Abstract
Pulmonary nodules and nodular infiltrates occur frequently during treatment of hematologic malignancies and after hematopoietic cell transplantation. In patients not receiving active immunosuppressive therapy, the most likely culprits are primary lung cancer, chronic infectious or inactive granulomata, or even the underlying hematologic disease itself (especially in patients with lymphoma). In patients receiving active therapy or who are otherwise highly immunosuppressed, there is a wider spectrum of etiologies with infection being most likely, especially by bacteria and fungi. Characterization of the pulmonary lesion by high-resolution CT imaging is a crucial first diagnostic step. Other noninvasive tests can often be useful, but invasive testing by bronchoscopic evaluation or acquisition of tissue by one of several biopsy techniques should be performed for those at risk for malignancy or invasive infection unless contraindicated. The choice of the optimal biopsy technique should be individualized, guided by location of the lesion, suspected etiology, skill and experience of the diagnostic team, procedural risk of complications, and patient status. Although presumptive therapy targeting the most likely etiology is justified in patients suspected of serious infection while evaluation proceeds, a structured evaluation to determine the specific etiology is recommended. Interdisciplinary teamwork is highly desirable to optimize diagnosis and therapy.
Introduction
Lung nodules and nodular infiltrates are common in patients treated for hematologic malignancy (HM) and in patients undergoing hematopoietic cell transplantation (HCT). Various case series suggest that 13% to 60% of patients develop a pulmonary infiltrate at some point in their treatment course, with the incidence varying considerably by different diseases and treatments.1-3 High-resolution CT (HRCT) scans are the preferred method for evaluation of a lung infiltrate over radiography because they are more sensitive in detecting infiltrates earlier and they are more capable of better characterization of the infiltrates. In patients treated for acute leukemia or undergoing HCT, HRCT scans have a high degree of sensitivity (> 85%), high negative predictive value (> 85%) in detecting pneumonia, and a gain of 5 days compared with chest radiography.4 Clinical management is often changed because of the HRCT findings.5
In general, pulmonary infiltrates can be categorized by their radiographic pattern broadly into diffuse and nodular infiltrates. This distinction is useful because the differential diagnostic possibilities are quite different (Table 1). Nodular lesions may be further characterized as solitary micronodules or macronodules with sharp or unsharp margins with or without halos, multiple nodules, masses, nodular infiltrates, and focal airspace disease. In reality, many patients have mixtures of multiple types of infiltrates simultaneously. A systematic approach to evaluation is necessary to determine the cause and develop a sensible management strategy. This report addresses the management of pulmonary nodules and nodular infiltrates and mentions diffuse infiltrates only for comparison.
Major etiologies of pulmonary infiltrates
| . | Diffuse infiltrates . | Nodules/nodular infiltrates . |
|---|---|---|
| Noninfectious | Pulmonary edema | Primary lung cancer |
| Acute respiratory distress syndrome | Lymphomas, Hodgkin lymphoma | |
| Congestive heart failure | Posttransplantation lymphoproliferative disease | |
| Pulmonary hemorrhage, diffuse alveolar hemorrhage | Thromboembolism | |
| Peri-engraftment respiratory distress syndrome | Leukemic infiltrates | |
| Idiopathic pneumonia syndrome | ||
| Interstitial pneumonitis, idiopathic or toxic | ||
| Cryptogenic organizing pneumonitis | ||
| Pulmonary alveolar proteinosis | ||
| Leukemic infiltrates | ||
| Infectious | Viruses (cytomegalovirus, community respiratory viruses, adenovirus, human herpesvirus VI) | Chronic/inactive infectious granulomata |
| P jiroveci | Bacteria, Gram-positive and Gram-negative | |
| Legionella | Nocardia | |
| Mycoplasma | Aspergillus | |
| Mucormycosis agents | ||
| Other molds | ||
| P jiroveci | ||
| Toxoplasmosis | ||
| Strongyloidiasis |
| . | Diffuse infiltrates . | Nodules/nodular infiltrates . |
|---|---|---|
| Noninfectious | Pulmonary edema | Primary lung cancer |
| Acute respiratory distress syndrome | Lymphomas, Hodgkin lymphoma | |
| Congestive heart failure | Posttransplantation lymphoproliferative disease | |
| Pulmonary hemorrhage, diffuse alveolar hemorrhage | Thromboembolism | |
| Peri-engraftment respiratory distress syndrome | Leukemic infiltrates | |
| Idiopathic pneumonia syndrome | ||
| Interstitial pneumonitis, idiopathic or toxic | ||
| Cryptogenic organizing pneumonitis | ||
| Pulmonary alveolar proteinosis | ||
| Leukemic infiltrates | ||
| Infectious | Viruses (cytomegalovirus, community respiratory viruses, adenovirus, human herpesvirus VI) | Chronic/inactive infectious granulomata |
| P jiroveci | Bacteria, Gram-positive and Gram-negative | |
| Legionella | Nocardia | |
| Mycoplasma | Aspergillus | |
| Mucormycosis agents | ||
| Other molds | ||
| P jiroveci | ||
| Toxoplasmosis | ||
| Strongyloidiasis |
What is the differential diagnosis?
Both infectious and noninfectious etiologies can be the cause of nodules and nodular infiltrates. The differential diagnosis varies according to the patient's treatment and immune status. We separate consideration of those receiving active chemotherapy or immunosuppressive therapy who are very immunocompromised (eg, those undergoing induction chemotherapy for acute leukemia or those within the first 6 months after allogeneic HCT or even later if still receiving immunosuppressive therapy for chronic GVHD from those not receiving active chemotherapy or off immunosuppressive therapy after HCT). The latter would also include those who present before treatment and after completion of their course of antineoplastic and/or immunosuppressive therapy, where host defenses are more intact.
Patients not receiving active chemotherapy or immunotherapy
Nodular lesions may be caused by the HM, especially in patients with Hodgkin or non-Hodgkin lymphoma, and much less so for patients with leukemia. Pulmonary nodular lesions are occasionally seen with plasmacytomas as extramedullary manifestations of multiple myeloma. There are case reports of acute myelogenous leukemia (AML) causing pulmonary nodules because of leukemia, but these are infrequent. More commonly, pulmonary infiltrates in patients newly presenting with AML are diffuse and may result from leukostasis in those with high leukocyte counts or even frank leukemic infiltration of tissues, pulmonary hemorrhage, and less commonly infection. Patients newly presenting with AML occasionally present with pulmonary infections. The types of infections that cause nodular infiltrates before start of chemotherapy have not been well described but, in our experience, are mostly bacterial. Both Gram-positive organisms (especially staphylococci and streptococci) and Gram-negative organisms (especially Pseudomonas aeruginosa, Escherichia coli, and Klebsiella spp.) are common.6 Fungal pneumonia is infrequent in newly diagnosed AML at initial presentation and, where seen, occurs predominantly in those with antecedent cytopenias or iron overload.7 However, consideration should be given to the possibility for endemic mycoses in certain high-risk geographic locations, such as coccidiomycosis in the southwest United States, histoplasmosis and blastomycosis principally in the Ohio and Mississippi river basins, and less commonly in other temperate zones.
Nodules in patients not highly immunosuppressed or myelosuppressed may also be caused by the same types of processes that cause pulmonary nodules in nonimmunosuppressed patients. In noncompromised patients, approximately half of nodules are caused by malignancy, chiefly primary lung cancer (usually solitary nodules) or less commonly metastases (usually multiple nodules).8-10 The other half are mostly the result of infectious granulomata from mycobacteria or fungi and much less commonly from other infrequent benign etiologies, such as hamartomas, sarcoidosis, or arteriovenous malformations. The differential diagnosis of pulmonary nodules in patients who are not immunocompromised has been reviewed in multiple publications.8-11 Radiographic features described in “How do I make the diagnosis” can be useful in distinguishing the likelihood of benign or malignant etiology. If there are no manifestations of acute infection, the crucial differential diagnosis should emphasize the exclusion of a malignant process,12 and its evaluation may proceed in a manner similar to that for the noncompromised patient (described in “Patients not receiving active chemotherapy or immunotherapy”).
After completion of antineoplastic therapy, pulmonary nodular lesions may represent residua of infections that occurred earlier during active therapy. Of course, in patients treated for lymphoma, the principal concern is recurrent disease. In the patient undergoing allogeneic HCT, secondary cancers must also be considered. There is a bimodal distribution of types of secondary cancers after HCT. During the first 6 to 12 months, the major concern is EBV-associated posttransplantation lymphoproliferative disorders.13 Risk factors for posttransplantation lymphoproliferative disorders include T-cell depletion of the stem cell graft, use of antithymocyte globulin, use of a mismatched or unrelated donor, cord blood stem cell source, and GVHD.14 The greater the number of risk factors, the greater the likelihood that posttransplantation lymphoproliferative disorders will occur. Epithelial cancers of various types occur at a higher rate than in the general population in survivors after allogeneic HCT, and the risk begins to increase appreciably 10 years after transplantation and thereafter climbs. Recently, an increased risk for lung cancer has been noted.15 Lung cancer has also been noted to occur at increased rates in survivors of Hodgkin and non-Hodgkin lymphoma and chronic leukemia, but this issue has been less well studied.16-19 Other potential noninfectious causes of nodular lesions include toxicity from the antineoplastic therapy, which more commonly causes diffuse fibrosis but in some instances can cause micronodular lesions.20,21
Patients receiving active chemotherapy or immunotherapy
Infections account for most nodular infiltrates in patients receiving active chemotherapy or immunotherapy or have severe compromise in immunity. Bacterial and fungal infections most commonly account for nodular infiltrates, whereas viruses, Pneumocystis jiroveci and Legionella most commonly account for diffuse infiltrates.
Bacterial pneumonia during neutropenia can be caused by both Gram-positive and Gram-negative organisms. Early after HCT, staphylococcus and Gram-negative pathogens are problematic, with a spectrum of pathogens similar to that for newly diagnosed AML as noted in “How do I make the diagnosis.” Later, the likely bacterial pathogens are different: in patients with chronic GVHD after allogeneic HCT, encapsulated bacteria are particularly problematic as causes of sinopulmonary infections.22 Nocardia is another bacterial pathogen that causes pulmonary nodular infiltrates late after HCT.23 Mycobacterial infections are also important but less frequent bacterial pathogens. In advanced countries, atypical mycobacteria are more common; whereas in underdeveloped countries, Mycobacterium tuberculosis is more likely.24 However, in inner city settings in the United States and various regions elsewhere in the world, M tuberculosis infections are becoming more common. Accordingly, in geographic areas with high rates of M tuberculosis, strong consideration should be given to this diagnostic possibility.
Invasive fungal infections, especially by mold pathogens, are particularly common, especially in patients with deep prolonged neutropenia during AML therapy. The onset is frequently later during neutropenia, typically occurring beyond 2 weeks of neutropenia.25 Approximately 45% of nodular infiltrates in AML treatment are the result of aspergillosis.26 In our experience, at least half of nodular infiltrates in AML therapy and HCT are the result of fungi, with 80% of the pulmonary fungal infections caused by aspergillosis, the remainder resulting from other molds, such as the agents of mucormycosis, and less frequently, fusarium, and Scedosporium species.
It is also important to recognize that nodular lesions may be the result of more than one organism. Mixed mold infections (most commonly aspergillosis and mucormycosis) can occur. In addition, aspergillosis can be accompanied by coinfection by bacteria or viruses. This growing recognition of mixed infections emphasizes the need for establishing a specific diagnosis.
Noninfectious causes must also be kept in mind, although they are less common. Noninfectious conditions include primary lung cancer, metastases from other epithelial cancers, lymphomas, EBV-associated posttransplantation lymphomas after HCT, and pulmonary thromboembolism. Rare conditions, such as Wegener granulomatosis, sarcoidosis, and pulmonary arteriovenous malformation, should also be considered.
Occasionally, infections and noninfectious entities that usually cause diffuse infiltrates (mentioned in Table 1) can also cause nodular infiltrates, especially micronodules, or even consolidation. Thus, one should be mindful of these possibilities as well.
How do I make the diagnosis?
Can Imaging distinguish various etiologies?
The HRCT scan is the best imaging technique to evaluate pulmonary infiltrates.27-29 A variety of studies in nonimmunocompromised patients have noted size, location, calcification pattern, change in size over time, edge characteristics, internal characteristics, number of nodules, attenuation, and contrast enhancement as features that provide important information.29 Lesions less than 1 cm are infrequently the result of neoplasm. Larger nodules are more likely to be malignant. Masses (lesions > 3 cm) are highly likely to be malignant. Malignant lesions are more likely to be in the upper lobes, whereas nonmalignant etiologies are more evenly distributed. Calcification is very suggestive of a benign granulomatous etiology if it has an organized diffuse, central, or laminar pattern. Lesions stable for more than 2 years are rarely malignant. Spiculated edges are suspicious for malignancy. Satellite nodules surrounding a central larger nodule are suggestive of granulomatous disease. An air-bronchogram within a nodule is suggestive of malignancy. Cavitation may be seen in both malignant and nonmalignant entities. Ground-glass opacification is suggestive of malignancy, such as adenocarcinoma in situ or minimally invasive adenocarcinoma of the lung. Figure 1 offers illustrative images of nodular lesions commonly observed.
Radiographs of different types of nodular lesions. (A) Solitary nodule. (B) Nodule with surrounding ground-glass halo. (C) Mass-like consolidation with surrounding halo of ground-glass opacity. (D) Peripheral pleural-based nodule with pleural effusion. (E) Large central irregular speculated density with surrounding ground-glass opacity and smaller more peripheral speculated lesion. (F) Cavitary nodule with sequestrum (air-crescent sign).
Radiographs of different types of nodular lesions. (A) Solitary nodule. (B) Nodule with surrounding ground-glass halo. (C) Mass-like consolidation with surrounding halo of ground-glass opacity. (D) Peripheral pleural-based nodule with pleural effusion. (E) Large central irregular speculated density with surrounding ground-glass opacity and smaller more peripheral speculated lesion. (F) Cavitary nodule with sequestrum (air-crescent sign).
Using such considerations, in noncompromised patients, lesions can categorized as demonstrating low, indeterminant, or high probability of malignancy. Those lesions judged to be high risk should undergo resection. Those at low risk (small < 8 mm, benign calcification pattern, stable over time, no smoking history) can be followed over time.30 Those judged to be indeterminant require further evaluation.
PET scans have been shown to be quite useful in the evaluation of the solitary pulmonary nodule (SPN) in the nonimmunocompromised patient.31-33 PET scans may be of particular value in lesions of indeterminant significance34 because malignancies typically have high uptake (> 2.5 SUV). PET scans have been found to be useful in lymphoma patients to distinguish active disease from inactive scar.35 However, PET has not been found to be useful for small lesions less than 1 cm because of false-negative results. Those at high risk require resection or biopsy. Infectious etiologies may also have high signal uptake by PET.36 Imaging using MRI and PET technologies has been poorly studied in the evaluation of new nodules and nodular infiltrates during active chemotherapy or immunotherapy in the hematologic malignancy and HCT setting where infection is highly likely.37,38
A number of studies have evaluated imaging findings as they relate to specific etiologies in patients treated for HM or undergoing HCT. For bacterial pneumonias, airspace consolidation is most common, but small centrilobular nodules and ground-glass opacities are also common; large nodules also are seen but are less common (20%).39-41 The halo sign appears to be the most useful radiologic sign for distinguishing aspergillosis from bacteria.40
In patients with invasive aspergillosis (IA), one large study, in mostly HM and HCT therapy, found that 94% had one or more macronodules (at least 1 cm in diameter).42 In 79% of cases, the nodules were multiple, and in 60%, there were multiple bilateral nodules. Halo signs (dense nodules surrounded by ground-glass perimeter) were found in 61%. Other findings noted with IA were consolidation (30%), infarct-shaped nodules (27%), cavities (20%), and air-crescent signs (10%). The nodular lesions of IA are often peripherally located.43 The presence of dense, well-circumscribed nodule, air-crescent sign, or cavity has been adopted as specific radiologic criteria that along with appropriate clinical setting and with supporting microbiology criteria establish the diagnosis of IA in consensus guidelines.44 In earlier studies, serial HRCT scans were performed in patients with IA: halo signs were most likely to be found early in infection, air-crescent signs much later, typically found at time of neutrophil recovery.45,46 Although IA is the most likely cause of halo lesions in this population, it is important to note that the halo is now known to not be specific for IA: other pathogens, such as P aeruginosa, the agents of mucormycosis, and other less common molds can also give rise to halo infiltrates.47 Mucormycosis can present with pulmonary nodules or nodular infiltrates similar to IA. There may be some distinguishing findings on CT scan. Comparing IA and mucormycosis, the presence of more than 10 nodules, pleural effusion, or sinus involvement, and a history of prior voriconazole use (an antifungal active against Aspergillus species but not mucormycosis) were conditions more likely to be found with mucormycosis than with IA.48,49 Important to note is that, although pulmonary nodules are highly likely in IA, other less-specific radiologic manifestations can also be caused by IA, including consolidation, ground-glass infiltrates, and occasionally pleural effusion.50 The reversed halo (circular focus of ground-glass density within a ring of dense consolidation) was initially described as highly suggestive of mucormycosis and subsequently also IA, but other infectious and noninfectious etiologies may also present with this radiologic picture.47,51
In studies of patients with AML who were neutropenic and patients who underwent HCT with lung opacities more than 5 mm, both IA and bacterial pneumonia were manifest frequently by both nodules and air-space consolidation in similar proportions.52,53 The halo sign was rarely seen in bacterial pneumonia, but cavities and air-crescent signs were present in both. A recent study suggests a role for CT pulmonary angiography in the diagnosis of pulmonary mold infections, exploiting the fact that such infections are angioinvasive.54
Can other noninvasive techniques be helpful?
Gram stain and culture of sputum should be performed if sputum is being expectorated; unfortunately, the patient who is neutropenic is frequently unable to expectorate sputum. Bacterial and fungal blood cultures should be performed; when positive, they are helpful; however, most have negative blood cultures. Mycobacterial blood cultures should also be considered in the evaluation of nodular lesions in non-neutropenic patients.
There are 2 commercial serum assays to assist in the diagnosis of invasive fungal infections. The serum galactomannan (GM) and β-glucan tests are useful in detection of invasive fungal infections. The serum GM test has been widely studied in the AML and HCT settings. GM is a constituent of the cell wall of Aspergillus species, and its presence in serum is strongly suggestive of IA. Its sensitivity and specificity have been found to be more than 80% in AML and HCT patients in the detection of IA.55 It is important to note that the test is recommended to be performed twice weekly prospectively in patients at risk, and the sensitivity and specificity of a single test result drawn at the time of a lung infiltrate are less. There are both false positives and false negatives reported. The most common reason reported for false-positive results is the concomitant use of piperacillin-tazobactam.56 There are suggestions recently that this may be less problematic, but more data are needed on this point. The use of antimold prophylaxis attenuates the value of the GM assay.57 There is also some cross-reactivity with penicillium (a rare pulmonary pathogen in the United States and Europe) and other endemic fungi, such as histoplasma and blastomyces, potential pathogens in certain geographic regions.58,59
β-glucan is also a cell wall constituent, and its presence in the blood occurs in invasive fungal infections. The serum glucan test is highly sensitive but less specific than the GM test, being positive in invasive infections by Candida, Aspergillus, Fusarium, Trichosporon, and Pneumocystis species. It has been best studied in Candida fungemia.60
Neither of these fungal serologic tests can detect the agents of mucormycosis. Although fungal PCR assays for various fungi are under study, none is commercially available.
Even given the limitations of the 2 commercial serum fungal assays, we recommend their use. When positive, we launch an investigation in search of further confirmation of an invasive fungal infection. Even when negative, if either the clinical scenario and/or imaging suggest fungal pneumona, we recommend proceeding to invasive techniques in search of an invasive fungal infection or to establish a specific alternative diagnosis.
Invasive techniques to establish the diagnosis
Invasive testing by bronchoscopic evaluation or acquisition of tissue by one of several biopsy techniques is usually needed to establish the diagnosis and should be done unless contraindicated. Studies have shown that, in highly immunosuppressed patients with HM or undergoing HCT, adjustments in antimicrobial therapy are frequently made as a result of invasive testing.6,12,61,62 The optimal type of invasive modality should be individualized. Factors that should guide the appropriate choice of procedure are location of the lesion, characteristics of the lesion, clinical presentation, the suspected etiology, the skill of the diagnostic team, risk of complications, and the patient's ability to undergo the procedure. Several diagnostic approaches are available.
Invasive procedures for evaluation of SPNs in the nonimmunocompromised patient include transthoracic needle aspirate (TTNA), flexible bronchoscopy (FB), or surgical resection by video-assisted thoracoscopic surgery (VATS) or open thoracotomy. Each procedure has strengths and limitations, and there are additional considerations for their usefulness in the HM and HCT patient population (Table 2).
Invasive diagnostic techniques
| Procedure . | Yield . | Risk . | Limitations and comments . | ||
|---|---|---|---|---|---|
| Noncompromised . | Hematologic malignancy and HCT . | Noncompromised . | Hematologic malignancy and HCT . | ||
| Bronchoscopy with BAL | 10%-50%62 | 15%-60% (90% for IPA if use noncultural diagnostics)60,61,72-74 | Major complications < 1%-35%, severe 10%62,70,71 | Most complications minor (< 2%-28%); major complications (< 1%-8%)6,60,61,74,75 ; 10% life-threatening complications if respiratory compromise67 | Higher yields with guided bronchoscopic techniques |
| Not suitable for patients with poor lung reserve | |||||
| Transthoracic needle biopsy | 80%-90%62,63 | 50%-78%25,46,66 | Bleeding, pneumothorax (15%-24%, chest tube (6%-7%)64,65 | 15%-38% with 6%-16% requiring chest tube25,46,66 | Lesions optimally should be peripheral Better with larger lesions (> 1 cm) |
| Not suitable for patients with poor lung reserve and thrombocytopenia | |||||
| Open biopsy/excision | Gold standard62 | 16%-60%45,46,85,86 | Considerable morbidity and mortality | 13%-21%; 30 day mortality 2%-45%45,46,85,86 | Not suitable for patients with poor lung reserve and thrombocytopenia |
| Video-assisted biopsy | Comparable with open lung biopsy62,87 | Not adequately studied | 10% morbidity, < 1% mortality87,88 | Not adequately studied | Not suitable for central or large (> 3 cm) lesions, lesions < 1 cm, deeper in lung parenchyma |
| Ground-glass lesions may require localization procedure | |||||
| Procedure . | Yield . | Risk . | Limitations and comments . | ||
|---|---|---|---|---|---|
| Noncompromised . | Hematologic malignancy and HCT . | Noncompromised . | Hematologic malignancy and HCT . | ||
| Bronchoscopy with BAL | 10%-50%62 | 15%-60% (90% for IPA if use noncultural diagnostics)60,61,72-74 | Major complications < 1%-35%, severe 10%62,70,71 | Most complications minor (< 2%-28%); major complications (< 1%-8%)6,60,61,74,75 ; 10% life-threatening complications if respiratory compromise67 | Higher yields with guided bronchoscopic techniques |
| Not suitable for patients with poor lung reserve | |||||
| Transthoracic needle biopsy | 80%-90%62,63 | 50%-78%25,46,66 | Bleeding, pneumothorax (15%-24%, chest tube (6%-7%)64,65 | 15%-38% with 6%-16% requiring chest tube25,46,66 | Lesions optimally should be peripheral Better with larger lesions (> 1 cm) |
| Not suitable for patients with poor lung reserve and thrombocytopenia | |||||
| Open biopsy/excision | Gold standard62 | 16%-60%45,46,85,86 | Considerable morbidity and mortality | 13%-21%; 30 day mortality 2%-45%45,46,85,86 | Not suitable for patients with poor lung reserve and thrombocytopenia |
| Video-assisted biopsy | Comparable with open lung biopsy62,87 | Not adequately studied | 10% morbidity, < 1% mortality87,88 | Not adequately studied | Not suitable for central or large (> 3 cm) lesions, lesions < 1 cm, deeper in lung parenchyma |
| Ground-glass lesions may require localization procedure | |||||
TTNA
TTNA has a high yield for the evaluation of the SPN in the noncompromised patient, approaching 80% to 90% and thus is generally preferred for tissue biopsy.63,64 TTNA is particularly useful in more peripherally located lesions. The yield is high for malignancy but much lower for benign lesions.63-65 Bleeding and pneumothorax are the major risks. Pneumothorax occurs in 15% to 24% of instances and requires a chest tube in 6% to 7%.65,66 Thus, TTNA would be ill advised in patients with poor lung reserve. Studies in HM and HCT patients indicate a lower yield in the range of 50% to 78%, where the likelihood of malignant etiologies is less.12,53,67 The complication rate is also higher, with complications occurring in up to 38% with chest tube being required in up to 16% (Table 2).12,53,67 TTNA is more likely to yield a definitive diagnosis with larger (> 1 cm) and cavitary lesions and is probably of more benefit in patients with lymphoma, where malignancy is more likely than with AML.
FB
FB has had a more limited role in the evaluation of the SPN in the noncompromised patient with lower yields in the range of 10% to 50%,63 but it can be useful in the evaluation of central airway lesions or mediastinal adenopathy. Navigation-guided bronchoscopy techniques have been evaluated in recent years and have been found to have higher yields, approximately 70% in a pooled analysis.68 However, FB with bronchoalveloar lavage (BAL) is a common approach for evaluation of nodular and diffuse infiltrates in HM and HCT patients. The reason is the low likelihood of serious complication, such as hemorrhage or pneumothorax. The presence of symptoms, location more centrally, presence of bronchus sign on CT, and visualization during bronchoscopy are associated with higher yields.69 Major complication rates have been low in noncompromised patients ranging between less than 1% to as high as 38%,63,70,71 with severe complications typically 10% or less.
The yield of FB in patients with HM or undergoing HCT before the advent of nonculture-based diagnostics varied widely, in the range of 15% to 60%.61,62,72-74 The yield was found to be substantially higher when performed promptly at the onset of pulmonary infection rather than later (87% vs 35%).61 Changes in therapy were made as a result of the FB in 51% to 65% of patients.61,62 The diagnostic yield was higher in patients with focal infiltrates compared with diffuse infiltrates (64% vs 47%).61
FB is associated with low major complications in the HM and HCT setting: less than 2% to 27% overall and less than 1% to 8% major.6,61,62,74,75 In general, FB has a lower risk for pneumothorax compared with TTNA. Bronchoscopic transbronchial biopsy (TBBx) has the potential to increase the yield75 but also increases the risk of hemorrhage and pneumothorax, and its utility remains controversial in this area. TBBx should generally be avoided in patients with significant thrombocytopenia. One study noted a yield of 55% for TBBx and 20% for BAL in patients with HM.75 In another study of non-HIV–immunosuppressed patients, of whom 46% had a HM, the diagnostic yield of BAL was 38%, TBBx was 38%, and both combined was 78%.76 Other studies, however, have not noted a significant improvement in diagnostic yields by including TBBx. In one HCT study, the diagnostic yield of FB with TBBx improved in only 8% of patients compared with BAL alone.61 Another HCT study noted that TBBx provided additional specific information in less than 10% of cases when added to BAL.77 In a study of neutropenic patients with infiltrates, TBBx added information in only 1 of 9 patients.78 The aforementioned studies did not include nonculture-based diagnostics. It is important to note, as mentioned, that there may be a higher risk of life-threatening complications with FB in patients treated for HM or undergoing HCT in the ICU with severe respiratory complications: in one study, 12 of 121 (10%) of patients had a life-threatening complication from FB.79 The high complication rate was compounded by low diagnostic yields for infectious etiologies: only 23% in patients with AML and 41% in patients with lymphoid malignancies.
Use of noncultural microbial testing can also increase the yield of BAL. The use of BAL GM testing26,80 has been associated with substantially increased yield in patients with IA. In one trial, BAL GM in patients with hematologic diseases had a sensitivity of 91% compared with culture and microscopy (50%-55%).26 Other studies have also noted the utility of BAL GM for diagnosis of IA.81 In a meta-analysis of BAL GM, summary estimates of the BAL-GM assay for proven or probable IA were as follows: sensitivity, 90%; specificity, 94%; positive likelihood ratio, 14.87; and negative likelihood ratio, 0.10.82 The estimates of the BAL GM assay for proven IA were as follows: sensitivity, 94%; and specificity, 79%.82 Analysis of β-glucan in BAL specimens in diagnosing fungal pneumonia in humans has not been evaluated as yet. Although not as yet used routinely in clinical practice, PCR-based testing of BAL specimens for IA is being investigated.83,84
Although the yield of FB in the diagnostic yield of focal lesions has been reported to be lower than TTNA in earlier studies, the use of CT guidance and the incorporation of newer diagnostic assays are pushing diagnostic yields of FB higher and make the need for adding a transbronchial biopsy to BAL unnecessary in many cases. Biopsy seems to be of best use in the diagnostic evaluation of lesions suspected to be malignancy, leukemic infiltrates, post-transplantation lymphoproliferative disorder, toxic pneumonitis, or cryptogenic organizing pneumonia (previously known as bronchiolitis obliterans organizing pneumonia) and is likely superior to BAL when those conditions are suspected.85 In our view, the greater safety of FB along with comparable yield has shifted the preference from TTNA and surgical lung biopsy to FB in most situations in the highly immunosuppressed HM and HCT setting, particularly where infection is most likely.
VATS or open thoracotomy
If the lesion cannot be diagnosed by either FB with BAL/TBBx or TTNA, VATS and open lung biopsy are options. Open lung biopsy offers the opportunity to examine a large piece of tissue and would be expected to give the greatest likelihood of establishment of the diagnosis.63 It is accompanied by considerable morbidity and occasionally mortality, and it is not a good option for patients with poor lung reserve and thrombocytopenia. Notwithstanding, the diagnostic yield in patients with HM has varied considerably in different reports, generally approximately 60%.52,53,86,87 However, it is associated with considerable morbidity and risk for serious complications. Its morbidity and particular danger in those with thrombocytopenia and reduced lung reserve make it much less appealing in most instances. VATS has supplanted the more extensive open thoracotomy lung biopsy in many instances because of fewer complications and shorter recovery time while preserving similar rates of yield.88,89 VATS is not suitable for centrally located lesions or large lesions more than 3 cm. Thoracotomy would be indicated for such lesions.
The choice of the diagnostic intervention must balance risk with likelihood of clearly establishing the diagnosis. In general, the involvement of a multidisciplinary team consisting of pulmonologist, radiologist, infectious disease specialist, and, if needed, thoracic surgeon, can facilitate reaching the best decision as to the optimal invasive procedure.
What are sensible evaluation/treatment strategies?
Patients not receiving active chemotherapy or immunotherapy
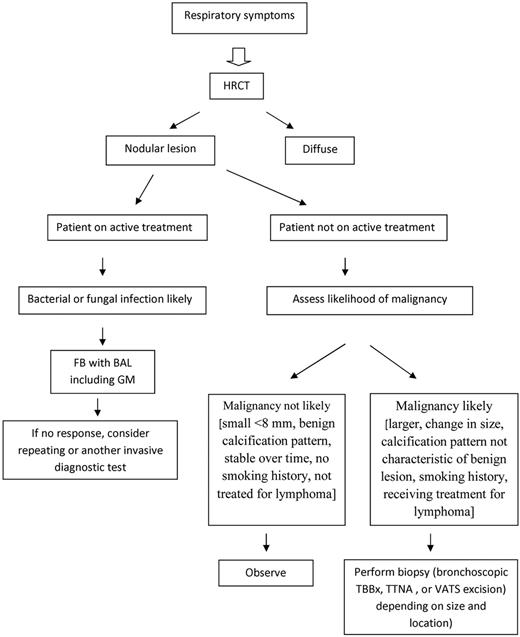
Drawing on lessons from noncompromised patients, after detection of a SPN in patients not on active therapy, there are 3 choices: (1) observe, (2) biopsy, or (3) surgical resection. The overarching principle is to aggressively pursue diagnosis and resection if the likelihood of cancer is substantial. Consensus guidelines have been developed to provide a structured approach in noncompromised patients.28,30,63 In patients with HM, particularly patients with lymphoma who have completed therapy, malignancy similarly is more likely than infection.12 Figure 2 offers a diagnostic algorithm to consider.
Patients receiving active chemotherapy or immunotherapy
Infections resulting from aggressive bacteria and fungi are key concerns. Prompt initiation of antimicrobial therapy is crucial. While evaluation is underway, presumptive therapy with broad-spectrum antibiotics is warranted and should generally follow the recommendations for healthcare-associated pneumonia90 or one of the consensus guidelines,91,92 modified by local antibiotic susceptibility patterns. We also recommend presumptive antimold therapy until the etiology is established while the evaluation proceeds. Early diagnosis and timely initiation of therapy have been shown to improve treatment outcomes for IA42,93 and mucormycosis.94 Some clinicians focus on the use of broad-spectrum antibiotics and antifungal therapy and do not pursue invasive diagnostic procedures unless the patient does not respond to the initial therapy. The downside for this approach is the uncertainty of the diagnosis, the need to subject the patient to therapy that he/she may not need along with its attendant costs and toxicities, and the possibility that the empirically chosen treatment may not target the true pathogen(s). We do not favor presumptive therapy as a substitute for invasive procedures but rather think that both are important.
The development of sensitive noncultural diagnostics has improved the yield of BAL.26,80-82,85 The use of HRCT to guide the optimal site for collection of BAL specimens has also been contributory to improved yields. Moreover, the delay in establishing a diagnosis not covered by the presumptive therapy is likely to result in poorer treatment outcome because delays are associated with lower responses.42,61,93,94 Further, delayed investigation is associated with lower diagnostic yields.61 A study of early (within 4 days of presentation) versus late bronchoscopy in HCT patients found a 2.5-fold higher yield compared with later bronchoscopy61 and greater mortality in patients subjected to late FB. The yield was highest (75%) when bronchoscopy was performed within 24 hours of presentation. For these reasons, we urge performance of an invasive procedure at presentation (Figure 1) rather than waiting to determine response to initial therapy, pursuing an invasive diagnostic only in those who are not responding.
How should I assess the response to therapy?
The first diagnostic decision is important, but it may not be the last diagnostic decision because the results of the tests and the subsequent clinical course to therapy may dictate the need for a second diagnostic intervention. For patients with small lesions at low risk for active infection or malignancy in which the initial decision was to observe, additional scanning at 2 to 3 months is advisable.
For those who were found to have an active infection, response to therapy dictates the type and frequency of additional subsequent testing. If clinically responding, repeat imaging should be done periodically until the infection is resolved. It is important to note that infiltrates may take several weeks to 1 to 2 months to fully resolve; thus, radiology by itself should not dictate the need for further diagnostic interventions. It is well recognized that the infiltrates in patients with IA worsen over the first week of therapy, even though with continued therapy, the patients respond.45 The patient with IA who is not clinically improving is the most challenging situation.95,96 The patient may have clinical and or radiologic deterioration, even while microbiologically responding. In large part, this is related to the changing immune status of the patient. For example, neutrophil recovery or withdrawal of immunosuppressive therapy may exacerbate inflammatory responses, leading to larger pulmonary infiltrates, persistent or worsening fever, and clinical manifestations, the so-called immune reconstitution syndrome. For patients with IA, it has been proposed that serial GM testing can be helpful in distinguishing those truly not responding and those who are responding in the face of clinical worsening.97 Early data are promising for this approach, but further study is needed.
If there is doubt as to whether the patient is responding or not, the diagnostic tests should be repeated. This is important because some infections are mixed and sometimes the initial assessment may not be conclusive. For example, in a patient who is documented to have IA at initial assessment but is not clinically responding and the radiography is not improving, consideration for a mixed infection by a respiratory virus, cytomegalovirus, bacteria (especially staphylococcus and pseudomonas), or another mold, such as the agents of mucormycosis, should be entertained and investigation to exclude these is warranted.
In conclusion, lung nodules and nodular infiltrates are often caused by malignancy or aggressive bacterial or mold infections. The principal goal of evaluation in patients not highly myelosuppressed or immunosuppressed is to distinguish malignant from nonmalignant causes, where relapse of the HM or a new primary lung cancer are major concerns. For patients receiving active chemotherapy or immunotherapy, acute infection is the chief concern. Where possible, a specific diagnosis should be established to optimize outcomes. The choice of the diagnostic intervention must balance risk with likelihood of clearly establishing the diagnosis. In general, the involvement of a multidisciplinary team consisting of an oncologist, pulmonologist, radiologist, infectious disease specialist, and, if needed, thoracic surgeon, can facilitate reaching the best decision as to the optimal invasive procedure and treatment strategy.
Authorship
Contribution: J.R.W. wrote the paper; and J.W.H. and M.A.J. edited and contributed to the text and tables.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John R. Wingard, University of Florida College of Medicine, Division of Hematology, PO Box 100278, Gainesville, FL 32610-0278; e-mail: wingajr@ufl.edu.