Key Points
ROSAKIT WT is a new human SCF-dependent FcεRI-positive mast cell line that converts to SCF-independence by KIT D816V-transfection.
The FcεRI-positive ROSAKIT D816V clone is a major tool for studying cellular aspects of mastocytosis and responses to targeted drugs.
Abstract
In systemic mastocytosis (SM), clinical problems arise from factor-independent proliferation of mast cells (MCs) and the increased release of mediators by MCs, but no human cell line model for studying MC activation in the context of SM is available. We have created a stable stem cell factor (SCF) –dependent human MC line, ROSAKIT WT, expressing a fully functional immunoglobulin E (IgE) receptor. Transfection with KIT D816V converted ROSAKIT WT cells into an SCF-independent clone, ROSAKIT D816V, which produced a mastocytosis-like disease in NSG mice. Although several signaling pathways were activated, ROSAKIT D816V did not exhibit an increased, but did exhibit a decreased responsiveness to IgE-dependent stimuli. Moreover, NSG mice bearing ROSAKIT D816V-derived tumors did not show mediator-related symptoms, and KIT D816V-positive MCs obtained from patients with SM did not show increased IgE-dependent histamine release or CD63 upregulation. Our data show that KIT D816V is a disease-propagating oncoprotein, but it does not activate MCs to release proinflammatory mediators, which may explain why mediator-related symptoms in SM occur preferentially in the context of a coexisting allergy. ROSAKIT D816V may provide a valuable tool for studying the pathogenesis of mastocytosis and should facilitate the development of novel drugs for treating SM patients.
Introduction
Mast cells (MCs) are tissue-resident cells that play an important role in inflammatory and allergic reactions.1 Two types of human MCs have been described: MCT containing only tryptase, and MCTC containing substantial amounts of tryptase and chymase.2,3 MCT cells are mostly found in mucosa, and MCTC cells are more commonly detected in the connective tissues.4-7 The development of tissue MCs from hematopoietic progenitors is regulated by stem cell factor (SCF), the ligand of KIT.8-10 MCs express large amounts of vasoactive and immunomodulatory mediators as well as high-affinity receptors for immunoglobulin E (IgE; FcεRI). As a result, MCs are potent effector cells of type I allergic reactions.11-13 Moreover, MCs play a pivotal role in the initiation and regulation of immune responses against various pathogens.14-17
The MC lineage can give rise to a distinct myeloid neoplasm, namely mastocytosis, characterized by MC expansion in one or more organ systems.18-21 In mastocytosis patients, clinical symptoms result from MC-derived mediators and from the destructive invasion of MCs into various organs.22 However, the exact mechanisms underlying MC activation in systemic mastocytosis (SM) remain unknown. In SM, the KIT D816V mutation in the catalytic domain of KIT is found in up to 90% of all patients.23 This mutant initiates a number of signal transduction events and is thought to trigger abnormal MC activation in patients with SM. However, not all patients with SM suffer from these symptoms, even if their neoplastic MCs display KIT D816V. Other studies have shown that such mediator-related symptoms are seen especially in SM patients who suffer from a coexisting allergy.22,24-26
Studies of the biology of human MCs have mainly been conducted by using MC lines or MCs obtained from primary cultures of CD34+ cells.27 The only 3 human MC lines available so far are HMC-1,28 LAD2,29 and LUVA.30 None of these cell lines coexpress KIT D816V and a functional IgE receptor. Here, we describe the establishment and characterization of a novel FcεRI-positive SCF-dependent human MC line, ROSAKIT WT, and of a factor-independent subclone, ROSAKIT D816V, produced by stably expressing KIT D816V in ROSAKIT WT cells. ROSAKIT D816V cells expressed the IgE receptor and engrafted NOD/SCID IL-2Rγ−/− (NSG) mice. However, unexpectedly, the responsiveness of ROSAKIT D816V cells to IgE-dependent stimuli was weak when compared with normal MCs, ROSAKIT WT cells, or normal basophils.
Materials and methods
Establishment and characterization of a new human SCF-dependent MC line, ROSAKIT WT
A sample of normal umbilical cord blood (CB) was collected after informed consent was given by the patient in September 2009 (Clinic Ambroise Paré, Bourg-la-Reine, France). Mononuclear cells were separated by density centrifugation (Ficoll-Hypaque; GE Healthcare). CD34+ cells were enriched by immunomagnetic sorting by using the MACS system (Miltenyi Biotec) and were cultured in the presence of recombinant human SCF (rhSCF) (80 ng/mL; R&D Systems) exactly as described.27 After 8 weeks, cells continued to grow in culture with virtually all cells resembling MCs.
The complete coding sequences of the KIT gene expressed in ROSA cells were analyzed as previously described31 and were found to be normal (wild-type). The resulting SCF-dependent MC line was termed ROSAKIT WT. This cell line was further transfected with KIT D816V, converting it into an SCF-independent clone, ROSAKIT D816V (see supplemental Methods available on the Blood Web site).
Morphologic and immunocytologic characterization of the ROSA cell lines
The 2 cell lines were analyzed by microscopy on cytospin preparations stained with May-Grünwald Giemsa (MGG) or toluidine blue, or on MGG-stained smears produced from a cell pellet. Transmission electronic microscopy (TEM) was performed on ROSAKIT WT cells by using standard techniques.32 Immunocytochemical staining was carried out on cytospin preparations of ROSA cells by using antibodies (Abs) against tryptase, chymase, and KIT (CD117) as described.33
Analysis of the effects of various cytokines on ROSA cells
In the first set of experiments, ROSAKIT WT or ROSAKIT D816V cells (3 × 105 per milliliter in 96-well plates; 100 μL per well) were incubated in medium with or without rhSCF (80 ng/mL) at 37°C and 5% CO2 for 4 days. On days 1, 2, 3, and 4, 10 μL of WST-1 (Water Soluble Tetrazolium salt-1; Roche Applied Science) were then added to the corresponding wells, and the cells were incubated for 3 additional hours at 37°C. The numbers of viable cells were measured by reading the absorbance at 570 nm using a Multiskan-MS plate reader (Thermo Labsystems). Because rhSCF has no significant (increasing) effects on the growth of ROSAKIT D816V cells, we treated ROSAKIT WT (cells with or without SCF) or ROSAKIT D816V (cells without SCF) with various cytokines, each at 100 ng/mL: interleukin-3 (IL-3), IL-4, IL-6, IL-9, interferon gamma (IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF), and nerve growth factor (NGF) (all from R&D Systems) at 37°C and 5% CO2 for 3 days. Then, 10 μL of WST-1 was added, and the cells were incubated for 3 additional hours at 37°C before the numbers of viable cells were measured.
Assays of cell activation and mediator release in ROSA cells
ROSAKIT WT or ROSAKIT D816V cells were first primed with recombinant human IL-4 (20 ng/mL; BioLegend) and human monoclonal IgE (2 μg/mL; Calbiochem) for 5 days to enhance the expression of FcεRI before stimulation.27 To determine the expression of CD203c and β-hexosaminidase release, cells were incubated in control medium or medium containing anti-human IgE (5 µg/mL) (BioValley) or 1 µM of calcium ionophore (Cai) A23187 (Sigma) for 1 hour. Then, cells were stained by an allophycocyanin-conjugated anti-human CD203c Ab. The fluorescence intensity was measured by using a FACSCalibur flow cytometer (BD Biosciences). For the β-hexosaminidase release assay, stimulated cells were centrifuged, and β-hexosaminidase activity was measured by spectrophotometry in the supernatants and in cell lysates.34 The percentage of β-hexosaminidase release was determined as described.35 To determine histamine release, cells were incubated in control buffer or buffer containing mouse anti-human IgE (clone Depsilon2; Immunotech) (0.1 to 10 µg/mL) for 30 minutes at 37°C. Thereafter, cells were centrifuged, and the histamine content of the cell-free supernatants and the cell pellets was analyzed by radioimmunoassay (Immunotech). The percentage of histamine release was calculated as described.36 To determine prostaglandin D2 (PGD2) and tumor necrosis factor α (TNF-α) release, cells were incubated in control medium, anti-IgE Ab (5 or 10 µg/mL), or Cai (1 µM) at 37°C for 1 hour (for PGD2 release) or 6 hours (for TNF-α release) and then centrifuged. PGD2 and TNF-α levels were determined in the supernatants by using a specific enzyme immunoassay for PGD2 (Cayman Chemical) and a specific enzyme-linked immunosorbent assay for TNF-α (R&D systems) according to the manufacturers’ instructions.
Western blotting
HMC-1.2 cells exhibiting 2 KIT point mutations, V560G and D816V (kindly provided by Dr J. H. Butterfield, Mayo Clinic, Rochester, MN), ROSAKIT WT cells, and ROSAKIT D816V cells were incubated in control medium or with rhSCF (80 ng/mL) for 5 minutes. Western blots were performed with Abs against total or phosphorylated STAT5 (all from Santa Cruz Biotechnology), total KIT, phosphorylated KIT, and total or phosphorylated AKT (all from Cell Signaling Technology). A mouse monoclonal IgG1 anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Ab (Santa Cruz Biotechnology) was used as loading control.
Assessment of the antiproliferative effects of tyrosine kinase inhibitors on ROSA cells
Cells (3 × 105 per milliliter; 100 μL per well) were incubated in 96-well plates with 1 μM of imatinib, 1 µM dasatinib (both supplied by Sequoia Research) or 1 µM midostaurin (Sigma) diluted in dimethylsulfoxide (DMSO; 0.1% final) or with DMSO alone (0.1%) in serum-free medium (with rhSCF at 80 ng/mL for ROSAKIT WT) at 37°C (5% CO2) for 48 hours. The number of viable cells was then determined in each condition as described in supplemental Methods.
Xenotransplantation of ROSA cells in NSG mice
ROSAKIT WT or ROSAKIT D816V cells (0.4 to 5 × 106) were either injected into the tail vein (intravenously) or transplanted under the skin (subcutaneously) bilaterally dorsocaudal of the scapula (3 to 5 mice per group) of adult NSG mice (The Jackson Laboratory). NSG mice were irradiated (2.4 Gy) 24 hours prior to intravenous injection. After injection, mice were inspected daily and euthanized as soon as disease-related symptoms appeared (intravenous group), the tumor grew to a maximum of 1 cm in diameter (subcutaneous group), or after a maximum observation period of 6 months. Bone marrow (BM) cells were obtained from flushed femurs, tibias, and humeri. Subcutaneous tumors were collected and dispersed by collagenase digestion. In addition, BM, lung, liver, spleen, and tumor samples were prepared for histologic and immunohistochemical analyses (supplemental Methods).
Flow cytometry analyses of ROSA cells
ROSAKIT WT cells and ROSAKIT D816V cells were analyzed by flow cytometry using a panel of monoclonal Abs directed against various leukocyte differentiation antigens (supplemental Table 1). In select experiments, xenotransplanted ROSA cells were quantified in BM samples and tumor dispersates obtained from NSG mice by flow cytometry as described37 by using phycoerythrin-labeled monoclonal Abs directed against human CD117 (BioLegend) and CD45 (BD Biosciences). Engrafted ROSA cells were defined as CD45+/CD117+ cells (at least 0.1% of all nucleated cells) detectable in mouse BM cell suspensions.
Statistics
Unless otherwise stated, data represent the mean values ± standard deviation of at least 3 independent experiments. When appropriate, data were analyzed by using the Student t test. Differences were considered as statistically significant if P < .05.
Animal studies
Animal studies were approved by the ethics committee of the Medical University of Vienna and were carried out in accordance with guidelines for animal care and protection and protocols approved by Austrian law (GZ 66.009/0040-II/10b/2009). This study was conducted in accordance with the Declaration of Helsinki.
Results
Establishment and growth characteristics of ROSAKIT WT, a new SCF-dependent human MC line, and of its SCF-independent subclone ROSAKIT D816V
ROSAKIT WT cells have a doubling time of 24 hours, and are split every 3 to 4 days. Optimal growth is obtained at 2 × 105 cells per milliliter in rhSCF (80 ng/mL). Because the KIT D816V mutant is commonly found in neoplastic MCs in patients with SM,38 we transfected ROSAKIT WT cells with a lentiviral vector encoding KIT D816V. The resulting SCF-independent line, named ROSAKIT D816V, exhibited the same doubling time as ROSAKIT WT cells cultured with rhSCF (80 ng/mL). The two ROSA cell lines have been deposited to the Collection National de Cultures de Microorganismes (CNCM, Institut Pasteur, Paris), under the number CNCM I-4551 for the KIT WT cell line and CNCM I4552 for the KIT D816V+ cell line. In addition, the two ROSA cell lines have been patented (Patent WO/2013/064639).
ROSAKIT WT and KIT D816V cells are MCs by morphology, TEM, and phenotype
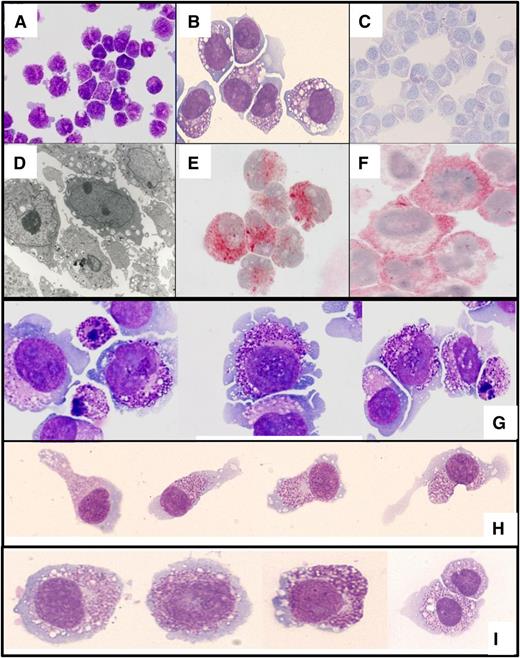
MGG-stained ROSAKIT WT cells are round cells with a relatively high nuclear-to-cytoplasm ratio (Figure 1A-B). The slightly basophilic cytoplasm contained purple granules, their number and size being variable from cell to cell. Metachromasia was documented by staining with toluidine blue (Figure 1C). TEM studies showed that ROSAKIT WT cells have a large nucleus with loose chromatin and 1 to 2 nucleoli (Figure 1D). The cytoplasm contained ribosomes, a well-developed Golgi apparatus, abundant mitochondria, and secretory granules with spherical electron-dense cores surrounded by electron-lucent space similar to those described in human CB-derived MCs (CBMCs).39 In addition, almost all cells stained strongly positive for tryptase (Figure 1E) and KIT (Figure 1F) but were negative for chymase (data not shown).
Phenotypic characterization of the ROSA cell lines. ROSAKIT WT cells were stained by (A-B) MGG or (C) toluidine blue. (D) The ultrastructure of ROSAKIT WT cells was analyzed by transmission electron microscopy. In addition, ROSAKIT WT cells were treated with an antibody against (E) tryptase or (F) KIT (CD117). Positivity was revealed by indirect immunocytochemistry as described in “Material and methods.” (G) Cytospin preparations or (H-I) glass smears of ROSAKIT D816V cells were stained by MGG. (G) Note the presence of many granules in ROSAKIT D816V cells and of apoptotic cells, a phenomenon likely to be related to terminal maturation induced by the introduction of the KIT D816V mutant. (H) On glass smear, a significant proportion of the ROSAKIT D816V cells presented with spindle-shaped appearance, which was not noticed for (I) ROSAKIT WT cells. Magnification: A and C, ×200; B, E, G, H, and I ×500; D, ×2000; F, ×1000.
Phenotypic characterization of the ROSA cell lines. ROSAKIT WT cells were stained by (A-B) MGG or (C) toluidine blue. (D) The ultrastructure of ROSAKIT WT cells was analyzed by transmission electron microscopy. In addition, ROSAKIT WT cells were treated with an antibody against (E) tryptase or (F) KIT (CD117). Positivity was revealed by indirect immunocytochemistry as described in “Material and methods.” (G) Cytospin preparations or (H-I) glass smears of ROSAKIT D816V cells were stained by MGG. (G) Note the presence of many granules in ROSAKIT D816V cells and of apoptotic cells, a phenomenon likely to be related to terminal maturation induced by the introduction of the KIT D816V mutant. (H) On glass smear, a significant proportion of the ROSAKIT D816V cells presented with spindle-shaped appearance, which was not noticed for (I) ROSAKIT WT cells. Magnification: A and C, ×200; B, E, G, H, and I ×500; D, ×2000; F, ×1000.
Morphologic analysis of ROSAKIT D816V cells showed significant differences when compared with ROSAKIT WT cells. Indeed, ROSAKIT D816V cells appear to be more granulated (Figure 1G). Interestingly, a significant percentage of ROSAKIT D816V cells exhibited a spindle-shaped morphology on smears (Figure 1H), which was not observed with ROSAKIT WT cells (Figure 1I). However, despite signs of maturation, ROSAKIT D816V cells retained an MCT phenotype because they expressed only tryptase but not chymase (data not shown).
Conventional cytogenetics and fluorescence in situ hybridization analyses of the ROSA cell lines
In initial cultures as well as after 12 months in culture, ROSAKIT WT cells exhibited a complex karyotype, with a derivative chromosome 1 (der(1)inv(1)(p31q21)del(1)(q24q32)) and 2 subclones: one with a complete trisomy 5, and the other was predominant with a partial trisomy 5 (+del(5)(q14q34)) (supplemental Figure 1A). Results from fluorescence in situ hybridization analysis confirmed the expression of aberrations observed by karyotyping in ROSA cells (supplemental Figure 1B-C). Interestingly, ROSAKIT D816V cells exhibited the same karyotype after lentiviral infection (not shown).
Analysis of cell surface antigens in ROSA cells and responses to IL-4
Both ROSA cell lines have a comparable phenotype with a strong positivity for FcεRIα and KIT (CD117) (supplemental Table 2). The level of KIT (CD117) was higher in ROSAKIT D816V cells compared with ROSAKIT WT cells. A number of other antigens known to be expressed by human cultured MCs were also detected on ROSA cells, such as CD32, CD44, CD50, CD66a, CD166, CD203c, and CD300a (supplemental Table 2).40 In addition, IL-4 induced a significant decrease in the expression of KIT as well as an increase in expression of FcεRI in both ROSA cell lines (supplemental Figure 2). These data strongly suggest that ROSA cells are MCs.
Response of ROSAKIT WT and ROSAKIT D816V cells to various cytokines
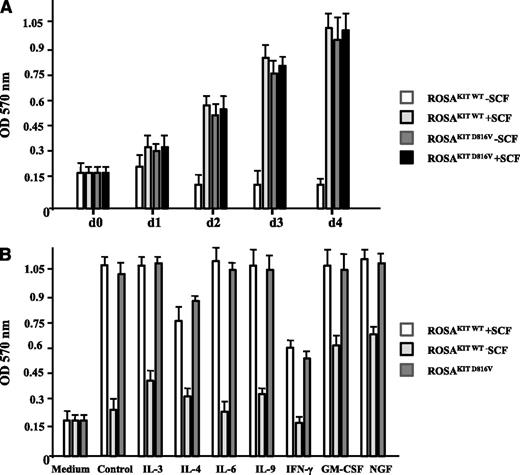
When comparing the effect of rhSCF (80 ng/mL) on the proliferation of ROSAKIT WT cells and of ROSAKIT D816V cells during a 4-day period, we observed that ROSAKIT WT cells deprived of SCF were not able to grow, whereas ROSAKIT WT cells cultured in the presence of SCF, as well as ROSAKIT D816V cells cultured in the absence of SCF or presence of rhSCF, proliferated at an apparently similar rate (Figure 2A), suggesting that constitutive activation of the mutant KIT D816V is sufficient to induce optimal growth of ROSA cells.
Effect of SCF and of various cytokines on the proliferation of the 2 different ROSA cell lines. (A) ROSAKIT WT or ROSAKIT D816V cells were treated or not treated with rhSCF (80 ng/mL) for 4 days at 37°C. At each time point, 10 μL of 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added in corresponding wells, and the cells were incubated for 3 additional hours in an incubator at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm. (B) ROSAKIT WT (with or without SCF) or ROSAKIT D816V cells (without SCF) were treated with various cytokines (all at 100 ng/mL) for 3 days. At that time, 10 μL of MTT was added in corresponding wells, and the cells were incubated for 3 additional hours in an incubator at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm. Data presented are the mean ± standard deviation (SD) from 3 independent experiments. OD, optical density.
Effect of SCF and of various cytokines on the proliferation of the 2 different ROSA cell lines. (A) ROSAKIT WT or ROSAKIT D816V cells were treated or not treated with rhSCF (80 ng/mL) for 4 days at 37°C. At each time point, 10 μL of 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added in corresponding wells, and the cells were incubated for 3 additional hours in an incubator at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm. (B) ROSAKIT WT (with or without SCF) or ROSAKIT D816V cells (without SCF) were treated with various cytokines (all at 100 ng/mL) for 3 days. At that time, 10 μL of MTT was added in corresponding wells, and the cells were incubated for 3 additional hours in an incubator at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm. Data presented are the mean ± standard deviation (SD) from 3 independent experiments. OD, optical density.
We then investigated the potential effects of IL-3, IL-4, IL-6, IL-9, IFN-γ, GM-CSF, or NGF (all at 100 ng/mL) on spontaneous or SCF-driven proliferation of ROSAKIT WT cells in the absence or presence of SCF (80 ng/mL) for 3 days. In addition, the same cytokines were added to ROSAKIT D816V cells cultured without SCF. As shown in Figure 2B, NGF, and to a lesser extent IL-3 and GM-CSF, were able to partly rescue ROSAKIT WT cells from cell death induced by SCF withdrawal. By contrast, none of the cytokines tested had any effect on increasing proliferation of ROSAKIT WT cells in the presence of SCF or on the proliferation of ROSAKIT D816V cells in the absence of SCF, except IL-4 and IFN-γ, which decreased the SCF-induced proliferation of ROSAKIT WT cells and the autonomous proliferation of ROSAKIT D816V cells (Figure 2B).
FcεRI cross-linked ROSAKIT WT cells release MC mediators
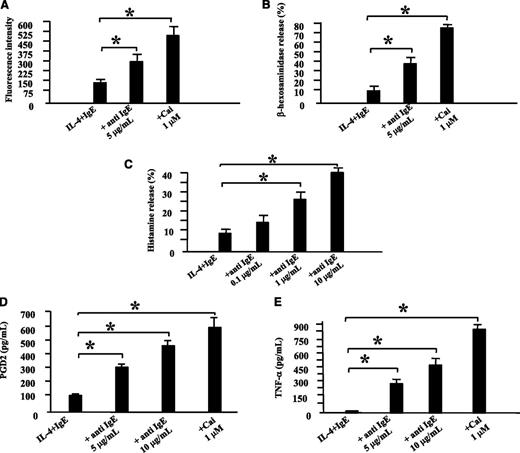
Anti-IgE–induced activation of ROSAKIT WT cells resulted in a significant upregulation of CD203c (Figure 3A), which was not the case neither for ROSAKIT D816V cells, nor for purified neoplastic BM MCs from SM patients (supplemental Results and Figures 5 and 7). Corresponding results were obtained in a β-hexosaminidase-release assay. In fact, anti-IgE–activated ROSAKIT WT cells released up to 38% of the enzyme after 1 hour (Figure 3B). As expected, calcium ionophore A23187 induced a strong increase in the expression of CD203c on ROSAKIT WT cells (Figure 3A) as well as a release of up to 80% of β-hexosaminidase (Figure 3B), which is consistent with previous data.41 In addition, anti-IgE–induced activation of ROSAKIT WT cells resulted in histamine release (Figure 3C). PGD2 and TNF-α were also synthesized and released by ROSAKIT WT cells upon stimulation with anti-IgE or Cai A23187 (Figure 3D-E). Besides, the cellular histamine level averaged approximately 15 pg per cell in ROSAKIT WT cells and ROSAKIT D816V cells, which is more than in primary CBMCs (4.5 pg per CBMC),42 LAD2 cells (3.1 pg per cell), or HMC-1 cells (0.9 pg per cell).29
Biologic effects of the cross-linking of FcεRI on ROSAKIT WT cells. ROSAKIT WT cells were primed first with recombinant human IL-4 (recombinant human IL-4; 20 ng/mL) and human monoclonal IgE (2 μg/mL) for 5 days to increase the expression of FcεRI. (A) To determine CD203c upregulation, ROSAKIT WT primed cells were stimulated by anti-human IgE (5 µg/mL) or by Ca-ionophore (Cai) (1 µM) for 1 hour. After labeling with an anti-human CD203c Ab coupled with allophycocyanin (APC), the fluorescence intensity was measured by using a FACSCalibur flow cytometer. (B) To determine β-hexosaminidase release, ROSAKIT WT primed cells were stimulated by anti-human IgE or by Cai for 1 hour. β-hexosaminidase enzymatic activity was measured in the cell-free supernatants and after cell lysis as described in Materials and methods. (C) To determine histamine release, primed ROSAKIT WT cells were stimulated with various concentrations of anti-human anti-IgE Ab at 37°C for 30 minutes. Thereafter, cells were centrifuged at 4°C, and the supernatants and cell pellets were analyzed for histamine content by using a specific radioimmunoassay kit. Results of histamine release are expressed as percentage of total histamine. (D) To examine synthesis of PGD2, primed cells were stimulated by anti-human IgE (5 or 10 µg/mL) or by Cai (1 µM) for 1 hour. Thereafter, cells were centrifuged, and the cell-free supernatants were analyzed for PGD2 content by using a specific enzyme immunoassay (EIA) kit. (E) To study the synthesis of TNF-α, primed ROSAKIT WT cells were stimulated by anti-human IgE (5 or 10 µg/mL) or by Cai (1 µM) for 6 hours. Thereafter, cells were centrifuged, and the cell-free supernatants were analyzed for TNF-α content by using a specific EIA kit. For each parameter, data presented are the mean ± SD from 3 independent experiments. *Significantly different from the control (IL-4 + IgE) at P < .05.
Biologic effects of the cross-linking of FcεRI on ROSAKIT WT cells. ROSAKIT WT cells were primed first with recombinant human IL-4 (recombinant human IL-4; 20 ng/mL) and human monoclonal IgE (2 μg/mL) for 5 days to increase the expression of FcεRI. (A) To determine CD203c upregulation, ROSAKIT WT primed cells were stimulated by anti-human IgE (5 µg/mL) or by Ca-ionophore (Cai) (1 µM) for 1 hour. After labeling with an anti-human CD203c Ab coupled with allophycocyanin (APC), the fluorescence intensity was measured by using a FACSCalibur flow cytometer. (B) To determine β-hexosaminidase release, ROSAKIT WT primed cells were stimulated by anti-human IgE or by Cai for 1 hour. β-hexosaminidase enzymatic activity was measured in the cell-free supernatants and after cell lysis as described in Materials and methods. (C) To determine histamine release, primed ROSAKIT WT cells were stimulated with various concentrations of anti-human anti-IgE Ab at 37°C for 30 minutes. Thereafter, cells were centrifuged at 4°C, and the supernatants and cell pellets were analyzed for histamine content by using a specific radioimmunoassay kit. Results of histamine release are expressed as percentage of total histamine. (D) To examine synthesis of PGD2, primed cells were stimulated by anti-human IgE (5 or 10 µg/mL) or by Cai (1 µM) for 1 hour. Thereafter, cells were centrifuged, and the cell-free supernatants were analyzed for PGD2 content by using a specific enzyme immunoassay (EIA) kit. (E) To study the synthesis of TNF-α, primed ROSAKIT WT cells were stimulated by anti-human IgE (5 or 10 µg/mL) or by Cai (1 µM) for 6 hours. Thereafter, cells were centrifuged, and the cell-free supernatants were analyzed for TNF-α content by using a specific EIA kit. For each parameter, data presented are the mean ± SD from 3 independent experiments. *Significantly different from the control (IL-4 + IgE) at P < .05.
KIT phosphorylation and KIT-dependent signaling are constitutively activated in ROSAKIT D816V cells
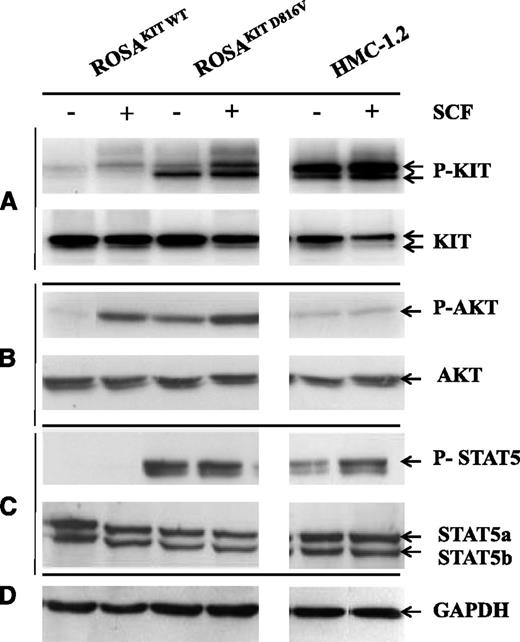
Western blotting was performed with anti-phospho-KIT Ab (Y703) on ROSA cells and on HMC-1.2 cells in the presence or absence of rhSCF. As shown in Figure 4A, in the absence of rhSCF, ROSAKIT D816V and HMC-1.2 cells exhibited a constitutively phosphorylated KIT (p-KIT), whereas ROSAKIT WT cells exhibited only low levels of p-KIT. Stimulation with rhSCF resulted in higher phosphorylation of KIT in ROSAKIT WT cells and increased phosphorylation of KIT in ROSAKIT D816V cells but not in HMC-1.2 cells (Figure 4A).
Detection of spontaneous phosphorylation of KIT, AKT, and STAT5 in ROSAKIT D816V cells by western blotting. Cell lysates from unstimulated ROSA cell lines and from the HMC-1.2 cell line or from the same cells stimulated for 10 minutes with rhSCF were subjected to electrophoresis in sodium dodecyl sulfate polyacrylamide gel electrophoresis and treated with Abs against (A) anti-human total KIT or p-KIT Y719, (B) anti-human total AKT or p-AKT S473, or (C) anti-human total STAT5 or p-STAT5 Y694. (D) An anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. The lanes were run on the same gel but were noncontiguous.
Detection of spontaneous phosphorylation of KIT, AKT, and STAT5 in ROSAKIT D816V cells by western blotting. Cell lysates from unstimulated ROSA cell lines and from the HMC-1.2 cell line or from the same cells stimulated for 10 minutes with rhSCF were subjected to electrophoresis in sodium dodecyl sulfate polyacrylamide gel electrophoresis and treated with Abs against (A) anti-human total KIT or p-KIT Y719, (B) anti-human total AKT or p-AKT S473, or (C) anti-human total STAT5 or p-STAT5 Y694. (D) An anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. The lanes were run on the same gel but were noncontiguous.
We also examined the phosphorylation of the serine residue 473 of AKT in the presence or absence of rhSCF in ROSA cells and in HMC-1.2 cells. In contrast to ROSAKIT WT cells, which require the presence of SCF to express phospho-AKT, ROSAKIT D816V cells exhibited a significant phosphorylation of AKT in the absence of rhSCF, which was even higher than in HMC-1.2 cells (Figure 4B).
Moreover, we demonstrated the constitutive phosphorylation of STAT5 in ROSAKIT D816V cells but not in ROSAKIT WT cells (even in the presence of rhSCF; Figure 4C). Finally, rhSCF did not alter p-STAT5 levels in ROSAKIT D816V, whereas it increased this expression in HMC-1.2 cells (Figure 4C). Anti-human glyceraldehyde-3-phosphate dehydrogenase demonstrated equal loading in all conditions (Figure 4D)
ROSA cells can be used as a major new model system for in vitro drug testing
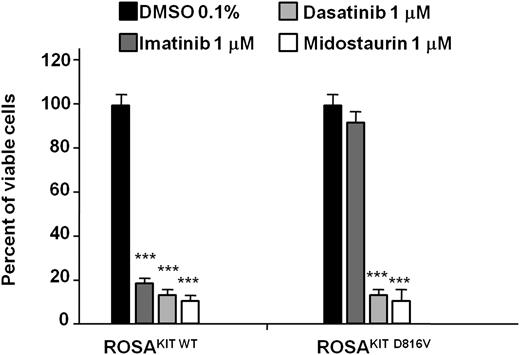
We evaluated the inhibitory effects of three tyrosine kinase inhibitors, imatinib, dasatinib, and midostaurin (PKC412), each at 1 μM, on the proliferation of ROSA cell lines. In line with published results,43 ROSAKIT WT cells were sensitive to imatinib, dasatinib, and midostaurin, whereas imatinib had no effects on ROSAKIT D816V cells (Figure 5). As expected, dasatinib and midostaurin were found to inhibit the growth of ROSAKIT D816V cells (Figure 5).
Effect of three tyrosine kinase inhibitors on the proliferation of the 2 ROSA cell lines. Cells were cultured for 48 hours in the presence of imatinib, dasatinib or midostaurin (1 μM, provided in dimethylsulfoxide (DMSO) 0.1% final concentration) or in the presence of DMSO alone (0.1% final concentration) in the established culture medium (containing rhSCF at 80 ng/mL for ROSAKIT WT cells but not for ROSAKIT D816V cells). At the end of this incubation, 10 μL of MTT was added in each well and the cells were incubated for 3 additional hours at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm, and the results were expressed as a percentage of living cells compared with the control (DMSO 0.1% alone). Data presented are the mean ± SD from 3 independent experiments. ***Significantly different from control (DMSO alone) at P < .0001.
Effect of three tyrosine kinase inhibitors on the proliferation of the 2 ROSA cell lines. Cells were cultured for 48 hours in the presence of imatinib, dasatinib or midostaurin (1 μM, provided in dimethylsulfoxide (DMSO) 0.1% final concentration) or in the presence of DMSO alone (0.1% final concentration) in the established culture medium (containing rhSCF at 80 ng/mL for ROSAKIT WT cells but not for ROSAKIT D816V cells). At the end of this incubation, 10 μL of MTT was added in each well and the cells were incubated for 3 additional hours at 37°C. The number of living cells was then measured for each condition by reading the absorbance at 570 nm, and the results were expressed as a percentage of living cells compared with the control (DMSO 0.1% alone). Data presented are the mean ± SD from 3 independent experiments. ***Significantly different from control (DMSO alone) at P < .0001.
The 2 ROSA cell lines differentially engraft NSG mice
BM samples (mice injected intravenously) or dispersed tumors (mice injected subcutaneously) were analyzed for the presence of CD117+/CD45+ cells. Nearly all the mice injected intravenously with the 2 different types of ROSA cells developed a significant BM engraftment, with a mean percentage of CD117+/CD45+ cells significantly higher in the mice injected with ROSAKIT D816V (52%) than in those injected with ROSAKIT WT cells (2.62%) (Table 1). Of 5 mice injected subcutaneously with ROSAKIT WT cells, 1 developed a subcutaneous tumor. This was similar to the other group, in which 1 of the 4 mice injected subcutaneously with ROSAKIT D816V cells presented a subcutaneous tumor (Table 1). Interestingly, cytocentrifuged BM-engrafted ROSAKIT WT cells stained with MGG kept a morphology comparable to the one observed in vitro, whereas BM-engrafted ROSAKIT D816V cells exhibited spindle-shaped features resembling those of MCs from patients with KIT D816V-positive SM (Figure 6).44 In addition, there was a highly significant correlation between the level of BM engraftment by CD117+/CD45+ cells and the level of tryptase-positive cells on BM biopsy (supplemental Figure 9). Finally, in line with previous data showing that the microenvironment can influence MC phenotype,3 there was a clear tendency toward increased expression of chymase in subcutaneous tumors compared with BM-grafted cells (supplemental Table 5).
Overview of ROSA NSG experiments
| . | Cells injected × 106 . | BM engraftment (positive/total)* . | Median engraftment of intravenously injected mice (%) . | Subcutaneous tumor growth (positive/total)† . |
|---|---|---|---|---|
| ROSAKIT WT | 5 | 4/5 (18 wk) | 2.62 | 1/5 |
| ROSAKIT D816V | 0.4 | 4/4 (21 wk) | 52 | 1/4 |
| . | Cells injected × 106 . | BM engraftment (positive/total)* . | Median engraftment of intravenously injected mice (%) . | Subcutaneous tumor growth (positive/total)† . |
|---|---|---|---|---|
| ROSAKIT WT | 5 | 4/5 (18 wk) | 2.62 | 1/5 |
| ROSAKIT D816V | 0.4 | 4/4 (21 wk) | 52 | 1/4 |
Table shows engraftment of ROSAKIT WT and of ROSAKIT D816V cells in NSG mice. Percentage of engrafted human MCs was assessed by flow cytometry using their double positivity for human CD45 and human CD117 antigens.
Number of mice showing an engraftment of ROSA cells >0.1% in bone marrow after intravenous injection of total mice injected that survived more than 12 weeks.
Number of mice showing a subcutaneous tumor formation after subcutaneous injection of total mice injected (2 sites per mouse) that survived more than 12 weeks.
Morphologic appearance of BM-engrafted ROSAKIT WT and ROSAKIT D816V cells in NSG mice. Whole BM cells from NSG mice engrafted intravenously with (A) ROSAKIT WT or (B) ROSAKIT D816V cells were analyzed for their content of CD117+/CD45+ engrafted human MCs (A and B, top panels). The cells were also cytocentrifuged on glass slides, stained with MGG and then observed by using light microscopy (bottom panels). Note the presence of spindle-shaped MCs with an abnormal distribution of granules in the BM of mice engrafted with ROSAKIT D816V cells (B, black arrows), whereas the BM MCs from mice engrafted with ROSAKIT WT cells presented with a rounded shape, similar to the one observed in culture (A, gray arrows). Magnification is ×200. FL2-H, FL2 peak emission values; PerCP, peridinin chlorophyll.
Morphologic appearance of BM-engrafted ROSAKIT WT and ROSAKIT D816V cells in NSG mice. Whole BM cells from NSG mice engrafted intravenously with (A) ROSAKIT WT or (B) ROSAKIT D816V cells were analyzed for their content of CD117+/CD45+ engrafted human MCs (A and B, top panels). The cells were also cytocentrifuged on glass slides, stained with MGG and then observed by using light microscopy (bottom panels). Note the presence of spindle-shaped MCs with an abnormal distribution of granules in the BM of mice engrafted with ROSAKIT D816V cells (B, black arrows), whereas the BM MCs from mice engrafted with ROSAKIT WT cells presented with a rounded shape, similar to the one observed in culture (A, gray arrows). Magnification is ×200. FL2-H, FL2 peak emission values; PerCP, peridinin chlorophyll.
Discussion
MC research suffers from a lack of FcεRI-positive human MC cell lines established from normal hematopoietic progenitors. Here we present a new tryptase-positive, chymase-negative, FcεRI-positive SCF-dependent human MC line, ROSAKIT WT, derived from CD34+ cells of a normal human CB, with a normal KIT receptor. Their morphology and phenotype closely resemble those of primary cultured CBMCs.29,42,45 However, in contrast to CBMCs, ROSAKIT WT cells can be cultured for years without loss of clonal stability, changes in phenotype, or significant alteration in their functional properties. Moreover, ROSAKIT WT cells are easy to freeze and thaw by using conventional techniques (supplemental Methods). All these features suggest that ROSA cells represent a novel tool for MC research.
So far, only 2 FcεRI-positive human cell lines have been established: LAD2 and LUVA.29,30 These 2 cell lines have several limitations, such as a long doubling time for LAD2 or a weak expression of FcεRI by LUVA cells. Thus, the establishment of a ROSAKIT WT cell line that has a short doubling time and stably expresses a functional FcεRI at high levels represents a new advanced tool for investigating human MC functions and for high-throughput screening of anti-allergic therapeutics.
KIT is a key receptor regulating the development of normal and neoplastic MCs, as evidenced by the presence of activating mutations in the KIT gene.22 In SM, the most frequent defect found, the KIT D816V point mutation, accounts for the pathophysiology of the disease.46,47 However, in vitro models available for studying the impact of KIT D816V mutation on human MCs are limited. Indeed, the only model available is the HMC-1 cell line and its 2 subclones HMC-1.1 and HMC-1.2.48 Both clones contain the KIT V560G mutation, whereas HMC-1.2 additionally exhibits the KIT D816V mutation.48 Unfortunately, HMC-1 cells do not bear the KIT D816V mutation alone, and there is no HMC-1 subclone with a normal KIT gene, making it difficult to compare signaling pathways activated by normal or mutant forms of KIT in the same cell model. To overcome these limitations, we established the KIT D816V-positive SCF-independent ROSAKIT D816V MC line, which constitutes the first in vitro model of human MC with KIT D816V mutant alone and for which a KIT WT equivalent exists.
ROSAKIT D816V cells express CD117, CD203c, and tryptase but they also express FcεRI at a level comparable to that of ROSAKIT WT cells. Interestingly, ROSAKIT D816V cells could not be activated by anti-IgE in the same way as ROSAKIT WT cells (data presented in supplemental Results and in Figure 5). These discrepant results led us to investigate whether malignant MCs from different subcategories of KIT D816V-positive SM were also insensitive to such activation. Interestingly, anti-IgE stimulated by BM MCs from such patients released little or no histamine and did not upregulate the activation marker CD63, and these data are presented in supplemental Results and in supplemental Figures 5 and 7. So far, the mechanisms underlying this phenomenon remain unknown. A decrease in expression of IgE receptors or other molecules relevant to MC activation in KIT D186V–transformed cells was excluded in our experiments. However, Itoh et al49 have recently shown that MCs chronically exposed to SCF display a marked attenuation of FcεRI-mediated degranulation and cytokine production. Such desensitization may also play a role in KIT D816V-positive cells in which the KIT receptor is chronically phosphorylated. Since FcεRI and KIT use common signaling pathways, cross-desensitization may occur.50 Alternatively, KIT D816V may induce the expression of negative regulators of MC activation, such as phosphatases. All in all, these findings might explain why many patients with SM, even with high MC burden, do not suffer at all from IgE-related mediator-related symptoms. Nevertheless, a number of patients may suffer from mediator-release symptoms related to non-IgE triggers, such as drugs, infection, or alcohol.51
Activation of KIT recruits a number of signaling pathways in MCs. Specifically, activation of the PI3-K/AKT pathway was shown to contribute to KIT-dependent proliferation, survival, maturation, adhesion, or activation.52 Interestingly, the introduction of KIT D816V in ROSA cells induced the constitutive activation of AKT, which is in line with previously reported data.53 In addition, KIT D816V recruits alternative signaling pathways not evoked by KIT WT, particularly STAT5.54-56 Interestingly, ROSAKIT D816V cells constitutively expressed p-STAT5, suggesting that they use the same alternative pathways as MCs in mastocytosis patients. Moreover, using inhibitors of AKT or STAT5, we showed that ROSAKIT D816V cells are more sensitive to their antiproliferative effects than the ROSAKIT WT cells (supplemental Methods, Results, and Figure 8). This is consistent with a previous report showing that (1) oncogenic KIT controls neoplastic MC growth through a STAT5/PI3-kinase signaling complex activating AKT and (2) knockdown of AKT or STAT5 activity inhibits growth of neoplastic KIT D816V-positive MCs.47 To the best of our knowledge, this is the first demonstration that de novo introduction of the KIT D816V mutation leads to constitutive phosphorylation of STAT5 in a model of human neoplastic MCs. Thus, ROSAKIT D816V cells represent valuable tools for exploring the mechanisms involved in mutant KIT-related signaling pathways and provide a robust model for screening potential STAT5 inhibitors. Moreover, in line with previously reported data,57,58 ROSAKIT WT cells, but not ROSAKIT D816V cells, were sensitive to imatinib. Of note, ROSA cell lines were equally sensitive to dasatinib and midostaurin, which is also consistent with previous reports.59,60 Thus, the 2 ROSA cell lines seem to be suitable models for in vitro screening of selective inhibitors of the different forms of KIT.
Mastocytosis research suffers from a lack of animal models mimicking human SM. Indeed in the 2 models already published, which were obtained by using mice transgenic for mutant KIT in which the transgenes were selectively expressed in MCs,46,61 the targeted cells in both cases were murine MCs. Here, we show that human neoplastic KIT D816V-positive MCs can engraft at a high rate in NSG mice, not only in the BM, but also in spleen and lung, giving rise to a disease mimicking, at best, the situation encountered in SM patients. Indeed, as evidenced by immunohistochemistry for human tryptase on BM sections of NSG mice injected intravenously with ROSAKIT D816V cells, compact infiltrates of tryptase-positive human MCs can be seen. Together with the spindle-shaped appearance of the neoplastic MCs found on BM smears of the animals, and with their positivity for KIT D816V, our animal model exhibits several features resembling SM in humans,62 making it the most similar model for human SM described so far.
In summary, we have established a new human FcεRI-positive MC line exhibiting the major characteristics of normal MCs, with several advantages over available human MC models in vitro, which might support future studies on the biology of MCs and on their pharmacologic modulation. In addition, we have derived a unique model of human MCs that expresses the KIT D816V mutant producing a mastocytosis-like disease in NSG mice. These noteworthy features should facilitate the development of new pathogenetic concepts and the testing of new anti-neoplastic drug therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tina Bernthaler, Sandra Brahimi, Caroline Ohana, Gabriele Stefanzl, Verena Suppan, and Daniela Berger for excellent technical assistance.
This work was supported in part by a grant from Fondation de France and in part by the Austrian Science Fund (FWF), SFB project F4611 and F4704-B20. Ghaith Wedeh is a fellow of the Marie Curie Eurocancer Stemcell (CSC) Training Network.
Authorship
Contribution: M.A. and P.V. designed the experiments; R.S., G.W., H.H., S.C.-R., I.S., K.B., E.H., S.B., S.J., C.B., E.C., F.S., and P.B. performed the experiments; M.A., M.L.-T., F.N.-K., H.M.-B., M.W., T.R., and P.V. analyzed the data; P.D. and V.D. contributed essential reagents; M.A. and P.V. wrote the paper; and all authors approved this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Arock, Laboratoire de Biologie et Pharmacologie Appliquée (LBPA), Centre National de la Recherche Scientifique (CNRS) Unité Mixte de Recherche (UMR) 8113, Ecole Normale Supérieure de Cachan, 61 Ave du Président Wilson, 94235 Cachan Cedex, France; e-mail: arock@ens-cachan.fr.
References
Author notes
R.S. and G.W. contributed equally to this work as joint first authors.