. ASCT indicates autologous stem cell transplantation; CRd, carfilzomib, lenalidomide, dexamethasone; LEN, lenalidomide monotherapy; and PR, partial response.
Study design and treatment schema. *Assessment on days 1 and 15 of cycle 1 and day 1 of each subsequent cycle using modified IMWG Uniform Criteria with the addition of nCR. †Patients achieving a PR after cycle 4 underwent SCC and then continued CRd with the option to proceed to ASCT. ‡Initial dose of 20 mg/m2 during cycle 1 days 1-2 for all patients regardless of dose cohort. §At the discretion of the investigator, patients could receive 4 mg of dexamethasone orally or intravenously on days 2, 9, and 16 (cycles 1 and 2) before the infusion of carfilzomib if signs of tumor flare-up were present. ¶Or the last tolerated dose. ASCT indicates autologous stem cell transplantation; CRd, carfilzomib, lenalidomide, dexamethasone; LEN, lenalidomide monotherapy; and PR, partial response.
During phase 1, the primary end points were to evaluate the safety and determine the MTD of carfilzomib in the context of CRd. Carfilzomib doses were escalated, whereas lenalidomide and dexamethasone were given at standard low-dose induction levels.31 Three dose levels for carfilzomib were planned—20, 27, and a maximum planned dose (MPD) of 36 mg/m2. These dose levels were determined in part on initial data from the phase 1/2 study of CRd in relapsed patients, which demonstrated the safety and tolerability of combining carfilzomib 27 mg/m2 with lenalidomide 25 mg and low-dose dexamethasone by the use of a similar dosing schedule without reaching MTD.28 On the basis of this information and emerging data that single-agent carfilzomib appeared to be tolerated at 36 mg/m2, we designed our dose escalation using 36 mg/m2 as the MPD. If 20 mg/m2 was deemed intolerable, then a dose of 15 mg/m2 dose would be assessed as the MTD. Dose escalation was designed with use of the time-to event continual reassessment method (TITE-CRM).32 The MTD of carfilzomib was defined by a target probability limit of 20% for dose-limiting toxicities (DLTs).
DLTs included any of the following treatment-related events that occurred during the first cycle: inability to begin cycle 2 because of drug-related toxicity; ≥ grade 2 neuropathy with pain; any AE ≥ grade 3 (excluding nausea, vomiting, diarrhea, dexamethasone-induced hyperglycemia, lenalidomide-induced maculopapular rash); ≥ grade 3 nausea, vomiting, or diarrhea despite maximal antiemetic/antidiarrheal therapy; grade 4 fatigue lasting longer than 7 days; any nonhematologic toxicity requiring dose reduction within cycle 1 except lenalidomide-induced maculopapular rash; grade 4 neutropenia (ANC < 0.5 × 109/L) longer than 7 days; febrile neutropenia (ANC < 1.0 × 109/L with fever ≥ 38°C); grade 4 thrombocytopenia (platelets < 25.0 × 109/L) longer than 7 days despite dose delay; grade 3/4 thrombocytopenia associated with bleeding; or any hematologic toxicity requiring dose reduction within cycle 1.
Once the MTD of carfilzomib was established, additional patients were enrolled in a phase 2 expansion cohort to reach a sample size of 36 patients treated at the MTD dose; if the MTD was not reached, the MPD could be used. The primary end point was the rate of at least near-complete response (nCR) after 4 cycles. Secondary end points included overall response rate defined as PR or better (≥ PR), time on study, duration of response, PFS, time to progression, OS, overall treatment toxicity and tolerability, and the feasibility of SCC after cycle 4.
For induction (cycles 1-8), carfilzomib (20, 27, and 36 mg/m2) was administered intravenously on days 1, 2, 8, 9, 15, and 16. The 20 and 27 mg/m2 doses were infused over 5-10 minutes, whereas the 36 mg/m2 dose was infused over 30 minutes.33 Patients slated to receive the 27 or 36 mg/m2 carfilzomib doses received 20 mg/m2 on days 1 and 2 of cycle 1 and the greater dose thereafter. Lenalidomide (25 g) was administered orally on days 1-21 of all cycles. Dexamethasone (40 mg for cycles 1-4, 20 mg for cycles 5-8) was administered orally or intravenously on days 1, 8, 15, and 22. During maintenance CRd, cycles 9-24, individual study drugs were continued at the same dose level as cycle 8; lenalidomide and dexamethasone were continued at the same dosing schedule, whereas carfilzomib was administered less frequently (days 1, 2, 15, and 16). From cycles 25+, it was recommended that patients continue maintenance with single-agent lenalidomide at the last tolerated dose.
Dose reductions during cycle 1 were considered DLTs, as described previously. After cycle 1, carfilzomib, lenalidomide, and dexamethasone dosing could be held for up to 21 days to resolve toxicity and then restarted at the same dose or the carfilzomib or lenalidomide dose could be reduced depending on the toxicity using dose reduction by 1 dose level (ie, 27, 20, 15, or 11 mg/m2 for carfilzomib and 20, 15, 10, or 5 mg for lenalidomide) or discontinued.
Patients were required to maintain adequate hydration and were treated prophylactically with ciprofloxacin or a similar antibiotic (cycle 1 only), valacyclovir or a similar antiviral, a proton pump inhibitor or H2 antagonist, and aspirin, a low-molecular-weight heparin, or clopidogrel. In patients with previous venous thrombosis, low-molecular-weight heparin or warfarin (international normalized ratio of 2-3) was required.
Assessments
For all patients receiving at least 1 dose of any required study drug, toxicity was assessed according to Common Terminology Criteria for Adverse Events Version 3.0.34 Disease response was assessed by local investigator review according to IMWG criteria, with categorized responses of sCR, CR, VGPR, PR, stable disease, and progressive disease,30 and the addition of nCR4 and minimal response.35 There was no independent central review of efficacy end points. M-protein was measured by serum or urine protein electrophoresis. Additional measurements included quantitative immunoglobulins, serum β2 microglobulin, serum-free light chains, plasmacytoma, and BM aspirate and biopsy as indicated. Assessments were performed at screening, on days 1 and 15 of cycle 1 and on day 1 of subsequent cycles. BM aspirate and biopsy were conducted at screening to quantify myeloma cell involvement, as well as for cytogenetics (ie, hypodiploidy, del 13) and FISH studies for t(4:14), t(11:14), and del 17p, and to confirm CR. Minimal residual disease (MRD) was evaluated in patients with suspected CR by the use of 10-color multiparameter flow cytometry as previously described.36,37 Neurologic assessments were performed on day 1 of each cycle.
Statistical analysis
The sample size for the estimation of the dose-toxicity function (phase 1) was 35 patients. Under the TITE-CRM paradigm, the relationship between dose and toxicity was summarized by a single-parameter (α) logistic model that represents the assumed relationship before data collection. The most current information about the relationship between dose and toxicity, including predictive intervals, was summarized by use of the distribution of α. The posterior distribution of toxicity, which displays the probability that a future patient will experience toxicity at a given dose on the basis of the current data, was calculated with 95% credible intervals for each dose level. The CRd dose closest to but not exceeding the target rate of toxicity (20%) was estimated as the MTD.
Response was determined at day 15 of cycle 1 and after each cycle. A rate of at least 45% for nCR or better after 4 cycles was considered promising, whereas a rate of 25% or lower was considered unworthy of future study. Patients unable to receive 4 cycles were considered nonresponders. This hypothesis was interrogated by the use of a Minimax 2-stage design for patients treated at the phase 2 dose. In the first stage, if at least 5 of 17 patients responded, the trial would continue to the second stage, adding patients for a total sample size of 36. If 14 or more of the 36 patients responded, then the null hypothesis of a 25% response rate would be rejected in favor of the alternative 45% response rate. The trial was designed to have 80% power for the hypothesis and 5% type I error.
Continuous and categorical data were summarized with descriptive statistics. The product-limit method of Kaplan-Meier was used to analyze time-to-event end points. Statistical analyses were conducted via use of the SAS System Version 9.2. All authors had access to clinical data and statistical analyses.
Results
Patients and treatment
Fifty-three patients were enrolled between October 27, 2009, and June 30, 2011. Data cutoff for this analysis was November 30, 2011. Phase 1 dosing cohorts included 4 patients at carfilzomib 20 mg/m2, 13 at 27 mg/m2, and 18 at 36 mg/m2. An additional 18 patients enrolled as part of the phase 2 expansion. The overall population was predominantly male (74%) and ranged in age from 35-81 years with 43% older than 65 years. Of 51 patients with available data, 33% had unfavorable cytogenetics defined as 1 or more abnormalities listed in Table 1.
Baseline characteristics
| Characteristic . | N = 53 . |
|---|---|
| Age | |
| Median, y (range) | 59 (35-81) |
| ≥ 65 y, n (%) | 23 (43) |
| Sex | |
| Male, n (%) | 39 (74) |
| Female | 14 (26) |
| ISS stage, n (%) | |
| I | 21 (40) |
| II | 18 (34) |
| III | 14 (26) |
| Durie-Salmon stage, n (%) | |
| I | 7 (13) |
| II | 12 (24) |
| III | 34 (63) |
| Unfavorable cytogenetics, n (%)* | 17/51 (33) |
| del 13†/hypodiploidy | 10/50 (20) |
| t(4;14) | 5/49 (10) |
| t(14;16) | 0/48 (0) |
| del 17p | 7/48 (15) |
| Characteristic . | N = 53 . |
|---|---|
| Age | |
| Median, y (range) | 59 (35-81) |
| ≥ 65 y, n (%) | 23 (43) |
| Sex | |
| Male, n (%) | 39 (74) |
| Female | 14 (26) |
| ISS stage, n (%) | |
| I | 21 (40) |
| II | 18 (34) |
| III | 14 (26) |
| Durie-Salmon stage, n (%) | |
| I | 7 (13) |
| II | 12 (24) |
| III | 34 (63) |
| Unfavorable cytogenetics, n (%)* | 17/51 (33) |
| del 13†/hypodiploidy | 10/50 (20) |
| t(4;14) | 5/49 (10) |
| t(14;16) | 0/48 (0) |
| del 17p | 7/48 (15) |
ISS indicates International Staging System.
One or more of the abnormalities listed.
del 13 by metaphase only.
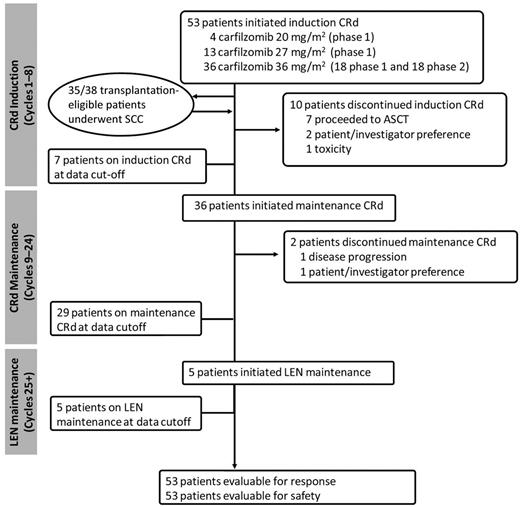
Median follow-up was 13 months (range, 4-25 months) with all 53 patients on treatment for more than 1 month and evaluable for response (Figure 2). Median treatment duration was 12 cycles (range, 1-25 cycles). A total of 10 patients discontinued treatment during induction—1 because of CRd toxicity at dose level 3 (pulmonary edema), 7 proceeded to ASCT, and 2 because of patient/investigator preference. Patients who proceeded to ASCT continue to be followed for time to progression, PFS, and OS. Thirty-six patients proceeded to maintenance CRd, with 1 patient discontinuing treatment because of PD and 1 patient preferring to discontinue treatment while in VGPR and then experiencing disease progression. At the data cut-off date, 29 patients were on maintenance CRd, and 5 patients proceeded to single-agent lenalidomide (median duration of 1 cycle).
Patient flow. Median duration of treatment (N = 53); 12 cycles (range, 1-25). One cycle = 28 days. ASCT indicates autologous stem cell transplantation; CRd, carfilzomib, lenalidomide, dexamethasone; LEN, lenalidomide monotherapy; and SCC, stem cell collection.
Patient flow. Median duration of treatment (N = 53); 12 cycles (range, 1-25). One cycle = 28 days. ASCT indicates autologous stem cell transplantation; CRd, carfilzomib, lenalidomide, dexamethasone; LEN, lenalidomide monotherapy; and SCC, stem cell collection.
The CRd regimen did not appear to have an adverse impact on SCC. Thirty-five transplantation-eligible patients who achieved at least a PR after cycle 4 underwent SCC. Three additional patients were considered for SCC but declined or did not proceed for insurance reasons. The median number of completed cycles before collection was 4 (range, 2-9 cycles), with 1 patient preferring to discontinue CRd after 2 cycles to undergo SCC and pursue alternative treatment. SCC was conducted with only growth factors in 30 patients, of whom 2 required the addition of cyclophosphamide, and with chemotherapy and growth factors in 5 patients. SCC was unsuccessful in 1 patient who was older than 70 years of age and underwent the procedure after 8 cycles of CRd. The median number of CD34+ cells harvested was 6.9 × 106/kg (range, 0.6-27.8 × 106/kg). Of 7 patients who underwent ASCT, all proceeded after initial induction per the participating center's preference (1 in the 27 mg/m2 cohort and 6 in the 36 mg/m2 cohort).
Determination of phase 2 dosing
There were no DLTs in the 20 mg/m2 dose cohort (Table 2). One patient in the 27 mg/m2 dose cohort experienced a DLT of grade 3 asymptomatic neutropenia, which resolved within a few days. Two patients experienced a DLT in the 36 mg/m2 dose cohort, including grade 4 pulmonary edema and grade 3 dyspnea. The probability of a DLT with the TITE-CRM algorithm was 5.9% at 20 mg/m2, 8.1% at 27 mg/m2, and 12% at 36 mg/m2. Although the DLT probability was less than the 20% set for MTD, these data indicated a dose-dependent trend of increasing DLTs.
Determination of the MTD during phase 1
| Dose level . | Carfilzomib dose, mg/m2 . | N = 35 . | n . | DLT probablilty estimate* . | 95% Credible interval . |
|---|---|---|---|---|---|
| 1 | 20 | 4 | 0 | 5.9% | 1.7-15.3 |
| 2 | 27 | 13 | 1† | 8.1% | 2.6-19.4 |
| 3 | 36 | 18 | 2‡ | 12.0% | 4.3-25.4 |
| Dose level . | Carfilzomib dose, mg/m2 . | N = 35 . | n . | DLT probablilty estimate* . | 95% Credible interval . |
|---|---|---|---|---|---|
| 1 | 20 | 4 | 0 | 5.9% | 1.7-15.3 |
| 2 | 27 | 13 | 1† | 8.1% | 2.6-19.4 |
| 3 | 36 | 18 | 2‡ | 12.0% | 4.3-25.4 |
DLT indicates dose-limiting toxicities; and MTD, maximum tolerated dose.
Time-to event continual reassessment method single-parameter logistic model.
Neutropenia, grade 3, asymptomatic, resolved within a few days.
One patient with grade 4 pulmonary edema; it resolved with diuresis but the patient withdrew consent and switched to alternate therapy achieving CR; the second patient with grade 3 dyspnea responded to diuresis and resumed treatment at a lower carfilzomib dose level; the patient continued on this dose without difficulty in subsequent cycles.
On the basis of the tolerability of this regimen, the trend observed in the DLT probability estimate with increasing doses of carfilzomib, the initial response data that revealed high activity at all 3 dose levels,38 the limited clinical experience with carfilzomib at greater dose levels, and the limitations of adding dose cohorts during an ongoing trial with TITE-CRM design in a posthoc fashion, we proceeded to phase 2 using carfilzomib at an MPD of 36 mg/m2 without determination of the MTD.
Efficacy
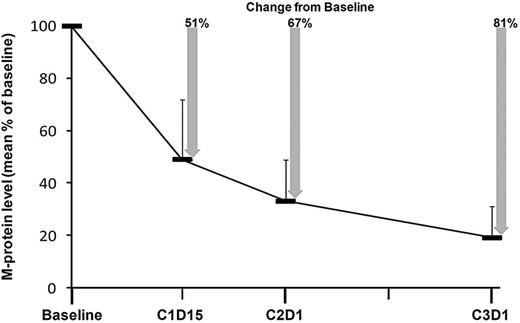
In the overall population (N = 53), 62% of patients achieved at least a nCR (42% sCR), 81% at least a VGPR, and 98% at least a PR after a median of 12 cycles (range, 1-25 cycles; Table 3). In general, responses were rapid, improved with continued treatment, and durable. At the end of cycle 1, the mean M-protein level was reduced by 67% from baseline, and at the end of cycle 2, the mean level was reduced by 81% from baseline (Figure 3). Prolonged treatment with CRd increased the proportion of patients in at least nCR. At the end of 4 cycles, 38% of patients were in at least nCR, with 6% in sCR. In patients who received 8 or more treatment cycles, 78% achieved at least a nCR with 61% in sCR. In the subset of patients who did not proceed to transplantation (n = 46), 67% achieved at least a nCR (48% sCR), 83% at least a VGPR, and 100% at least a PR after a median of 12 cycles (range, 1-25). Of the 7 patients who proceeded to ASCT, best responses before transplant were 2 CR, 1 VGPR, 3 PR, and 1 minimal response. In 22 patients with CR or suspected CR (including 2 in nCR), there was no evidence of MRD in 20 (91%). Of 2 patients with positive MRD, 1 was in CR and the other was in nCR.
Best response to treatment in evaluable patients
| . | Response, n (%)* . | |||
|---|---|---|---|---|
| ≥ PR . | ≥ VGPR . | ≥ nCR . | sCR . | |
| All patients (N = 53) | 52 (98) | 43 (81) | 33 (62) | 22 (42) |
| Treatment duration | ||||
| 4+ cycles (n = 49) | 49 (100) | 43 (88) | 33 (67) | 22 (45) |
| 8+ cycles (n = 36) | 36 (100) | 33 (92) | 28 (78) | 22 (61) |
| 12+ cycles (n = 29) | 29 (100) | 25 (86) | 21 (72) | 18 (62) |
| . | Response, n (%)* . | |||
|---|---|---|---|---|
| ≥ PR . | ≥ VGPR . | ≥ nCR . | sCR . | |
| All patients (N = 53) | 52 (98) | 43 (81) | 33 (62) | 22 (42) |
| Treatment duration | ||||
| 4+ cycles (n = 49) | 49 (100) | 43 (88) | 33 (67) | 22 (45) |
| 8+ cycles (n = 36) | 36 (100) | 33 (92) | 28 (78) | 22 (61) |
| 12+ cycles (n = 29) | 29 (100) | 25 (86) | 21 (72) | 18 (62) |
IMWG indicates International Myeloma Working Group; nCR, near-complete response; PR, partial response; sCR, stringent complete response; and VGPR, very good partial response.
Assessed by Modified IMWG Uniform Criteria with the addition of nCR.
Change in M-protein levels compared with baseline. Error bars indicate SD.
International Staging System stage and cytogenetics did not impact rate or depth of responses (Table 4), but these analyses are limited by patient numbers. Although response rates in the 36 mg/m2 cohort were lower compared with the 20 and 27 mg/m2 dose cohorts combined, the median duration of treatment at data cut-off was 8 cycles (range, 1-19 cycles) versus 21 cycles (range, 4-25 cycles), respectively. At equivalent time points, response rates were generally comparable across the 20, 27, and 36 mg/m2 dose cohorts, although achieving sCR appeared to be dependent on the dose (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Best response to treatment by carfilzomib dose, ISS stage, and cytogenetics (N = 53)
| . | Response, n (%)* . | |||
|---|---|---|---|---|
| ≥ PR . | ≥ VGPR . | ≥ nCR . | sCR . | |
| Carfilzomib dose, mg/m2 | ||||
| 20 (n = 4) | 4 (100) | 4 (100) | 3 (75) | 1 (25) |
| 27 (n = 13) | 13 (100) | 13 (100) | 10 (77) | 7 (54) |
| 36 (n = 36) | 35 (97) | 26 (72) | 20 (55) | 14 (39) |
| ISS stage | ||||
| I (n = 21) | 21 (100) | 16 (76) | 12 (57) | 7 (33) |
| II (n = 18) | 18 (100) | 15 (75) | 10 (55) | 8 (44) |
| III (n = 14) | 13 (93) | 12 (86) | 11 (79) | 7 (50) |
| Cytogenetics | ||||
| Normal/favorable (n = 34)† | 34 (100) | 26 (76) | 20 (59) | 13 (38) |
| Unfavorable (n = 17)† | 16 (94) | 13 (76) | 11 (65) | 9 (53) |
| . | Response, n (%)* . | |||
|---|---|---|---|---|
| ≥ PR . | ≥ VGPR . | ≥ nCR . | sCR . | |
| Carfilzomib dose, mg/m2 | ||||
| 20 (n = 4) | 4 (100) | 4 (100) | 3 (75) | 1 (25) |
| 27 (n = 13) | 13 (100) | 13 (100) | 10 (77) | 7 (54) |
| 36 (n = 36) | 35 (97) | 26 (72) | 20 (55) | 14 (39) |
| ISS stage | ||||
| I (n = 21) | 21 (100) | 16 (76) | 12 (57) | 7 (33) |
| II (n = 18) | 18 (100) | 15 (75) | 10 (55) | 8 (44) |
| III (n = 14) | 13 (93) | 12 (86) | 11 (79) | 7 (50) |
| Cytogenetics | ||||
| Normal/favorable (n = 34)† | 34 (100) | 26 (76) | 20 (59) | 13 (38) |
| Unfavorable (n = 17)† | 16 (94) | 13 (76) | 11 (65) | 9 (53) |
ISS indicates International Staging System; nCR, near-complete response; PR, partial response; and VGPR, very good partial response.
Assessed by Modified IMWG Uniform Criteria with the addition of nCR.
Any of del 13 by metaphase or hypodiploidy or t(4;14) or t(14;16) or del 17p considered as unfavorable; all others considered normal/favorable.
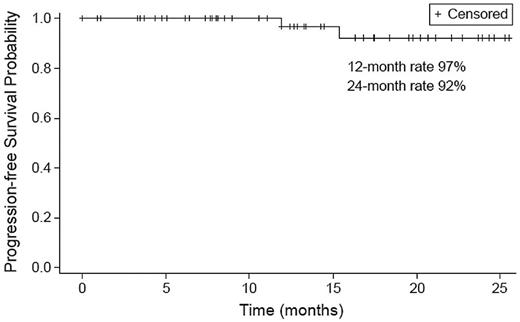
Assessment of time-to-event end points was limited because of the small number of events. Only 2 patients progressed, 1 after discontinuation of treatment while in VGPR after completion of 8 cycles as noted earlier and another after completion of 15 cycles while in PR. The PFS rate was 97% at 12 months and 92% at 24 months (Figure 4). All patients who achieved sCR have maintained response for a median of 9 months (range, 1-20 months). The patient who progressed after discontinuing treatment eventually died because of disease progression; all others are alive.
Safety and tolerability
Table 5 summarizes the incidence of AEs that occurred during induction (cycles 1-8). The most common toxicities of any grade were hyperglycemia (72%), thrombocytopenia (68%), anemia (60%), edema (47%), hypophosphatemia (45%), and fatigue (38%). Grade 3/4 nonhematologic AEs included hypophosphatemia (25%), hyperglycemia (23%), deep-vein thrombosis/pulmonary embolism (DVT/PE; 9%), rash (8%), and elevated liver function test (8%). Hematologic grade 3/4 toxicities included anemia (21%), thrombocytopenia (17%), and neutropenia (17%). Peripheral neuropathy was experienced by 23% and was limited in severity to grades 1 (17%) and 2 (6%).
Treatment-emergent adverse events during induction (cycles 1-8; N = 53)
| . | Any grade, n (%) . | Grade 3/4, n (%) . |
|---|---|---|
| Nonhematologic | ||
| Hyperglycemia | 38 (72) | 12 (23) |
| Edema | 25 (47) | 2 (4) |
| Hypophosphatemia | 24 (45) | 13 (25) |
| Fatigue | 20 (38) | 1 (2) |
| Muscle cramping | 17 (32) | 0 (0) |
| Rash | 15 (28) | 4 (8) |
| Elevated liver function test | 15 (28) | 4 (8) |
| Diarrhea | 14 (26) | 0 (0) |
| Infection* | 12 (23) | 2 (4) |
| Phlebitis | 12 (23) | 0 (0) |
| Peripheral neuropathy | 12 (23)† | 0 (0) |
| Dyspnea | 8 (15) | 2 (4) |
| Deep vein thrombosis | 6 (11) | 2 (4) |
| Pulmonary embolism | 3 (6) | 3 (6) |
| Nausea | 7 (13) | 0 (0) |
| Renal | 5 (9) | 1 (2) |
| Constipation | 5 (9) | 0 (0) |
| Mood alterations | 5 (9) | 1 (2) |
| Hematologic | ||
| Thrombocytopenia | 36 (68) | 9 (17) |
| Anemia | 32 (60) | 11 (21) |
| Neutropenia | 16 (30) | 9 (17) |
| . | Any grade, n (%) . | Grade 3/4, n (%) . |
|---|---|---|
| Nonhematologic | ||
| Hyperglycemia | 38 (72) | 12 (23) |
| Edema | 25 (47) | 2 (4) |
| Hypophosphatemia | 24 (45) | 13 (25) |
| Fatigue | 20 (38) | 1 (2) |
| Muscle cramping | 17 (32) | 0 (0) |
| Rash | 15 (28) | 4 (8) |
| Elevated liver function test | 15 (28) | 4 (8) |
| Diarrhea | 14 (26) | 0 (0) |
| Infection* | 12 (23) | 2 (4) |
| Phlebitis | 12 (23) | 0 (0) |
| Peripheral neuropathy | 12 (23)† | 0 (0) |
| Dyspnea | 8 (15) | 2 (4) |
| Deep vein thrombosis | 6 (11) | 2 (4) |
| Pulmonary embolism | 3 (6) | 3 (6) |
| Nausea | 7 (13) | 0 (0) |
| Renal | 5 (9) | 1 (2) |
| Constipation | 5 (9) | 0 (0) |
| Mood alterations | 5 (9) | 1 (2) |
| Hematologic | ||
| Thrombocytopenia | 36 (68) | 9 (17) |
| Anemia | 32 (60) | 11 (21) |
| Neutropenia | 16 (30) | 9 (17) |
Grade 3/4 events were pneumonia, and grade 1/2 events were upper respiratory infections
Three (6%) grade 2, remaining grade 1.
Overall, the CRd regimen was well tolerated during induction. Dose modifications were limited (31%), and more than 50% of patients remained on originally assigned doses. Generally, AEs were effectively managed with supportive measures. As noted previously, only 1 patient discontinued treatment because of toxicity (pulmonary edema). Grade 3/4 dyspnea was observed only during phase 1 and within the first 3 cycles. All cases of dyspnea except for 1 were associated with vigorous hydration, and patients promptly responded to diuresis; 1 case was associated with the development of methemoglobinemia secondary to dapsone given as Pneumocystis carinii pneumonia prophylaxis. No cases of dyspnea were reported after vigorous hydration was discontinued. Most cases of hyperglycemia and hypophosphatemia (65% and 61% of all respective events) were observed after dexamethasone dosing (ie, on days 2, 9, and 16) and had no clinical implications. All cases of DVT/PE developed while patients were receiving aspirin prophylaxis and after the first few cycles. One patient who experienced a DVT had 2 risk factors (previous DVT and ongoing smoking), and DVT was deemed possibly related to carfilzomib infusion in 2 patients. All 3 cases of PE required hospitalization.
Extended treatment in the CRd maintenance phase (9-24 cycles) was also generally well tolerated. The most common toxicities (all grades) during maintenance were lymphopenia (30%), leukopenia (26%), and fatigue (25%). Peripheral neuropathy remained limited (11%, all grade 1/2) as did dose modifications (19% carfilzomib, 28% lenalidomide, and 31% dexamethasone). Throughout the duration of treatment, there were no treatment-related deaths, no incidents of acute renal failure and only limited grade 1 and 2 transient changes in serum creatinine, and only 1 incident of febrile neutropenia.
Discussion
This phase 1/2 study demonstrated that CRd is well tolerated and highly active in patients with newly diagnosed MM. During phase 1, all 3 dose levels of carfilzomib were safely combined with standard doses of Rd. Response rates were excellent at all carfilzomib dose levels with no apparent dose–response relationship. Given the safety and high activity of the CRd regimen with the MPD of carfilzomib (36 mg/m2), the dose-dependent trend of DLTs, the limited experience with carfilzomib at greater dose levels, and the limitations of adding additional dose cohorts during an ongoing trial using the TITE-CRM design, we decided to proceed with phase 2 using the 36 mg/m2 dose rather than amending the protocol to escalate the dose further.
The efficacy data from the combined phase 1 and 2 populations indicated a rapid and deep response with CRd. Analysis of response demonstrated a significant and rapid decline in M-protein levels within the first few cycles. Responses improved as patients continued treatment with the majority achieving nCR or better, which exceeded 75% after treatment of 8 cycles or more. The impact of prolonged treatment on the sCR rate was notable with an exceptional rate of 61% in patients who completed at least 8 cycles. In addition, the depth of response included a significant number of patients suspected to be in CR without evidence of MRD. Although time-to-event data continue to mature, the lack of disease progression in all but 2 patients after a median of 13 months of follow-up as well as all patients who achieved sCR remaining in remission for a median of 9 months suggest that responses were also durable.
There was no notable difference in response when assessed with the International Staging System or the presence of unfavorable cytogenetic factors. Although the proportion of patients achieving at least nCR appears to be lower in the 36 mg/m2 cohort compared with the other dose cohorts, this was likely impacted by the shorter duration of treatment for the MPD cohort, greater proportion of patients proceeding early to transplantation, and the limited number of patients. Response trends during the study suggest that as patients are treated longer with carfilzomib 36 mg/m2, best response data will improve. However, given the sample size, we cannot preclude that as more patients are treated for a longer duration that this trend will dissipate. The response data also suggested that achievement of sCR was dose dependent, but with the small number of patients in the lower dose cohorts and limited follow-up in the 36 mg/m2 dose cohort, it is difficult to definitively establish such a relationship.
Although direct comparison between studies should be viewed cautiously, these response data with frontline CRd compare favorably with results from studies with frontline Rd and RVD. In the phase 3 Eastern Cooperative Oncology Group E4A03 study, transplantation-eligible and -ineligible patients with newly diagnosed MM (N = 445) were randomized to receive at least 4 cycles of treatment with lenalidomide (25 mg) in combination with high-dose dexamethasone or low-dose dexamethasone and followed for a median of 35.8 months.31 After 4 cycles, the rate of patients achieving at least a VGPR was 50% in the high-dose group and 40% in the low-dose group (P = .04), and the rate of at least a PR was 81% and 70% (P = .009). Of 140 patients in the low-dose group who continued Rd after 4 cycles, 57% achieved at least a VGPR and 91% at least a PR with a median duration of treatment of 11.2 months. The PFS rate was 50% at 3 years. In a phase 1/2 dose escalation study of RVD for newly diagnosed MM (N = 66), 39% of patients in the overall population achieved at least a nCR, 67% at least a VGPR, and 100% at least a PR with a median of 10 cycles of treatment9 ; corresponding values in the phase 2 population (n = 35) who received the MTD were 57%, 74%, and 100%. Of note, 28 patients (42%) proceeded to ASCT (13 before the end of cycle 8). With a median follow-up of 21 months, the 18-month PFS rate was 75%.
The CRd regimen was well tolerated, with only 1 patient discontinuing treatment because of toxicity after 1 cycle. Some patients required dose modifications, but the majority were able to maintain dose intensity. It is possible that the ability to tolerate the CRd regimen allowed for prolonged treatment at or near the assigned dose, effectively reducing the disease to nondetectable levels and ultimately reaching rates of CR and sCR that are close to or that possibly exceed rates observed after sequential therapy, including transplantation and posttransplantation consolidation.10,39 The types of AEs generally were consistent with those reported in previous studies, with Rd in newly diagnosed MM,31 single-agent carfilzomib in relapsed and relapsed/refractory myeloma,24-27 and CRd in relapsed/refractory myeloma.28
There were no AEs that would preclude the use of the CRd regimen with carfilzomib dosed at 36 mg/m2. The myelosuppressive effect of the regimen was limited and tolerable, with a small proportion of patients requiring dose modifications to manage these events. Despite the prolonged use of lenalidomide at the originally assigned dose of 25 mg, only 4 patients required a dose reduction of lenalidomide because of myelosuppression, and there was only 1 case of neutropenic fever. Renal toxicity was infrequent and transient. Dyspnea occurred early during the study and appeared to correspond to fluid overload. Once overhydration was addressed, the incidence of dyspnea declined, and no grade 3/4 dyspnea was reported during phase 2. The incidence of DVT/PE may also be mitigated in the future with improved risk stratification and the use of heparin or full-dose warfarin as per current guidelines.40 Most patients experiencing an event were receiving only aspirin (81 mg) prophylaxis from the start of treatment, and in at least 3 cases additional risk factors were retrospectively noted.
Comparison of tolerability and AEs between CRd and RVD or other frontline regimens should also be viewed with caution, but the difference in peripheral neuropathy is notable. In the frontline RVD phase 1/2 study, the rate of sensory neuropathy was 80% for all grades and 2% for grade 3 with rates of 18% and 2%, respectively, for motor neuropathy, whereas in our study the rate for any grade of peripheral neuropathy was only 23% with no grade 3 events. Furthermore, the study investigators attributed most neuropathic events to lenalidomide, although it was deemed to be related to carfilzomib in 1 patient.
The results of this phase 1/2 study are very encouraging but are limited by the sample size, the single-arm nonrandomized design, the lack of independent central review of response results, and a study population that included both transplantation-eligible and -ineligible patients. These results will require validation in the randomized controlled setting to definitively demonstrate the benefit of adding carfilzomib to Rd. A phase 3 trial of CRd compared with Rd for the treatment of patients with relapsed MM (ASPIRE) is ongoing (clinicaltrials.gov ID NCT01080391).
It is worth noting that only 7 of the 35 transplantation-eligible patients proceeded to ASCT. It appears that with the depth and duration of responses observed with frontline combination therapy with a proteasome inhibitor, immunomodulatory drug, and corticosteroid, select patients may opt to defer ASCT for a maintenance regimen until disease progression or intolerable toxicity. This approach is being evaluated in an ongoing Intergroupe Francophone du Myelome/Dana-Farber Cancer Institute randomized study with RVD (clinicaltrials.gov ID NCT01191060) and in a study conducted by the European Network using a sequence of combination regimens (clinicaltrials.gov ID NCT01208766). The tolerability and efficacy data reported here support a similar study with CRd. Conversely, it is of clinical interest to evaluate whether incorporating ASCT into sequential treatment with CRd before and after transplantation may further improve outcomes beyond those reported here. Our observation that CRd does not appear to adversely impact SCC is the first for a carfilzomib combination in the frontline setting and consistent with similar reports of bortezomib regimens.7,9,41 Longer follow-up from our study and results from separate studies will help to determine the most effective strategies for CRd in the frontline setting. Given the challenges of long-term twice-weekly infusions of carfilzomib in the CRd regimen reported here, more convenient dosing schedules will likely be explored in future studies.
In conclusion, the CRd regimen was well tolerated and highly active as frontline therapy in patients with newly diagnosed MM. Long-term follow-up will help to better characterize the durability of the response with this regimen, and the relationship of response to PFS and OS, as well as long-term tolerability. On the basis of our study results, a carfilzomib dose of 36 mg/m2 for CRd treatment appears appropriately tolerated in the frontline setting and should be considered for future studies. The CRd regimen would be a welcomed addition to frontline treatment options. These data support a phase 3 trial to validate the benefit of adding carfilzomib to Rd as a frontline therapy for MM.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients who participated in this study; all of the investigators, nursing staff, and research support staff; and the data management staff at participating sites for their collection and timely entry of trial data. In addition, they acknowledge Sandra Wear and Robert Ott and the Multiple Myeloma Research Consortium (MMRC), under whose auspices this multisite trial was conducted. Finally, they thank Homa Yeganegi (Onyx Pharmaceuticals) and Mohamad Hussein (Celgene Corporation) and the research teams at Onyx Pharmaceuticals and Celgene Corporation. This was an investigator-initiated study that was led by A.J.J. (Principal Investigator) and coordinated by the University of Michigan Comprehensive Cancer Center. They also acknowledge and thank Colleen Harvey for help with data collection and analysis.
This work was supported in part by Onyx Pharmaceuticals, Celgene Corporation, the MMRC, and a University of Michigan Clinical/Translational Resource Allocation Committee (CTRAC) grant. Onyx and Celgene provided carfilzomib and lenalidomide, respectively, free of charge. Editorial assistance was provided by Melanie Watson and Michael Raffin (Fishawack Communications), which was supported by Onyx.
Authorship
Contribution: A.J.J. designed and performed research, contributed vital new reagents or analytical tools, analyzed data, and wrote the paper; D.D. performed research, contributed vital new reagents or analytical tools, and analyzed data; K.A.G. designed research, analyzed data, and wrote the paper; D.L., D.H.V., and S.J. performed research and contributed vital new reagents or analytical tools; A.A.-Z. performed research; T.A., B.N., K.D.-S., K.S.-G., A.A., and T.J. performed research; D.E.D. contributed vital new reagents or analytical tools and analyzed data; K.M. contributed vital new reagents or analytical tools and analyzed data; M.M. performed research and contributed vital new reagents or analytical tools; D.C. performed research; M.K. performed research; and R.V. performed research and contributed vital new reagents or analytical tools.
Conflict-of-interest disclosure: A.J.J. serves as a consultant for Ortho Biotech, Celgene, Millennium, Onyx Pharmaceuticals, Bristol-Myers Squibb, and Exelixis; receives honoraria from Ortho Biotech, Celgene, Millennium, Bristol-Myers Squibb, and Exelixis; serves on speakers' bureaus for Ortho Biotech, Celgene, and Millennium; is a member of the boards of directors for Millennium, Onyx Pharmaceuticals, and Bristol-Myers Squibb and of the advisory committees for Onyx Pharmaceuticals and Bristol-Myers Squibb. D.H.V. serves on the advisory board for, receives honorarium from, and is a member of the speakers' bureau for Celgene. S.J. serves as a consultant for and receives honoraria from Celgene and serves as a consultant for Millenium/Takeda and Merck. R.V. serves as a consultant for and receives research funding from Onyx Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Andrzej J. Jakubowiak, MD, PhD, Professor of Medicine, Director, Myeloma Program, Section of Hematology/Oncology, University of Chicago Medical Center, 5841 S Maryland Ave, MC 2115, Chicago, IL 60637-6613; e-mail: ajakubowiak@medicine.bsd.uchicago.edu.