Key Points
Implanting autologous FVIII-expressing BOECs results in sustained FVIII antigen in hemophilia dogs.
Anti-FVIII immunoglobulin G2 antibodies develop.
Abstract
Ex vivo gene therapy strategies avoid systemic delivery of viruses thereby mitigating the risk of vector-associated immunogenicity. Previously, we delivered autologous factor VIII (FVIII)-expressing blood outgrowth endothelial cells (BOECs) to hemophilia A mice and showed that these cells remained sequestered within the implanted matrix and provided therapeutic levels of FVIII. Prior to translating this strategy into the canine (c) model of hemophilia A, we increased cFVIII transgene expression by at least 100-fold with the use of the elongation factor 1 alpha (EF1α) promoter and a strong endothelial enhancer element. BOECs isolated from hemophilia A dogs transduced with this lentiviral vector express levels of cFVIII ranging between 1.0 and 1.5 U/mL per 106 cells over 24 hours. Autologous BOECs have been implanted into the omentum of 2 normal and 3 hemophilia A dogs. These implanted cells formed new vessels in the omentum. All 3 hemophilia A dogs treated with FVIII-expressing autologous BOECs developed anti-FVIII immunoglobulin G2 antibodies, but in only 2 of the dogs were these antibodies inhibitory. FVIII antigen levels >40% in the absence of FVIII coagulant function were detected in the circulation for up to a year after a single gene therapy treatment, indicating prolonged cellular viability and synthesis of FVIII.
Introduction
Although gene transfer studies for hemophilia B have shown recent success,1 hemophilia A gene therapy is a more complex challenge, as illustrated by the fact that none of the 5 approved human trials (http://www.wiley.com//legacy/wileychi/genmed/clinical/) have progressed beyond phase I. Two of the obstacles to success are low levels of factor VIII (FVIII) expression and the anti-FVIII immune response. Although the size and composition of the F8 gene contribute to inefficient expression2,3 and secretion,4,5 changes to the transgene cassette have improved both parameters.6-10 In contrast, the anti-FVIII immune response remains a significant obstacle to successful gene therapy for hemophilia A. This adverse event occurs in ∼25% of severely affected patients treated with replacement therapy and is associated with both genetic and acquired pathogenic factors.11,12 An added risk factor associated with gene therapy is the use of viral vectors, which act as adjuvants to initiate the anti-FVIII immune response.13
Currently, no F8 gene delivery strategy stands out as superior. In general adeno-associated viral and lentiviral vectors are more commonly used but both have limitations, including difficulty in producing the high yields of vectors required for clinical use and challenges centered on the antiviral immune response.1,14-17 The recent observation of anti-FVIII antibodies in nonhuman primates after adeno-associated virus delivery of F8 (albeit with a human F8 transgene) suggests that the transition to clinical trial successes may still face challenges.18 In light of these difficulties, new strategies need to be evaluated. There is interest within the hemophilia community for the use of gene therapy as a treatment modality, provided it is safe.19 With this in mind, we have developed an ex vivo gene delivery strategy that uses autologous blood outgrowth endothelial cells (BOECs) transduced with a lentiviral vector to express FVIII.20 We demonstrated efficacy with this strategy in the murine model of hemophilia A,20 and in the current study, we are translating this strategy into the larger canine (c) model of hemophilia A. Here we present levels of FVIII expression along with an evaluation of the immune response after delivering the FVIII-expressing cells to 3 dogs.
Material and methods
Lentiviral vector construction and production
The lentiviral plasmids, pLenti-CMV-cFVIII and pLenti-TM-cFVIII, containing the cytomegalovirus (CMV) and thrombomodulin (TM) promoters, respectively, and canine B domain–deleted (BDD) F8 complementary DNA have previously been described.20 The CMV promoter was excised and replaced with the human elongation factor 1 alpha (EF1α) promoter. Three additional constructs were engineered that contain either 1, 2, or 3 copies of the 164-bp endothelial-specific protein C receptor enhancer element (–5.5HSCR)21 (Figure 1A). Lentiviral vectors were produced, and titers were determined as previously described.20
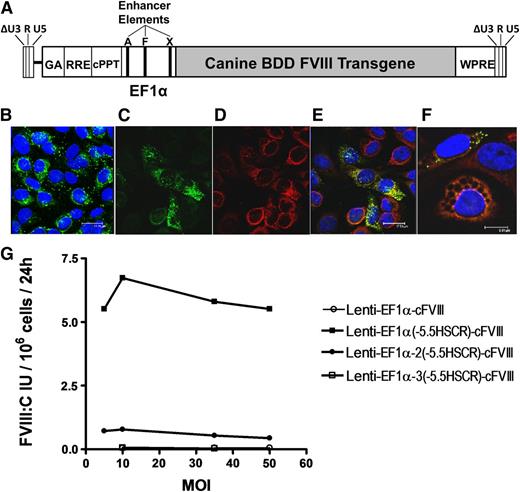
Evaluation of modified lentiviral vectors for FVIII expression in cBOECs. (A) Schematic representation of the third-generation, self-inactivating lentiviral vector containing the cFVIII transgene under the control of the human EF1α promoter into which 1 to 3 copies of the 164-bp endothelial-specific enhancer element (–5.5HSCR were inserted. Unique AgeI (A), XhoI (X), and FseI (F) restriction sites in the promoter were used to insert the endothelial enhancer element into either the AgeI, or AgeI and XhoI, or AgeI and XhoI and FseI sites for constructs containing 1, 2, or 3 copies, respectively. (B) Confocal analysis of cultured BOECs representative of those isolated from hemophilia A dogs. BOECs were immunostained with vWF (green) and were used only if >90% of the cells stained positive for vWF. (C-F) Confocal microscopic analysis of BOECs transduced with Lenti-EF1α-(–5.5HSCR)-cFVIII and immunostained with vWF (green), 4,6 diamidino-2-phenylindole (DAPI) nuclear counterstain (blue), and FVIII (red). (E-F) Merged images of FVIII and vWF staining. (G) cBOECs (1 × 105 cells) cultured in 6-well plates were transduced with Lenti-EF1α-cFVIII, Lenti-EF1α(–5.5HSCR)-cFVIII, Lenti-EF1α-2(–5.5HSCR)-cFVIII, or Lenti-EF1α-3(–5.5HSCR)-cFVIII containing 0, 1, 2, or 3 copies, respectively, of the endothelial enhancer element. Culture media was collected 3 days later and FVIII activity (FVIII:C) was measured. Experiments were repeated at least 3 times.
Evaluation of modified lentiviral vectors for FVIII expression in cBOECs. (A) Schematic representation of the third-generation, self-inactivating lentiviral vector containing the cFVIII transgene under the control of the human EF1α promoter into which 1 to 3 copies of the 164-bp endothelial-specific enhancer element (–5.5HSCR were inserted. Unique AgeI (A), XhoI (X), and FseI (F) restriction sites in the promoter were used to insert the endothelial enhancer element into either the AgeI, or AgeI and XhoI, or AgeI and XhoI and FseI sites for constructs containing 1, 2, or 3 copies, respectively. (B) Confocal analysis of cultured BOECs representative of those isolated from hemophilia A dogs. BOECs were immunostained with vWF (green) and were used only if >90% of the cells stained positive for vWF. (C-F) Confocal microscopic analysis of BOECs transduced with Lenti-EF1α-(–5.5HSCR)-cFVIII and immunostained with vWF (green), 4,6 diamidino-2-phenylindole (DAPI) nuclear counterstain (blue), and FVIII (red). (E-F) Merged images of FVIII and vWF staining. (G) cBOECs (1 × 105 cells) cultured in 6-well plates were transduced with Lenti-EF1α-cFVIII, Lenti-EF1α(–5.5HSCR)-cFVIII, Lenti-EF1α-2(–5.5HSCR)-cFVIII, or Lenti-EF1α-3(–5.5HSCR)-cFVIII containing 0, 1, 2, or 3 copies, respectively, of the endothelial enhancer element. Culture media was collected 3 days later and FVIII activity (FVIII:C) was measured. Experiments were repeated at least 3 times.
Animals
Isolation, culture, and transduction of BOECs
Venous blood was collected into heparinized cell preparation tubes (BD, Mississauga, Canada) from normal and hemophilia A dogs and canine BOECs (cBOECs) were isolated as previously outlined.20 One million cBOECs were transduced at increasing multiplicities of infection (MOI) by using a Viraductin lentivirus transduction kit (Cell Biolabs, San Diego, CA). The transduced cells were cultured in 6-well plates, and cFVIII activity was assayed as outlined below. Immunostaining for immunohistochemistry and confocal analysis was carried out by using sheep anti-cFVIII (Affinity Biologicals, Ancaster, Canada) as primary antibodies and Cy3-conjugated anti-sheep immunoglobulin G (IgG) (Abcam, Cambridge, MA) as secondary antibodies, respectively. Rabbit anti-human von Willibrand factor (vWF) and fluorescein isothiocyanate–conjugated anti-rabbit IgG antibodies (DakoCytomation, Glostrup, Denmark) were used to stain canine vWF.
Preparation of autologous fibrinogen
After 0.15 g of MgSO4 and 3.0 g BaSO4 were added to 30 mL of citrated plasma, the mix was agitated for 1 hour and centrifuged at 1500g for 20 minutes. The pellet was resuspended in 28 mL of 55 mM sodium citrate (pH 7.4), and 5 g of glycine was added. The sample was agitated for 30 minutes, centrifuged at 1800g for 20 minutes, and resuspended in Hank’s balanced salt solution (HBSS) to a final concentration of 2 mg/mL.
Preparation of BOECs for implanting into the omentum
After washing cBOECs 3 times with HBSS containing 0.6% citrate, cells were suspended in 6 mL HBSS and placed on ice. To this was added 6 mL of a solution consisting of 20 mM CaCl2, 500 U/mL thrombin (Beriplast P Combi Set, CLS Behring, King of Prussia, PA), 0.1 μg/mL vascular endothelial growth factor (R&D Systems, Minneapolis, MN), and 0.1 μg/mL fibroblast growth factor 2 (FGF2) (R&D Systems). The cells and autologous fibrinogen were each drawn into individual syringes of the Pantaject dual syringe from the Beriplast P Combi Set.
Implanting the FVIII-expressing BOECs into the omentum
Dogs were pretreated with cFVIII (10 U/kg in the form of canine cryoprecipitate) and butorphonal-acepromazine-glycopyrrolate (0.1 mL/kg2), anesthetized with an intravenous infusion of ketamine-valium (0.1 mL/kg2), and maintained on isoflurane. Animals were intubated and ventilated, and they received lactated Ringer’s solution throughout the procedure. An abdominal ventral midline incision was made to exteriorize the greater omentum, and a total volume of 1 mL of the cBOEC-thrombin mixture and fibrinogen was injected into each site (between 10 and 14 sites were injected). Upon completion, the omentum was placed back into the abdomen and the incision was closed. Additional infusions of cFVIII (10 U/kg) were administered 12 and 24 hours after the procedure or as required to stop bleeding. Pain was treated prophylactically with buprenorphine (0.01 mg/kg) for up to 4 days after the procedure.
Transient immune suppression
An intravenous infusion of cyclophosphamide (200 to 250 mg/m2) was administered 1 day prior to the day of surgery and once per week thereafter for 6 weeks. To prevent urothelial toxicity, 3 mg/kg furosemide and 20 mg/mL 2-mercaptoethane sulfonate was given intravenously over 5 to 10 minutes.
FVIII activity and antigen assays
Canine blood was collected into 10% buffered citrate. Blood was centrifuged at 2500g for 20 minutes at 4°C, and plasma was stored at −80°C. cFVIII activity in plasma and tissue culture media was determined by one-stage activated partial thromboplastin time–based assay in a semiautomated coagulometer (Diagnostica Stago). Samples were diluted in cFVIII-deficient plasma, and cFVIII antigen levels were measured by an enzyme-linked immunosorbent assay (ELISA). Plates (Immulon 4HBX; Thermo Scientific, Waltham, MA) were coated with 10 µg/mL of a polyclonal sheep anti-cFVIII antibody (SAC8C-IG; Affinity Biologicals), and cFVIII was detected with a horseradish peroxide–conjugated polyclonal sheep anti-cFVIII antibody (SAC8C-HRP; Affinity Biologicals) diluted in Stabilizyme HRP:Ultrapure H2O (SZ02-1000; SurModics, Eden Prairie, MN) and nonimmune sheep IgG (NIS-Ig; Affinity Biologicals). Plasma samples were diluted in Specimen Diluent Green II with 0.85 M NaCl (C10201; AlerCHECK, Springvale, ME). Protein concentrations were measured at 490 nm using a microplate reader (VersaMax; Molecular Devices, Downingtown, PA). Normal canine pooled plasma was used to generate the standard curve for both assays.
Anti-FVIII IgG1, IgG2, and inhibitor assays
FVIII inhibitors were measured with a Bethesda assay as previously outlined.24 The sensitivity of this assay is 0.5 BU/mL. Anti-FVIII IgG1 and IgG2 levels were determined by ELISA. Microtiter plates (Immulon 4HBX; Thermo Scientific) were coated with 1.5 µg/mL recombinant cFVIII (gift from Dr R. Camire, University of Pennsylvania) and blocked with Low Cross Buffer (Candor Bioscience GmbH, Wangen, Germany). Plasma samples were diluted 1:4 and 1:20 in Low Cross Buffer for IgG1 and IgG2 assays, respectively, and anti-FVIII IgG1 and IgG2 antibodies were detected with horseradish peroxidase–conjugated polyclonal goat anti-cIgG1 (A40-120P; Bethyl Laboratories, Montgomery, TX) and polyclonal sheep anti-cIgG2 (A40-121P; Bethyl Laboratories). The standard curve was generated by using standardized canine serum (RS10-105; Bethyl Laboratories).
Assessing BOEC interactions with thrombin
Because antibodies for canine protease-activated receptor 1 (PAR1) are not commercially available, human BOECs (hBOECs) were used instead. The cells included in this study were >90% positive for CD31, CD144, and CD146 and had <3% CD14+ and CD45+ cells. hBOECs were exposed to 5 or 10 U/mL of thrombin (Bayer, Toronto, ON, Canada) or 1 μg/mL lipopolysaccharide (LPS) for 24 hours, and 1 × 106 cells were assessed by flow cytometry for surface expression of uncleaved PAR1 (IOTest Anti-Thrombin Receptor PE [clone SPAN12]; Beckman Coulter, Fullerton, CA), ICAM-1 (anti-human CD54 [ICAM-1] PE; eBioscience, USA), CD40 (eBioscience; #11-0409), CD80 (eBioscience; #12-0809), CD86 (eBioscience; #15-0869), and major histocompatibility complex (MHC) class II (HLA DP, DQ, DR; Dako; F0817). THP-1 cells (monocytes) and Raji cells (B cells) were used as positive controls for CD40, CD80, CD86, and MHC class II. Isotype controls were included for all antibodies used. Levels of interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) secreted by cultured hBOECs exposed to thrombin or LPS were measured by using an ELISA-based assay (R&D Systems, Minneapolis, MN).
Purification of Ig antibodies and screening a cFVIII peptide library
Results
Lentiviral vectors with improved cFVIII expression
With a view toward improving levels of cFVIII expression, we replaced the original TM promoter with the stronger EF1α promoter and assessed expression in cBOECs isolated from hemophilia A dogs. Antibodies routinely used to characterize hBOECs are not available for dogs and therefore vWF expression was assessed to ensure that >95% of the cBOECs displayed an endothelial phenotype (Figure 1B). cBOECs isolated from hemophilia A dogs showed no evidence of FVIII expression as assessed by confocal analysis (supplemental Figure 1). These cells were transduced with lentiviral vectors (MOI 20) containing either the CMV, TM, or EF1α promoters, and functional cFVIII expression was measured in culture media 2 weeks after transduction. Relative to pooled canine plasma, levels of cFVIII expression were 0.1, 0.25, and 0.4 U per 106 cells over 24 hours for constructs containing the TM, CMV, and EFIα promoters, respectively. Confocal analysis demonstrated colocalization of cFVIII with the endogenous canine vWF in Weibel-Palade bodies and a reduction in the percentage of cells that expressed vWF (Figure 1C-E).
To further improve cFVIII expression, we included an endothelial enhancer element (–5.5HSCR)21 in 3 separate locations within the EF1α promoter. Increases of 104- and 10.6-fold were observed when 1 and 2 copies, respectively, of –5.5HSCR were incorporated into the EF1α promoter; no apparent increase in cFVIII was observed with 3 copies of the enhancer element (Figure 1G). However, we observed considerable cell death, particularly in cBOECs transduced with vectors containing 2 and 3 copies of –5.5HSCR, and within 48 hours, only 50% and 20%, respectively, of the cells survived. It has been reported that elevated levels of FVIII expression are associated with initiation of the unfolded protein response and apoptosis,27,28 and therefore we speculate that the increased cell death we observed was likely caused by the markedly elevated FVIII expression that triggered the unfolded protein response. A small number of transduced cells with an abnormal appearance suggestive of apoptosis were still seen when using a single enhancer element (Figure 1F). Even though initial cFVIII expression was high, levels declined with time, and by 2 weeks, an average of 1.0 to 1.5 U/mL per 106 cells over 24 hours of cFVIII was observed (supplemental Figure 2). In addition, this reduction in levels of FVIII expression was also associated with increased viability and minimal cell death. Therefore, given that levels of FVIII expression were consistently higher when cells were transduced with Lenti-EF1α-(–5.5HSCR)-cFVIII and because these cells could be readily expanded to very large numbers, we chose to use this construct to evaluate our gene delivery strategy in dogs.
Sustained expression of FVIII from cBOECs implanted into the omentum of two normal dogs
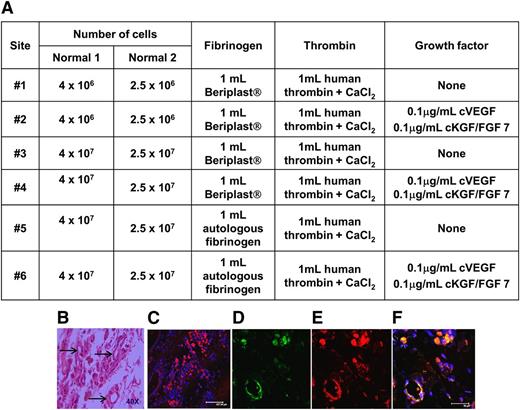
To exploit its angiogenic capacity, we used the greater omentum as the site to implant cFVIII-expressing cBOECs. To avoid complications associated with an anti-FVIII immune response and ensure that biopsies could be safely retrieved, we initially evaluated this strategy in 2 normal dogs. Different densities of transduced autologous cBOECs were implanted, with or without growth factors, in two different fibrin-based support scaffolds into six individual sites in each normal dog (Figure 2A). Biopsies obtained 1 or 3 months after implantation showed no evidence of lymphocyte infiltration or structures that resembled blood vessels (Figure 2B). Immunohistochemical analysis revealed significant numbers of cFVIII-expressing cells (Figure 2C). The cells within structures that resembled vessels expressed both cFVIII and canine vWF (Figure 2D-F). Because more apparent structures resembling vessels were identified in the biopsy obtained from site #6, these conditions were used to evaluate the efficacy of this strategy in the hemophilia A dogs.
Evidence of neovascularization after implanting autologous BOECs mixed in various fibrin-based matricies into the omentum. (A) Autologous BOECs isolated from 2 normal dogs (Normal 1 and Normal 2) were implanted into 6 individual sites (sites #1 to #6) in the greater omentum in each of the dogs. Various numbers of BOECs (2.5 × 106 to 4 × 107) were implanted in each site. The fibrin-based matrix consisted of fibrinogen (either autologous or obtained from the Beriplast kit) and human thrombin from the Beriplast kit. Growth factors were included with the cells implanted into sites #2, #4, and #6. (B) Normal dog #2 was biopsied 3 months after implanting the autologous BOECs. Hematoxylin and eosin staining of a representative sample isolated from site #6. Arrows indicate areas of neovascularization. (C) Confocal microscopic analysis of a biopsy sample taken from site #6 and immunostained for FVIII (red). (D-F) Representative images of one of the structures appearing to be a small blood vessel immunostained with antibodies for FVIII (red), vWF (green), and DAPI (blue).
Evidence of neovascularization after implanting autologous BOECs mixed in various fibrin-based matricies into the omentum. (A) Autologous BOECs isolated from 2 normal dogs (Normal 1 and Normal 2) were implanted into 6 individual sites (sites #1 to #6) in the greater omentum in each of the dogs. Various numbers of BOECs (2.5 × 106 to 4 × 107) were implanted in each site. The fibrin-based matrix consisted of fibrinogen (either autologous or obtained from the Beriplast kit) and human thrombin from the Beriplast kit. Growth factors were included with the cells implanted into sites #2, #4, and #6. (B) Normal dog #2 was biopsied 3 months after implanting the autologous BOECs. Hematoxylin and eosin staining of a representative sample isolated from site #6. Arrows indicate areas of neovascularization. (C) Confocal microscopic analysis of a biopsy sample taken from site #6 and immunostained for FVIII (red). (D-F) Representative images of one of the structures appearing to be a small blood vessel immunostained with antibodies for FVIII (red), vWF (green), and DAPI (blue).
Transient immune suppression does not prevent the anti-FVIII immune response
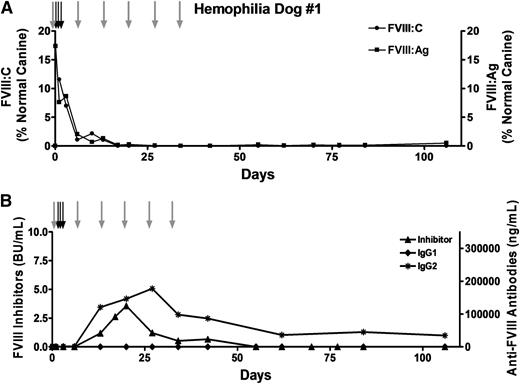
Because transient immune suppression prevented the anti-FVIII immune response in the murine model of hemophilia A20 as well as in hemophilia B dogs,29,30 we treated hemophilia A dog #1 with 6 once-per-week intravenous infusions of cyclophosphamide (200 to 250 mg/m2). Autologous FVIII-expressing cBOECs were implanted into the omentum, and although functional FVIII activity and antigen were initially detected (Figure 3A), levels dropped rapidly and were undetectable within 3 weeks. The loss of FVIII activity was attributed to inhibitory and IgG2 anti-FVIII antibodies that first appeared at week 2 (Figure 3B). Inhibitors peaked by 3 weeks and then dropped slowly and were undetectable by week 8. Levels of IgG2 antibodies also dropped slowly but persisted throughout the duration of the study.
Transient immune suppression does not prevent the anti-FVIII immune response. Hemophilia dog #1 was 17 months old and weighed 10.6 kg when the procedure was performed. A total of 2 × 109 autologous BOECs that expressed 1.0 U FVIII per 106 cells over 24 hours in vitro were implanted into 18 sites (1.1 × 108 cells per site). To prevent bleeding problems, the dog received 3 infusions of canine cryoprecipitate (black arrows) before and after the procedure. This dog received 6 once-per-week infusions of cyclophosphamide (200 to 250 mg/m2) (gray arrows), the first of which occurred on the day before the cells were implanted. Plasma samples were collected at each of the indicated time points. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured, and the results were compared against pooled normal canine plasma samples isolated from 8 healthy dogs. The FVIII:C and FVIII:Ag values presented in the figure are relative to the canine pooled plasma sample that was arbitrarily set at 100%. (B) Anti-FVIII inhibitory antibodies were detected by using a standard Bethesda assay. Anti-cFVIII IgG1 and IgG2 antibodies were detected by a cFVIII-specific ELISA.
Transient immune suppression does not prevent the anti-FVIII immune response. Hemophilia dog #1 was 17 months old and weighed 10.6 kg when the procedure was performed. A total of 2 × 109 autologous BOECs that expressed 1.0 U FVIII per 106 cells over 24 hours in vitro were implanted into 18 sites (1.1 × 108 cells per site). To prevent bleeding problems, the dog received 3 infusions of canine cryoprecipitate (black arrows) before and after the procedure. This dog received 6 once-per-week infusions of cyclophosphamide (200 to 250 mg/m2) (gray arrows), the first of which occurred on the day before the cells were implanted. Plasma samples were collected at each of the indicated time points. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured, and the results were compared against pooled normal canine plasma samples isolated from 8 healthy dogs. The FVIII:C and FVIII:Ag values presented in the figure are relative to the canine pooled plasma sample that was arbitrarily set at 100%. (B) Anti-FVIII inhibitory antibodies were detected by using a standard Bethesda assay. Anti-cFVIII IgG1 and IgG2 antibodies were detected by a cFVIII-specific ELISA.
Prolonged survival of implanted FVIII-expressing BOECs in hemophilia A dogs
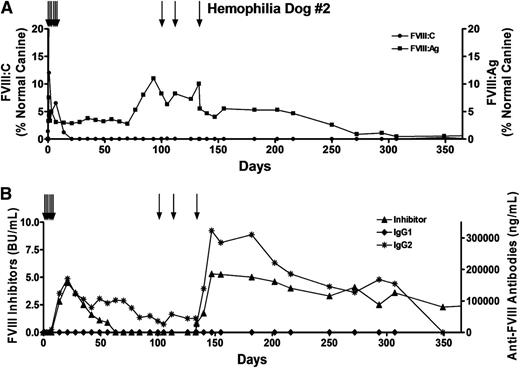
Because transient immune suppression failed to prevent the anti-FVIII immune response, we chose not to treat hemophilia A dog #2 with cyclophosphamide. Instead, this dog received 2 infusions of canine cryoprecipitate (35 and 38 U FVIII) 8 and 1 month prior to surgery. However, although functional FVIII was initially detected after the autologous FVIII-expressing cBOECs were implanted, levels dropped rapidly and were undetectable by 3 weeks (Figure 4A). Once again, low titers of FVIII inhibitors and IgG2 antibodies appeared by week 2 (Figure 4B). In both of the treated hemophilia A dogs, the appearance of inhibitory antibodies was accompanied by a dramatic increase (∼100-fold) in anti-FVIII IgG2 antibodies that persisted throughout the duration of the study. In dog #2, FVIII inhibitors peaked by day 28 and then dropped slowly to undetectable levels. However, these inhibitors reappeared after this dog received an infusion of FVIII on day 133 posttreatment. Notably, despite the presence of anti-FVIII antibodies, FVIII antigen was detected in the plasma (1% to 10% levels) for at least 250 days, indicating that the implanted cBOECs were viable and continued to express cFVIII for an extended period of time.
Prolonged survival and expression of FVIII:Ag from genetically modified BOECs implanted into the omentum. Hemophilia dog #2 was 10 months old and weighed 9.4 kg when the procedure was performed. Approximately 4.5 × 107 cells were injected into each of 11 sites. In vitro these cells expressed 1.5 IU of cFVIII per 106 cells over 24 hours. To prevent bleeding problems, the dog received multiple infusions of canine cryoprecipitate (black arrows) before and after the procedure, including 3 infusions of 56, 48, and 140 U FVIII on days 100, 113, and 133, respectively. Plasmas samples were obtained at the indicated time points. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured, and the results were compared with pooled normal canine plasma samples that were arbitrarily set at 100%. Plasma samples from 13 untreated hemophilia A dogs showed no detectable FVIII:Ag. (B) Levels of inhibitory antibodies were measured by a Bethesda assay, and anti-FVIII IgG1 and IgG2 antibodies were measured by ELISA.
Prolonged survival and expression of FVIII:Ag from genetically modified BOECs implanted into the omentum. Hemophilia dog #2 was 10 months old and weighed 9.4 kg when the procedure was performed. Approximately 4.5 × 107 cells were injected into each of 11 sites. In vitro these cells expressed 1.5 IU of cFVIII per 106 cells over 24 hours. To prevent bleeding problems, the dog received multiple infusions of canine cryoprecipitate (black arrows) before and after the procedure, including 3 infusions of 56, 48, and 140 U FVIII on days 100, 113, and 133, respectively. Plasmas samples were obtained at the indicated time points. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured, and the results were compared with pooled normal canine plasma samples that were arbitrarily set at 100%. Plasma samples from 13 untreated hemophilia A dogs showed no detectable FVIII:Ag. (B) Levels of inhibitory antibodies were measured by a Bethesda assay, and anti-FVIII IgG1 and IgG2 antibodies were measured by ELISA.
Thrombin may contribute to the initiation of the anti-FVIII immune response
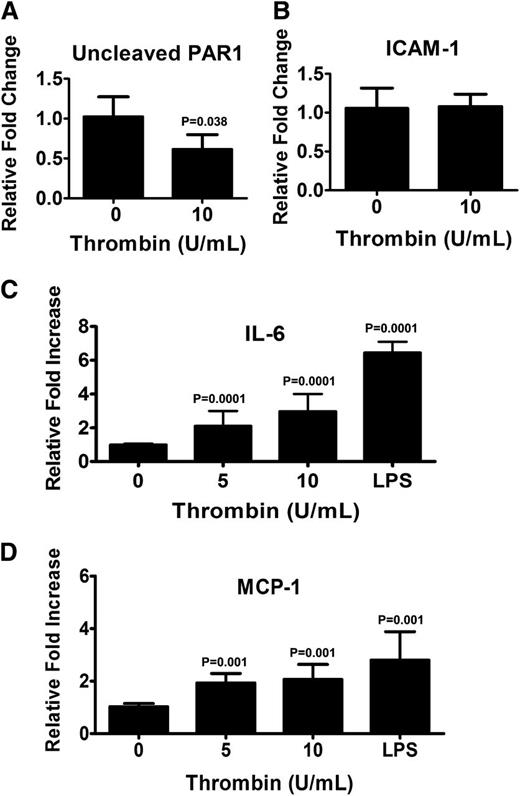
Although it is known that thrombin activates PARs on endothelial cells, it is unclear how this affects their function and whether these affects are uniform for all types of endothelial cells. Because thrombin was used to generate the fibrin-based matrix, we investigated what affect thrombin signaling through PAR1 has on the phenotype of BOECs. Antibodies that react with canine PAR1 are not available, and therefore hBOECs were used. hBOECs were cultured in levels of thrombin comparable to those used to generate the fibrin matrix. As assessed by flow cytometry, we observed no difference in the levels of expression of PAR1 between hBOECs cultured with or without thrombin (data not shown). Thrombin activation of PAR1 can be assessed with the PAR1 (clone SPAN12) antibody because this antibody interacts only with noncleaved PAR1. As the results in Figure 5A illustrate, there was a significant reduction in the relative median fluorescence when the cells were cultured with thrombin, indicating that thrombin does activate PAR1 on hBOECs. The phenotypic effects of this activation were assessed through determination of the surface expression of the adhesive glycoprotein ICAM-1, MHC class II, and the costimulatory molecules CD80 and CD86. No increase in ICAM-1 expression was observed (Figure 5B), and the lack of upregulation of CD80, CD86, and MHC class II molecules suggests that an antigen-presenting phenotype was not attained (data not shown). Activation of PAR1 can cause endothelial cells to release inflammatory cytokines and chemokines such as IL-6 and MCP-1, respectively. Analysis of culture supernatants from thrombin-exposed hBOECs is shown in Figure 5C-D and demonstrates significant increases in levels of both IL-6 and MCP-1.
Thrombin activates endothelial PAR1 resulting in release of IL-6 and MCP-1. Confluent monolayers of hBOECs were cultured with the indicated concentrations of thrombin for 24 hours. Flow cytometric analysis was used to evaluate surface expression of (A) uncleaved PAR1 and (B) ICAM-1. Values represent the fold increase in mean fluorescence relative to untreated cells and are expressed as a mean, with vertical bars indicating standard deviation from the mean. Experiments were repeated at least 3 times. Culture media from confluent BOECs exposed to various amounts of thrombin or LPS was isolated and assayed for (C) IL-6 and (D) MCP-1 by ELISA. Values represent the mean of 3 experiments with vertical bars indicating the standard deviation.
Thrombin activates endothelial PAR1 resulting in release of IL-6 and MCP-1. Confluent monolayers of hBOECs were cultured with the indicated concentrations of thrombin for 24 hours. Flow cytometric analysis was used to evaluate surface expression of (A) uncleaved PAR1 and (B) ICAM-1. Values represent the fold increase in mean fluorescence relative to untreated cells and are expressed as a mean, with vertical bars indicating standard deviation from the mean. Experiments were repeated at least 3 times. Culture media from confluent BOECs exposed to various amounts of thrombin or LPS was isolated and assayed for (C) IL-6 and (D) MCP-1 by ELISA. Values represent the mean of 3 experiments with vertical bars indicating the standard deviation.
The removal of the matrix modulates the FVIII immune response
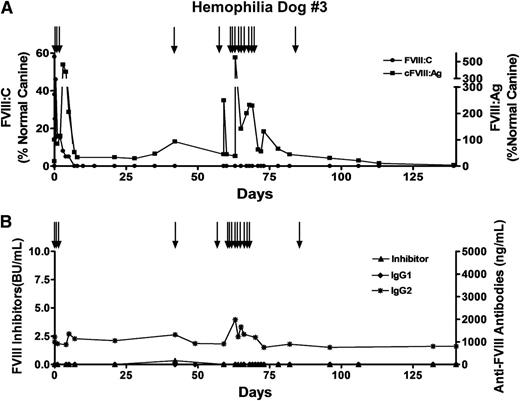
Because we had evidence suggesting that thrombin may contribute to the development of inhibitors, we decided to implant the autologous cBOECs directly into the omentum of hemophilia dog #3. Prior to the procedure, this dog received 8 infusions of 10 U/kg recombinant cFVIII over a 4-month period. Levels of anti-FVIII IgG2 antibodies slowly increased after each infusion, and levels of 1220 ng/mL were detected on the day of the procedure. However, these levels are still very low relative to those in a dog with low titers of FVIII inhibitory antibodies. Furthermore, there was no indication of a loss of functional FVIII with each infusion, and no inhibitory antibodies were detected on the day of cBOEC implantation. Two weeks after the last infusion of recombinant cFVIII, cFVIII-expressing autologous cBOECs were implanted in the omentum, and although 5% normal functional FVIII levels were detected on day 5 postimplantation, these levels fell and were undetectable by day 7 (Figure 6A). Factor VIII inhibitory antibodies were not present at this time, although nonneutralizing anti-FVIII IgG2 antibodies were detected (Figure 6B). The levels of these antibodies were significantly lower than those observed when the cells were implanted into the fibrin-based matrix and support a possible role for thrombin in contributing to the anti-FVIII immune response. We assayed circulating FVIII antigen in dog #3 and detected substantial levels ranging between 29% and 44% normal (Figure 6A), indicating that the implanted cells were viable and continued to express FVIII despite the lack of a supporting scaffold matrix.
Significant levels of FVIII:Ag in plasma of hemophilia A dog treated with gene delivery. Hemophilia dog #3 weighed 9 kg and was 2 years old when 3.28 × 109 autologous cBOECs that expressed 1.5 U/mL per 106 cells over 24 hours in vitro were implanted into 13 sites. Prior to receiving the genetically modified cells, 8 infusions of recombinant FVIII were administered by intravenous injections. This dog received 3 infusions of recombinant FVIII (black arrows) on the day of the treatment. An additional 2 infusions of FVIII were given on days 42 and 59 after gene delivery. A second gene delivery treatment of 2 × 109 autologous FVIII-expressing cBOECs was implanted into 11 sites 63 days after the first treatment. Plasma samples were taken at the indicated times. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured. Normal canine plasma from 8 healthy dogs was pooled and used to generate the standard curve. Normal pooled plasma was arbitrarily set at 100%. Plasma samples from 13 untreated hemophilia A dogs showed no detectable FVIII:Ag. (B) Levels of inhibitory antibodies were measured by a Bethesda assay, and anti-FVIII IgG1 and IgG2 antibodies were measured by ELISA.
Significant levels of FVIII:Ag in plasma of hemophilia A dog treated with gene delivery. Hemophilia dog #3 weighed 9 kg and was 2 years old when 3.28 × 109 autologous cBOECs that expressed 1.5 U/mL per 106 cells over 24 hours in vitro were implanted into 13 sites. Prior to receiving the genetically modified cells, 8 infusions of recombinant FVIII were administered by intravenous injections. This dog received 3 infusions of recombinant FVIII (black arrows) on the day of the treatment. An additional 2 infusions of FVIII were given on days 42 and 59 after gene delivery. A second gene delivery treatment of 2 × 109 autologous FVIII-expressing cBOECs was implanted into 11 sites 63 days after the first treatment. Plasma samples were taken at the indicated times. (A) FVIII activity (FVIII:C) and FVIII antigen (FVIII:Ag) levels were measured. Normal canine plasma from 8 healthy dogs was pooled and used to generate the standard curve. Normal pooled plasma was arbitrarily set at 100%. Plasma samples from 13 untreated hemophilia A dogs showed no detectable FVIII:Ag. (B) Levels of inhibitory antibodies were measured by a Bethesda assay, and anti-FVIII IgG1 and IgG2 antibodies were measured by ELISA.
At 9 weeks after gene delivery, hemophilia dog #3 received a second treatment of 2 × 109 autologous FVIII-expressing cBOECs mixed with growth factors (KGF/FGF-7, vascular endothelial growth factor, FGF-2, and FGF-9) and implanted into the fibrin matrix in 11 sites. Postoperative internal bleeding occurred that required infusions of cFVIII for 10 days. Although no functional FVIII activity could be detected 2 weeks after the second treatment, significant levels (66% normal) of FVIII antigen were observed that persisted for 10 weeks after this second treatment. Throughout the duration of the study, no inhibitory antibodies were detected by the Bethesda assay in this dog.
Nonneutralizing anti-FVIII antibodies interact with functional sites on FVIII
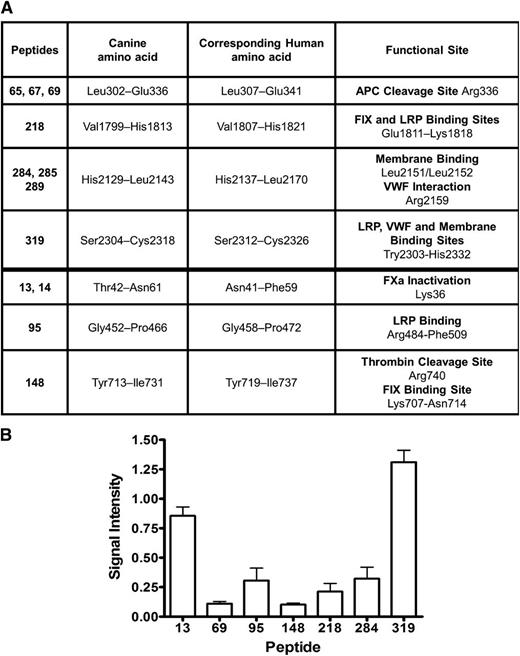
The lack of functional FVIII after treating hemophilia dog #3, despite the presence of relatively high levels of circulating FVIII antigen with no detectable FVIII inhibitors, was perplexing, and therefore a peptide library was screened to identify the IgG2 antibody epitopes on the cFVIII. Plasma samples taken from this dog during the period of time when IgG2 antibodies first appeared until week 7 after gene delivery were pooled, and total IgG antibodies were isolated. Anti-FVIII IgG2 antibody levels during this period ranged between 347 and 673 ng/mL. An ELISA-based assay was used to screen 321 peptides spanning the canine BDD FVIII molecule, and a total of 12 peptides were identified (Figure 7A, column 1) that interact with the anti-FVIII IgG2 antibodies from dog #3. These peptides identify 7 unique locations on the FVIII molecule. To get some indication of the relative abundance of each of the different IgG2 antibodies, the signal intensity of the most abundant peptide from each of the 7 locations was compared with the intensity of the signal obtained for recombinant BDD cFVIII (Figure 7B).
Anti-FVIII IgG2 antibodies isolated from hemophilia dog #3 interact with functional sites on the therapeutic cFVIII protein. (A) Screening a peptide library consisting of 321 peptides spanning the canine BDD FVIII with an ELISA-based assay revealed 12 peptides that interacted with the purified anti-FVIII IgG2 antibodies isolated from dog #3 (column 1). The canine and corresponding human amino acids along with the known functional sites are indicated. (B) After subtracting the background signal (negative control) for each of the peptides, the signal intensity for each peptide was compared with the signal intensity obtained from recombinant cFVIII. For overlapping peptides, the peptide with the strongest signal intensity is displayed on the horizontal axis. APC, activated protein C; FIX, factor IX; FXa, factor Xa.
Anti-FVIII IgG2 antibodies isolated from hemophilia dog #3 interact with functional sites on the therapeutic cFVIII protein. (A) Screening a peptide library consisting of 321 peptides spanning the canine BDD FVIII with an ELISA-based assay revealed 12 peptides that interacted with the purified anti-FVIII IgG2 antibodies isolated from dog #3 (column 1). The canine and corresponding human amino acids along with the known functional sites are indicated. (B) After subtracting the background signal (negative control) for each of the peptides, the signal intensity for each peptide was compared with the signal intensity obtained from recombinant cFVIII. For overlapping peptides, the peptide with the strongest signal intensity is displayed on the horizontal axis. APC, activated protein C; FIX, factor IX; FXa, factor Xa.
An alignment of the cFVIII and hFVIII amino acid sequence was used to assign functional sites to the canine molecule. On the basis of this alignment, the IgG2 anti-FVIII antibodies isolated from dog #3 would directly interfere with APC cleavage site at Arg336,31 factor IX, and low-density lipoprotein (LDL) receptor-related protein (LRP) binding at Glu1811-Lys1818,32 interaction of vWF at Arg2159, and membrane binding at Leu2151/Leu2152 within the C1 domain33 as well as binding to vWF, LRP, and phospholipid, which are all sites mapped within Trp2303-His2332 of the C2 domain.34-36 Additionally, 3 of the identified anti-FVIII antibodies map in close proximity to the factor Xa inactivation site at Lys36,37 LRP binding site at Arg484-Phe509,38 the thrombin cleavage site at Arg740,39 and factor IX binding site at Lys707-Asn714.32 To confirm an inhibitory function of these antibodies, the purified and concentrated IgG2 antibodies from dog #3 were used in a Bethesda assay and 1.3 BUs of inhibitory function was detected.
Discussion
The anti-FVIII immune response is a crucial consideration for both FVIII protein replacement and gene therapy. In the murine model of hemophilia A, the anti-FVIII immune response was avoided by transient immune suppression or preexposure to FVIII prior to transduced murine BOEC implantation. However, inbred murine models are not always predictive of adverse responses in larger animal models,18 and neither of these immunomodulatory strategies prevented the development of inhibitors in the hemophilic dogs. One explanation for this difference in outcomes is the use of distinct support matrixes in the dogs and mice. The Matrigel used in mice is a heterogeneous mixture of structural proteins and growth factors. Although it is known to stimulate cellular proliferation and angiogenesis, its effects on the immune system are unknown, and given that growth factors are associated with immunologic tolerance,40 it is possible that the Matrigel had an immunomodulatory effect on the anti-FVIII immune response in the mice. In contrast, we demonstrated that the thrombin needed to generate the fibrin-based matrix activates PAR1 on hBOECs and this results in secretion of IL-6 and MCP-1, both of which stimulate immune responses. The overall role of thrombin in initiating an anti-FVIII immune response remains unclear.41,42 In our study, inhibitors developed in the two hemophilia A dogs when a thrombin-induced matrix was used, although no inhibitors were detected in dog #3 after the first gene therapy treatment that used no matrix. However, it is important to note that dog #3 did not develop inhibitors after the second gene therapy treatment when a thrombin-based matrix was used and when the internal bleeding would have produced a proinflammatory milieu. Therefore, in this gene delivery setting, the development of FVIII immunogenicity cannot simply be attributed to the thrombin that was used to generate the matrix.
Evaluating the immune response in the hemophilia A dogs at Queen’s University is complicated because 30% of them have an inherited predisposition to develop inhibitors,43 and no predictive markers of this tendency are currently available. When these dogs develop inhibitors after gene delivery, it is difficult to weigh the relative contributions of the gene therapy protocol vs the dog’s genetic predisposition for inhibitor development. Prior to gene delivery, all 3 hemophilia dogs were exposed to cFVIII, and on the day of treatment, none had detectable inhibitors. Although these dogs all developed persistent anti-FVIII IgG2 antibodies, notable differences in the levels of these antibodies were observed. Dogs #1 and #2 had approximately 100-fold higher levels of IgG2 antibodies relative to dog #3, and these higher levels were associated with detectable inhibitors. In dog #3, the IgG2 antibodies first appeared prior to the gene delivery and therefore clearly resulted from the previous infusions of FVIII. Surprisingly, despite the presence of these anti-FVIII antibodies, no inhibitors were ever detected in this dog, even with further infusions of FVIII after implanting the cells. Because inhibitors were detected only when levels of anti-IgG2 antibodies were >100 000 ng/mL and because IgG2 antibody levels never rose much above 1000 ng/mL in dog #3, it is likely that the threshold for detection of FVIII inhibitory activity with the Bethesda assay was never achieved in dog #3. This hypothesis is supported by the fact that we could detect inhibitory function in the concentrated purified antibodies and that some of the epitopes for these antibodies mapped to functional sites on cFVIII.
Given the considerations above, it is surprising that no functional FVIII was detected in dog #3 insofar as inhibitor levels were so low (below detection with the Bethesda assay) and circulating levels of FVIII antigen were relatively high. Prior to gene delivery and after the sixth infusion of FVIII, we noted a 10-fold increase in anti-FVIII IgG2 antibodies along with an extended period of time that the FVIII antigen persisted in the circulation. We hypothesize that the high levels of FVIII antigen detected in dog #3 resulted from FVIII immune complexes. We are unable to confirm this since the n-caprylic acid used in the antibody purification process causes dissociation of antigen-antibody complexes. However, persistent circulating IgG immune complexes are not uncommon and are found in patients with different types of allergies,44 and in some patients with acquired hemophilia A.25 Consistent with our observations, the acquired hemophilia patients in Nogami et al25 had disproportionally higher levels of FVIII antigen relative to activity, and antibodies that interacted with the APC cleavage site. Furthermore, dog #3 had antibodies that interacted with the LRP and membrane phospholipid binding sites which may inhibit FVIII clearance from the circulation and contribute to the elevated levels of circulating FVIII antigen.
In conclusion, we have demonstrated that implanted, autologous FVIII-expressing BOECs are viable for a prolonged period of time and appear to form new vessels at the site of omental implantation. These new vessels presumably join the existing vasculature because the FVIII antigen can be detected in the circulation. Although this therapy was associated with the development of FVIII inhibitors, antibody levels are low and far less than what we have previously observed with systemic delivery of viral vectors.24 Although they clearly require additional modifications to the transgene delivery protocol, the results of this study provide further support for the evaluation of genetically modified autologous endothelial cells as a future therapy for hemophilia A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP-10912 and IRO-90122) and the Canadian Stem Cell Network. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis.
Authorship
Contribution: M.C.O. assisted in designing the study, performed research and interpreted data; B.V., C.B., C. Hegadorn, C.N., and J.A. performed research and interpreted data; S.W., L.H., A.W., and J.H. cared for the dogs and assisted with or carried out surgeries; V.R.A. provided the canine peptide library; C. Hough assisted in the design of the study, interpreted data, and wrote the paper; and D.L. designed research, interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, Queen’s University, Kingston, ON, Canada K7L 3N6; e-mail: dpl@queensu.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal