Key Points
In a 3-year follow-up of the DASatinib versus Imatinib Study In treatment-Naive CML patients trial, first-line dasatinib resulted in faster and deeper responses compared with imatinib.
Deeper responses at 3, 6, and 12 months were associated with better 3-year progression-free survival and overall survival.
Abstract
This analysis explores the impact of early cytogenetic and molecular responses on the outcomes of patients with chronic myeloid leukemia in chronic phase (CML-CP) in the phase 3 DASatinib versus Imatinib Study In treatment-Naive CML patients trial with a minimum follow-up of 3 years. Patients with newly diagnosed CML-CP were randomized to receive 100 mg dasatinib (n = 259) or 400 mg imatinib (n = 260) once daily. The retrospective landmark analysis included patients evaluable at the relevant time point (3, 6, or 12 months). Median time to complete cytogenetic response was 3 vs 6 months with dasatinib vs imatinib. At 3 and 6 months, the proportion of patients with BCR-ABL transcript levels ≤10% was higher in the dasatinib arm. Deeper responses at 3, 6, and 12 months were observed in a higher proportion of patients on dasatinib therapy and were associated with better 3-year progression-free survival and overall survival in both arms. First-line dasatinib resulted in faster and deeper responses compared with imatinib. The achievement of an early molecular response was predictive of improved progression-free survival and overall survival, supporting new milestones for optimal response in patients with early CML-CP treated with tyrosine kinase inhibitors. This study was registered at www.clinicaltrials.gov as NCT00481247.
Introduction
The DASatinib versus Imatinib Study In treatment-Naive CML patients (DASISION) study is a randomized phase 3 trial comparing treatment with dasatinib 100 mg once daily (qd) or imatinib 400 mg qd in patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP). Primary results showed superior efficacy with dasatinib vs imatinib during the first year of therapy, including significantly higher percentages of complete cytogenetic response (CCyR; 83% vs 72%, P = .001), confirmed CCyR (cCCyR [primary end point]; 77% vs 66%, P = .007), and major molecular response (MMR; 46% vs 28%, P < .0001) by 12 months, as well as an acceptable safety and tolerability profile.1 The 3-year update has continued to demonstrate the efficacy and tolerability of dasatinib.2
The achievement of an early response remains the major surrogate end point predicting for improved long-term outcome in patients with CML-CP treated with tyrosine kinase inhibitors (TKIs).3-8 The value of earlier and deeper response in outcome prediction has emerged in patients with CML-CP receiving first-line imatinib.9,10 Similarly, in recent pooled11 and unpooled12 analyses of patients treated with second-generation TKIs (dasatinib or nilotinib) as initial therapy for CML, patients who achieved early major cytogenetic response (MCyR) and molecular response (MR) had significantly better outcomes than those who did not.
The aim of this analysis was to assess the predictive value of early responses for the outcomes of patients treated in the DASISION trial with a minimum follow-up of 3 years.
Materials and methods
Patients, study design, and treatment
Eligibility criteria and study design have been described previously.1,13 Adults with newly diagnosed Philadelphia chromosome (Ph)–positive CML-CP who had adequate organ function and no other serious medical conditions were eligible. The trial was approved by the relevant institutional review boards and ethics committees. All patients gave written informed consent before randomization in accordance with the Declaration of Helsinki.
DASISION is an ongoing multinational, open-label, phase 3 trial. Patients were stratified by Euro (Hasford) risk score14 and randomized 1:1 to receive either oral dasatinib (100 mg daily) or imatinib (400 mg daily). Treatment interruptions and dose reductions were permitted for managing adverse events (AEs). Dose escalations to dasatinib 140 mg daily or imatinib 600 to 800 mg daily were permitted for suboptimal response (European LeukemiaNet 2006 definitions) at 3 to 18 months.15 The primary end point was the proportion of patients with cCCyR by 12 months. Secondary end points were time in cCCyR, rates of MMR at any time, times to cCCyR or MMR, progression-free survival (PFS), and overall survival (OS).
Evaluations
CCyR was defined as lack of Ph chromosome in ≥20 bone marrow (BM) metaphases. Confirmed CCyR was defined as CCyR in 2 consecutive cytogenetic assessments ≥28 days apart. MMR was defined as a BCR-ABL transcript level ≤0.1%. Missing samples were counted as no response. A centralized laboratory (MolecularMD, Portland, OR) performed molecular assessments on the international scale.16 Transformation to accelerated phase/blast phase (AP/BP) CML was defined using European LeukemiaNet 2006 criteria and did not include clonal evolution.15 Patients who survived after discontinuation and agreed to follow-up were followed for transformation and survival at least yearly for up to 5 years. AEs were assessed continuously and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical considerations
All patients registered and enrolled were assessed for responses. Comparisons of response rates in the current manuscript are post hoc analyses; P values (all 2-sided) are descriptive and have not been adjusted for multiple comparisons. All measurements of time to a response or to an event were reported in days relative to the date of randomization (converted to months for this analysis). PFS and OS were estimated using Kaplan-Meier analysis. Progression was defined as doubling of white blood cell count to >20 × 109/L, loss of complete hematologic response (CHR), increase in Ph-positive BM metaphases to ≥30% from nadir, transformation to AP/BP, or death from any cause. Failure was defined as no hematologic response by 3 months, no CHR or cytogenetic response by 6 months, no partial cytogenetic response (PCyR) by 12 months, or no CCyR by 18 months. For PFS analyses, patients who discontinued without progression/failure were censored at the date of their last on-study hematologic or cytogenetic evaluation. Information on progression after failure or study discontinuation was obtained to identify patients who had progressed and would be censored on the date of progression. Transformation was collected as part of progression information; patients who transformed were identified if they had not already progressed by other criteria. For OS analyses, patients who had not died were censored on the date they were last known to be alive, which may have been after study discontinuation if off-study survival was reported by the investigator. Safety analyses were performed in all patients who received at least 1 dose of study drug. Hazard ratios, where provided, were based on the Cox model without assumption of hazard form; hazard ratios accounted for competing risks.
Landmark analyses conducted retrospectively at 3, 6, and 12 months were restricted to patients with assessments at the corresponding time points. All patients with the appropriate response assessment (molecular or cytogenetic) at the landmark time were included, and the event of interest was considered at or after the landmark time. After the landmark time, patients both on and off study were considered. Using the Kaplan-Meier method, landmark analyses estimated the distributions of PFS and OS by BCR-ABL transcript levels at 3 and 6 months, by cytogenetic response at 3 and 6 months, and by MMR and/or CCyR at 12 months. Unlike the 3-year efficacy analyses, landmark PFS analyses included all events, regardless of whether the patient had discontinued at the time of progression.
Results
Patient disposition and treatment characteristics
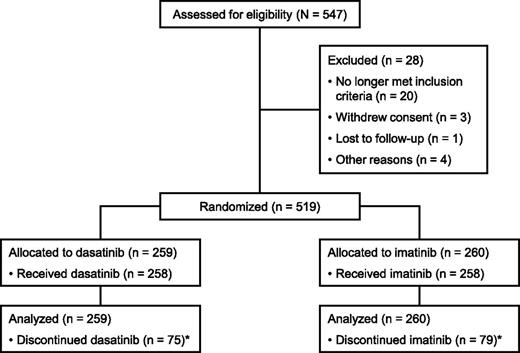
Between September 2007 and December 2008, 519 patients were randomized to receive dasatinib or imatinib (Figure 1). Baseline characteristics have been published1 and were well balanced between the dasatinib and imatinib arms. At the time of analysis (with database lock February 3, 2012), 183 patients (71%) receiving dasatinib and 179 patients (69%) receiving imatinib remained in the study, with more than 90% of patients continuing to be followed for OS. Reasons for study discontinuation are shown in Table 1. In the dasatinib and imatinib arms, respectively, median durations of follow-up from randomization to last assessment (including after study discontinuation) were 36.9 months (range, 0.2-49.8) and 37.0 months (0.7-49.7); median durations of treatment were 36.8 months (0.03-49.7) and 36.8 months (0.3-49.7). During dasatinib or imatinib treatment, respectively, 162 patients (63%) and 117 (45%) had a treatment interruption, 79 (31%) and 42 (16%) had a dose reduction, and 22 (9%) and 57 (22%) had a dose escalation. Median dose intensities were 99 mg/d (range, 21-200) for dasatinib and 400 mg/d (125-728) for imatinib.
CONSORT diagram for the DASISION trial after a minimum follow-up of 36 months. *Reasons for discontinuation are provided in Table 1. Adapted from Kantarjian et al13 and reproduced with permission from the American Society of Hematology.
Patient disposition and discontinuations
| . | Treated patients, n (%) . | |
|---|---|---|
| . | Dasatinib 100 mg qd, n = 258 . | Imatinib 400 mg qd, n = 258 . |
| Still on treatment | 183 (71) | 179 (69) |
| Discontinued | 75 (29) | 79 (31) |
| Progression* | 17 (7) | 18 (7) |
| Treatment failure† | 8 (3) | 12 (5) |
| Adverse event | 27 (10) | 16 (6) |
| Nonhematologic | 20 (8) | 12 (5) |
| Hematologic | 7 (3) | 4 (2) |
| Unrelated adverse event | 6 (2) | 2 (<1) |
| Death‡ | 4 (2) | 1 (<1) |
| Poor/nonadherence§ | 0 (0) | 4 (2) |
| Other|| | 13 (5) | 26 (10) |
| . | Treated patients, n (%) . | |
|---|---|---|
| . | Dasatinib 100 mg qd, n = 258 . | Imatinib 400 mg qd, n = 258 . |
| Still on treatment | 183 (71) | 179 (69) |
| Discontinued | 75 (29) | 79 (31) |
| Progression* | 17 (7) | 18 (7) |
| Treatment failure† | 8 (3) | 12 (5) |
| Adverse event | 27 (10) | 16 (6) |
| Nonhematologic | 20 (8) | 12 (5) |
| Hematologic | 7 (3) | 4 (2) |
| Unrelated adverse event | 6 (2) | 2 (<1) |
| Death‡ | 4 (2) | 1 (<1) |
| Poor/nonadherence§ | 0 (0) | 4 (2) |
| Other|| | 13 (5) | 26 (10) |
Progression was defined as doubling of white blood cell count to >20 × 109/L, loss of CHR, increase in Ph-positive BM metaphases to ≥30% from nadir, transformation to accelerated phase/blast phase, or death from any cause.
Failure was defined as no hematologic response by 3 months, no CHR or cytogenetic response by 6 months, no PCyR by 12 months, or no CCyR by 18 months.
Patient discontinuations because of death (shown in table) represent a subset of total deaths (17 in dasatinib-treated patients and 20 in imatinib-treated patients at time of database lock).
As reported by investigators.
Includes consent withdrawal, loss to follow-up, pregnancy, patient request, and other reasons.
Efficacy and outcome
The higher rates of response with dasatinib compared with imatinib that were observed at 1 year were maintained at 3 years. The percentage of patients achieving CCyR at any time (unconfirmed, ≥20 metaphases) was 87% with dasatinib vs 83% with imatinib; the rate difference was 3.8% (95% confidence interval [CI], −2.4% to 10.1%; P = .23). The percentage of patients achieving cCCyR at any time (≥20 metaphases) was 82% vs 77% (dasatinib vs imatinib), with a rate difference of 4.5% (95% CI, −2.5% to 11.5%; P = .20). Median time to cCCyR was 3.1 months (95% CI, 3.0-3.1) with dasatinib and 5.8 months (95% CI, 5.6-6.0) with imatinib. The cumulative MMR rate by 36 months (calculated using the cumulative incidence approach–Kaplan-Meier method and allowing for competing events) was 69% for dasatinib vs 55% for imatinib (hazard ratio, 1.62; P < .0001) in randomized patients (Table 2). Likewise, cumulative rates of MR4 (MR with 4-log reduction from standardized baseline; BCR-ABL transcript level ≤0.01%) and MR4.5 (BCR-ABL transcript level ≤0.0032%) were higher with dasatinib vs imatinib (P = .0064 and P = .0007, respectively).
Cumulative molecular response rates by 36 months
| . | Randomized patients, % . | . | |
|---|---|---|---|
| Outcome . | Dasatinib 100 mg qd, n = 259 . | Imatinib 400 mg qd, n = 260 . | P value . |
| MMR | 69 | 55 | <.0001 |
| MR4 | 35 | 22 | .0064 |
| MR4.5 | 22 | 12 | .0007 |
| MMR by Euro score* | |||
| Low | 83 | 65 | .0068 |
| Intermediate | 65 | 57 | .1968 |
| High | 61 | 43 | .0808 |
| . | Randomized patients, % . | . | |
|---|---|---|---|
| Outcome . | Dasatinib 100 mg qd, n = 259 . | Imatinib 400 mg qd, n = 260 . | P value . |
| MMR | 69 | 55 | <.0001 |
| MR4 | 35 | 22 | .0064 |
| MR4.5 | 22 | 12 | .0007 |
| MMR by Euro score* | |||
| Low | 83 | 65 | .0068 |
| Intermediate | 65 | 57 | .1968 |
| High | 61 | 43 | .0808 |
For polymerase chain reaction–negative samples, ≥4.5-log test sensitivity was estimated if the total ABL (control gene) transcript level was ≥25 614/reaction volume complementary DNA.
MMR rates in Euro score subgroups are presented as simple rates (percentages of patients), whereas MMR, MR4, and MR4.5 rates are presented as cumulative incidence rates. Low risk, n = 170 (84 dasatinib, 86 imatinib); intermediate risk, n = 242 (121 dasatinib, 121 imatinib); high risk, n = 98 (49 dasatinib, 49 imatinib).
Transformation to AP/BP during study treatment occurred in a lower percentage of patients in the dasatinib arm (3% [95% CI, 1.3% to 6.0%]; 8 patients) compared with the imatinib arm (5% [95% CI, 2.7% to 8.4%]; 13 patients). The rate difference between arms (dasatinib – imatinib) was −1.9% (95% CI, −5.7% to 1.8%). A similar trend was observed throughout study follow-up, including events during and after study treatment: 4.2% (11/259 patients) in the dasatinib arm vs 6.2% (16/260 patients) in the imatinib arm transformed to AP/BP.
At the time of database cut, 17 patients randomized to dasatinib and 20 patients randomized to imatinib had died. The 3-year OS rates were 93.7% and 93.2% for patients receiving dasatinib and imatinib, respectively. The 3-year PFS rates were 91.0% and 90.9%, respectively, according to the study definition. For this calculation of PFS, the events considered were doubling of white blood cell count to >20 × 109/L, loss of CHR, increase in Ph-positive BM metaphases to ≥30% from nadir, transformation to AP/BP, or death from any cause. Rates of survival without transformation to AP/BP at 3 years were 94.1% and 93.5% for dasatinib and imatinib, respectively. The percentage of patients discontinuing therapy because of disease progression or treatment failure was 10% for dasatinib and 12% for imatinib.
Adverse events
Treatment was well tolerated in both arms, with most AEs occurring within the first year and minimal increases from 1 to 2 years and from 2 to 3 years of therapy. With the exception of pleural effusion, all of the most common (≥10%, any grade) drug-related nonhematologic AEs were either lower with dasatinib or comparable between arms (Table 3). Infection (drug-related and not drug-related) occurred in 33% of patients receiving dasatinib and 25% of patients receiving imatinib by 36 months. Pulmonary hypertension was reported in 8 patients receiving dasatinib and none receiving imatinib based on echocardiography performed under the direction of the investigator. Right heart catheterization, required for diagnosis,17,18 was performed in 1 patient and did not confirm pulmonary arterial hypertension. In the dasatinib arm, 1 patient discontinued study treatment as a result of pulmonary hypertension.
Rates of drug-related nonhematologic adverse events that occurred in ≥10% of patients
| . | Treated patients, % . | |||
|---|---|---|---|---|
| . | Dasatinib, n = 258 . | Imatinib, n = 258 . | ||
| . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . |
| Fluid retention | 31 | 3 | 44 | <1 |
| Superficial edema | 13 | 0 | 37 | <1 |
| Pleural effusion | 19 | 2 | <1 | 0 |
| Myalgia* | 23 | 0 | 41 | <1 |
| Nausea | 10 | 0 | 24 | 0 |
| Diarrhea | 21 | <1 | 22 | 1 |
| Vomiting | 5 | 0 | 11 | 0 |
| Rash | 13 | 0 | 18 | 2 |
| Headache | 13 | 0 | 11 | 0 |
| Fatigue | 9 | <1 | 11 | 0 |
| . | Treated patients, % . | |||
|---|---|---|---|---|
| . | Dasatinib, n = 258 . | Imatinib, n = 258 . | ||
| . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . |
| Fluid retention | 31 | 3 | 44 | <1 |
| Superficial edema | 13 | 0 | 37 | <1 |
| Pleural effusion | 19 | 2 | <1 | 0 |
| Myalgia* | 23 | 0 | 41 | <1 |
| Nausea | 10 | 0 | 24 | 0 |
| Diarrhea | 21 | <1 | 22 | 1 |
| Vomiting | 5 | 0 | 11 | 0 |
| Rash | 13 | 0 | 18 | 2 |
| Headache | 13 | 0 | 11 | 0 |
| Fatigue | 9 | <1 | 11 | 0 |
Includes myalgia, muscle spasms, and musculoskeletal pain.
Regarding grade 3/4 hematologic AEs, the rate of thrombocytopenia was higher for patients receiving dasatinib (19%) vs imatinib (11%); rates of neutropenia (24% vs 21%) and anemia (12% vs 9%) were similar. Except for grade 3/4 hypophosphatemia (dasatinib, 7%; imatinib, 28%), other types of grade 3/4 biochemical abnormalities occurred in ≤3% of patients and with a similar frequency in the dasatinib and imatinib arms.
Landmark analyses
Cytogenetic and molecular responses at 3 and 6 months are summarized in supplemental Table 1 (available on the Blood Web site).
Three-month landmark analysis.
At 3 months, treatment with dasatinib resulted in a higher proportion of patients with deeper cytogenetic and molecular responses. Dasatinib was associated with a higher percentage of patients achieving BCR-ABL transcript levels ≤10% compared with imatinib (84% vs 64%; P < .0001). Similarly, a higher percentage of patients achieved MCyR (≤35% Ph-positive cells in metaphase in BM; CCyR + PCyR) with dasatinib vs imatinib (81% vs 67%; P = .0006). Of note, transcript levels ≤10% and ≤1% were not fully concordant with PCyR and CCyR, respectively. Among patients with MCyR, 96% and 83% of those treated with dasatinib and imatinib, respectively, had BCR-ABL transcript levels ≤10%. Among patients with CCyR, 68% (dasatinib) and 26% (imatinib) had BCR-ABL transcript levels ≤1%.
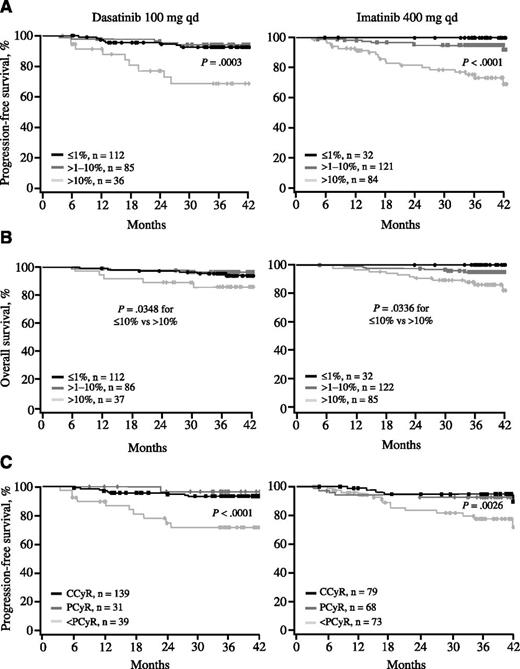
An MR, defined by a BCR-ABL transcript level ≤10% at 3 months, was predictive of PFS in both treatment arms. The 3-year PFS rates for patients with transcript levels ≤10% and >10% at 3 months were 93% and 68%, respectively, with dasatinib (Figure 2A, left panel; P = .0003) and 96% and 75%, respectively, with imatinib (Figure 2A, right panel; P < .0001). Transcript levels ≤10% were predictive of OS in both treatment arms. The 3-year OS rates for patients with transcript levels ≤10% and >10% at 3 months were 96% and 86%, respectively, with dasatinib (Figure 2B, left panel; P = .0348) and 96% and 88%, respectively, with imatinib (Figure 2B, right panel; P = .0036). No differences in PFS or OS were observed when comparing patients with transcript levels ≤1% and >1% to 10% at 3 months.
PFS and OS based on response at 3 months. (A) PFS according to BCR-ABL transcript levels at 3 months. Among patients receiving dasatinib, 48%, 37%, and 16% achieved BCR-ABL transcript levels ≤1%, >1% to 10%, and >10% at 3 months, respectively. Among patients receiving imatinib, the percentages were 13%, 51%, and 36%, respectively. Four patients (2 in each treatment arm) progressed before the 3-month molecular landmark and were not included in this analysis. (B) OS according to BCR-ABL transcript levels at 3 months. (C) PFS according to cytogenetic response at 3 months. Among patients receiving dasatinib, 67%, 15%, and 19% achieved CCyR, PCyR, and less than PCyR at 3 months, respectively. Among patients receiving imatinib, the percentages were 36%, 31%, and 33%, respectively. In all panels, patients with response data at 3 months and outcome data after 3 months were included in the analysis.
PFS and OS based on response at 3 months. (A) PFS according to BCR-ABL transcript levels at 3 months. Among patients receiving dasatinib, 48%, 37%, and 16% achieved BCR-ABL transcript levels ≤1%, >1% to 10%, and >10% at 3 months, respectively. Among patients receiving imatinib, the percentages were 13%, 51%, and 36%, respectively. Four patients (2 in each treatment arm) progressed before the 3-month molecular landmark and were not included in this analysis. (B) OS according to BCR-ABL transcript levels at 3 months. (C) PFS according to cytogenetic response at 3 months. Among patients receiving dasatinib, 67%, 15%, and 19% achieved CCyR, PCyR, and less than PCyR at 3 months, respectively. Among patients receiving imatinib, the percentages were 36%, 31%, and 33%, respectively. In all panels, patients with response data at 3 months and outcome data after 3 months were included in the analysis.
MCyR was a significant predictor of PFS in both arms and OS in the dasatinib arm. The 3-year PFS rates for patients with and without MCyR at 3 months were 94% and 71%, respectively, with dasatinib (Figure 2C, left panel; P < .0001) and 94% and 77%, respectively, with imatinib (Figure 2C, right panel; P = .0026). The 3-year OS rates for patients with and without MCyR at 3 months were 96% and 84%, respectively, with dasatinib (supplemental Figure 1, left panel; P = .0115) and 94% and 93%, respectively, with imatinib (supplemental Figure 1, right panel; P = .3681). In both arms, PCyR and CCyR led to similar outcomes.
Six-month landmark analysis.
At 6 months, 89% of patients receiving dasatinib had achieved BCR-ABL transcript levels ≤10%, compared with 83% in the imatinib arm (P = .0523). More patients reached a transcript level ≤1% with dasatinib (69% with dasatinib and 49% with imatinib; P < .0001). MCyR was observed in 91% of patients receiving dasatinib vs 81% of patients receiving imatinib (supplemental Table 1; P = .0028).
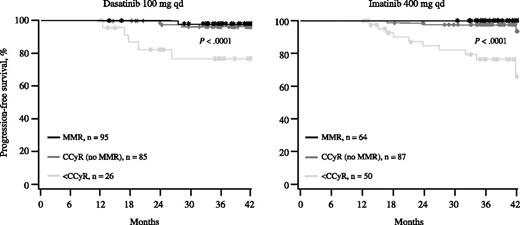
As with MR at 3 months, transcript levels ≤10% at 6 months were predictive of better PFS. Among patients receiving dasatinib, the 3-year PFS rates were 94% and 66% for those achieving BCR-ABL transcript levels ≤10% and >10%, respectively (Figure 3, left panel; P < .0001). The corresponding rates for imatinib were 95% and 65% (Figure 3, right panel; P < .0001). Achieving BCR-ABL transcript levels ≤1% was also predictive of PFS. The 3-year PFS rates for patients achieving ≤1% and >1% were 95% and 85%, respectively, in the dasatinib arm (P = .0020) and 97% and 84%, respectively, in the imatinib arm (P = .0016). Both arms showed a trend toward a difference in PFS in those achieving transcript levels ≤1% compared with >1% to 10%, but this difference was not significant (95% vs 91% for the dasatinib arm [P = .1383] and 97% vs 92% for the imatinib arm [P = .2021]).
PFS according to BCR-ABL transcript levels at 6 months. Among patients receiving dasatinib, 69%, 19%, and 11% achieved BCR-ABL transcript levels ≤1%, >1% to 10%, and >10% at 6 months, respectively. Among patients receiving imatinib, the percentages were 49%, 34%, and 17%, respectively. Patients with response data at 6 months and outcome data after 6 months were included in the analysis.
PFS according to BCR-ABL transcript levels at 6 months. Among patients receiving dasatinib, 69%, 19%, and 11% achieved BCR-ABL transcript levels ≤1%, >1% to 10%, and >10% at 6 months, respectively. Among patients receiving imatinib, the percentages were 49%, 34%, and 17%, respectively. Patients with response data at 6 months and outcome data after 6 months were included in the analysis.
BCR-ABL transcript levels ≤10% at 6 months were predictive of better OS in both treatment arms. The 3-year OS rate was 97% with transcript levels ≤10% and 84% with transcript levels >10% in patients treated with dasatinib (supplemental Figure 2A, left panel; P = .0091). These rates were 96% and 88% for patients receiving imatinib therapy (supplemental Figure 2A, right panel; P = .0004). No differences in OS were observed when comparing patients with transcript levels ≤1% and >1% to 10% at 6 months.
The achievement of MCyR at 6 months was significantly predictive of PFS in both arms and OS in the imatinib arm. The 3-year PFS rates for patients with and without MCyR at 6 months were 94% and 83%, respectively, with dasatinib (supplemental Figure 2B, left panel; P = .0282) and 95% and 69%, respectively, with imatinib (supplemental Figure 2B, right panel; P < .0001). The 3-year OS rates for patients with and without MCyR at 6 months were 97% and 94%, respectively, with dasatinib (supplemental Figure 2C, left panel; P = .6649) and 96% and 90%, respectively, with imatinib (supplemental Figure 2C, right panel; P = .0124). PCyR and CCyR led to similar outcomes.
Transformation risk by 3- and 6-month landmark MRs.
Risk of transformation was increased 6-fold in both treatment arms for patients who did not achieve transcript levels ≤10% at 3 months (supplemental Table 2). The risk increased to 13- and 10-fold in the dasatinib and imatinib arms, respectively, for patients who did not achieve these levels at 6 months. Not achieving BCR-ABL transcript levels ≤1% at 6 months was associated with a 6-fold increase in risk of transformation among patients receiving dasatinib and a 12-fold increase among patients receiving imatinib.
Twelve-month landmark analysis.
At 12 months, a higher proportion of patients on dasatinib had achieved MMR (46% with dasatinib vs 28% with imatinib) or CCyR (83% with dasatinib vs 72% with imatinib).1 In both treatment arms, patients who achieved MMR or CCyR had significantly better (P < .0001) PFS than those who did not (Figure 4). There was no difference in outcome between patients who achieved MMR and those who achieved only CCyR. Similarly, the achievement of CCyR at 12 months was predictive of OS in both treatment arms regardless of achievement of MMR (supplemental Figure 3).
PFS according to response at 12 months. Patients with response data at 12 months and outcome data after 12 months were included in the analysis.
PFS according to response at 12 months. Patients with response data at 12 months and outcome data after 12 months were included in the analysis.
Discussion
The 3-year follow-up of the DASISION trial continues to support earlier response with dasatinib compared with imatinib. At 3 and 6 months, dasatinib treatment was associated with a higher proportion of MCyR and BCR-ABL transcript levels ≤10%. Fluid retention, infection, and hematologic toxicity seen during long-term follow-up were manageable in both arms and did not impede overall positive outcomes. Other than hypophosphatemia, severe biochemical abnormalities were rare in both arms. There were no reports of elevations in lipase, amylase, or glucose; however, these laboratory parameters were not collected systematically in all patients.
Although cytogenetic response rates were higher with dasatinib at 36 months, the statistically significant difference that was observed at earlier time points (12 months and 24 months) was no longer present at 36 months. Patients who did not achieve CCyR with dasatinib likely discontinued, whereas additional patients in the imatinib arm may have achieved CCyR later. The difference in MR rates between arms remained statistically significant at 36 months, with higher cumulative incidence rates of MMR (P < .0001), MR4 (P = .0064), and MR4.5 (P = .0007) with dasatinib vs imatinib.
Response during TKI therapy is the most important prognostic factor for long-term outcome in CML.5-8 Our analysis has shown that the achievement of early cytogenetic responses and MRs at 3, 6, and 12 months was associated with better PFS, OS, and reduced risk of transformation. Response rates were higher with dasatinib therapy, although overall PFS and OS rates were not significantly higher in the dasatinib arm. Three-year rates of survival without transformation to AP/BP were also similar between arms.
There are several reasons why early responses with dasatinib may not have translated into superiority of PFS or OS at this database lock. First, the responses of patients treated with imatinib improve by 6 months overall. Also, more patients discontinued imatinib because of treatment failure, which is not counted as progression, and these patients could have been rescued by other drugs, including dasatinib, off study. It may have been more challenging to rescue patients with failure on dasatinib, a more potent treatment, with secondary therapies available during the study. Use of secondary therapies limited our ability to attribute PFS and OS to the randomly assigned treatments in these post hoc landmark analyses. A difference in PFS or OS rates between arms may emerge with longer follow-up.
Overall, our findings provide a global confirmation of single-institution and regional experiences.19-21 Hanfstein et al20 reported significant improvement in PFS and OS in patients who achieved MCyR or BCR-ABL transcript levels ≤10% with 3 months of imatinib therapy. Marin et al21 reported that achieving BCR-ABL transcript levels ≤9.84% at 3 months increased the likelihood of achieving CCyR, MMR, and MR4.5 and significantly improved PFS and OS in patients treated with imatinib. Our observation that achieving 6-month BCR-ABL transcript levels ≤1% vs >1% was highly predictive of better 3-year PFS further strengthens the assertion that fast, deep responses are associated with improved outcomes. However, these data are preliminary, and a clinically meaningful separation of outcomes according to transcript levels ≤1% vs >1% to 10% at 6 months may emerge with longer follow-up.
The present study adds to the evidence that the achievement of MCyR at 3 and 6 months has a significant impact on long-term outcomes with second-generation TKIs.11,12 Of note, MCyR at 3 months was predictive of OS for dasatinib, but not imatinib, whereas MCyR at 6 months was predictive of OS for imatinib, but not dasatinib. This finding indicates that some treatment milestones for second-generation TKIs may be earlier than those for imatinib.
Our study has confirmed, in a large international sample, the importance of 12-month CCyR, with or without MMR, as a major surrogate end point for survival outcomes. This finding is consistent with recent reports,5,7,8,11 including a study by Hehlmann et al22 that found that the achievement of a BCR-ABL transcript level ≤1% on the international scale—a level that closely correlates with CCyR—at 12 months confers better PFS and OS at 3 years compared with the achievement of a BCR-ABL transcript level >1%.
In summary, dasatinib used in first-line therapy induces fast and deep cytogenetic and molecular responses that translate to better outcomes. The findings of our analysis demonstrate the prognostic value of MCyR at 3 to 6 months and CCyR at 12 months, supporting the assertion that these milestones reflect optimal responses to first-line therapy. Our data also support that BCR-ABL transcript levels >10% at 3 months are associated with worse outcomes. BCR-ABL transcript levels ≤1% at 6 months could be considered a new prognostic threshold because of the association of this cutoff with reduced risk for transformation and a trend for better PFS. These milestones for the optimal response of patients with newly diagnosed CML-CP treated with TKIs may have practical applications, including the early identification of patients with an excellent prognosis and those who may require careful monitoring and/or intervention.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participating study sites for this Bristol-Myers Squibb−sponsored study (CA180-056). Professional medical writing support, funded by Bristol-Myers Squibb, was provided by Zenab Amin of StemScientific. The authors received no financial compensation for authoring the manuscript.
Authorship
Contribution: H.M.K., G.S., N.P.S., J.C., M.B., and A.H. designed the research; G.S., J.L.S., C.B., C.C., J.M., M.B., and D.-W.K. enrolled patients and collected data; E.J., H.M.K., G.S., N.P.S., C.P., J.M., J.C., M.B., D.-W.K., M.B.B.-G., H.M., M.W., and A.H. analyzed and interpreted data; and all authors wrote and/or critically reviewed the manuscript and agreed upon the final version.
Conflict-of-interest disclosure: E.J. has received honoraria from Bristol-Myers Squibb (BMS), Novartis, Pfizer, and Ariad. H.M.K. has received research funding from BMS, Novartis, Pfizer, and Ariad and acted as a consultant for Novartis. G.S. has acted as a consultant for and received honoraria from BMS and Novartis. J.L.S. has acted as a consultant for and received honoraria and research funding from BMS, Novartis, and Pfizer. N.P.S. has acted as a consultant for BMS, Novartis, and Ariad and received research funding from BMS and Ariad. C.B. has received honoraria from BMS. C.C. has acted as a consultant for BMS and Novartis and received honoraria from BMS. C.P. has received research funding from Novartis. J.M. has acted as a consultant for and received honoraria and research funding from BMS and Novartis. J.C. has acted as a consultant for and received research funding from BMS, Novartis, Pfizer, and Ariad. M.B. has acted as a consultant for and received honoraria from BMS, Novartis, and Pfizer. D.-W.K. has acted as a consultant for BMS and Novartis and received honoraria and research funding from BMS, Novartis, and Wyeth. M.B.B.-G. and H.M. are employees of BMS and declare competing financial interest. M.W. was an employee of BMS at the time this analysis was conducted and declared a competing financial interest. A.H. has acted as a consultant for and received research funding from BMS, Novartis, Pfizer, Ariad, and MSD.
Correspondence: Hagop M. Kantarjian, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.