Key Points
Adding siltuximab to VMP did not improve CR, progression-free survival, or overall survival but improved very good partial response in MM.
This suggests that the association of less than CR with long-term outcomes and the role of IL-6 in MM should be reassessed.
Abstract
Because interleukin-6 (IL-6) is considered important in the proliferation of early multiple myeloma (MM), we hypothesized that the addition of the anti-IL-6 monoclonal antibody siltuximab to the bortezomib-melphalan-prednisone (VMP) regimen would improve outcomes in transplant-ineligible patients with newly diagnosed MM. One hundred and six patients were randomized to receive 9 cycles of VMP or VMP plus siltuximab (11 mg/kg every 3 weeks) followed by siltuximab maintenance. Baseline characteristics were well balanced except for immunoglobulin A subtype and 17p deletions. With a complete response (CR) rate of 27% on siltuximab plus VMP (S+VMP) and 22% on VMP, the study did not confirm its hypothesis that the addition of siltuximab would increase the CR rate by at least 10%. Overall response rate was 88% on S+VMP and 80% on VMP, and at least very good partial response rates were 71% and 51% (P = .0382), respectively. Median progression-free survival (17 months) and 1-year overall survival (88%) were identical in the 2 arms. Grade ≥3 adverse-event incidence was 92% on S+VMP and 81% on VMP (P = .09), with trends toward more hematologic events and infections on S+VMP. Maintenance therapy with siltuximab was well tolerated. In conclusion, the addition of siltuximab to VMP did not improve the CR rate or long-term outcomes. This study was registered at http://clinicaltrials.gov as #NCT00911859.
Introduction
For patients with newly diagnosed multiple myeloma (MM) ineligible for autologous stem cell transplantation (ASCT), bortezomib-melphalan-prednisone (VMP) is a standard treatment regimen.1,2 In the VISTA study, the VMP regimen improved the complete response (CR) rate (30% vs 4%) and overall survival (median 56.4 months vs 43.1 months) over melphalan-prednisone (MP). These results were the basis for the regulatory approval of VMP in newly diagnosed MM in both the United States and the European Union.3,4 However, these results are still inferior to the outcomes in younger patients with newly diagnosed MM treated with high-dose chemotherapy and ASCT,5 and further improvements in treatment modalities for the transplantation-ineligible population are needed.
Interleukin-6 (IL-6) is a cytokine known to enhance proliferation and survival of malignant plasma cells.6-9 Because the role of IL-6 is considered important in the early development of MM,10,11 the addition of anti-IL-6–directed treatment to current standard regimens would be a logical approach to improve results in newly diagnosed MM. Siltuximab (formerly CNTO 328) is a chimeric monoclonal antibody with high binding affinity for human IL-612 and has been shown in preclinical experiments to enhance the antimyeloma activity of bortezomib, melphalan, and corticosteroids.13-15 In a single-agent phase 1 study in hematologic malignancies, a dose schedule of 11 mg/kg every 3 weeks was determined to be the recommended regimen based on the high radiologic response rate observed in multicentric Castleman disease (MCD), an IL-6–driven lymphoproliferative disorder, and on the sustained suppression of systemic C-reactive protein (CRP), a downstream marker of IL-6 activity.16,17 In a recent randomized study in MCD, siltuximab at this dose and schedule provided significant improvements in disease symptoms, lymphadenopathy, and inflammatory parameters.18 The good safety profile established in these single-agent studies allowed for the combination of siltuximab with cytotoxic agents.
Two combination studies have been performed with siltuximab in relapsed and refractory MM. In a single-arm phase 2 study in combination with dexamethasone in heavily pretreated patients, a 17% partial response (PR) rate was observed, including responses in patients previously refractory to dexamethasone.19 In a large, randomized, phase 2 study of siltuximab in combination with bortezomib vs bortezomib alone in relapsed MM, an overall response rate (≥PR) of 55% was seen with the combination as compared with 47% with single-agent bortezomib, but there was no improvement in progression-free survival (PFS) with the addition of siltuximab (median PFS, 8.0 months vs 7.6 months).20 This moderate additional activity of siltuximab in relapsed MM could be interpreted as advanced MM having become increasingly independent of the bone marrow microenvironment in general, and of IL-6 in particular, and left open the question of whether IL-6 blockade would be more relevant in newly diagnosed MM.
Here, we report the results of a randomized phase 2 study of siltuximab in combination with VMP vs VMP alone in patients with newly diagnosed MM ineligible for ASCT. Because siltuximab had not been previously combined with VMP in clinical studies, this randomized study was designed with a run-in to assess the safety of the combination. Bortezomib, as part of the VMP regimen, was given intravenously according to regulatory prescribing information at that time; the study had completed accrual by the time subcutaneous use of bortezomib was approved.21
Methods
Patients
The study population consisted of previously untreated patients with symptomatic MM not eligible for high-dose chemotherapy with stem cell transplantation due to age (≥65 years) or important comorbid conditions. Patients were required to have measurable disease, defined as quantifiable M-protein in either serum (≥1 g/dL) or urine (light-chain protein ≥200 mg/24 hours) and adequate liver function (total bilirubin ≤1.5 times the upper limit of normal and transaminases ≤2.5 times the upper limit of normal) and bone marrow function (absolute neutrophil count ≥1.0 × 109/L and platelets ≥ 70 × 109/L). Patients with known infection with HIV or hepatitis B or C, with recent live vaccinations, or with creatinine clearance <20 mL/min were excluded. All patients provided written informed consent. Review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice.
Study design and treatment groups
This was an international, randomized, open-label, multicenter, phase 2 study of siltuximab plus VMP (S+VMP) vs VMP. This study was conducted in 2 parts: part 1 was a single-arm lead-in to evaluate the safety of S+VMP, whereas in part 2, patients were randomized 1:1 to S+VMP or VMP and were to be treated up to a maximum of 9 cycles (54 weeks). Patients who achieved at least a PR in the S+VMP arm could enter maintenance treatment with single-agent siltuximab for a maximum of 18 months or until disease progression. Patients were stratified according to International Staging System (ISS) stage based on central-laboratory–determined serum β2-microglobulin and albumin.22 High-risk cytogenetic abnormalities were determined by central review of local laboratory fluorescence in situ hybridization and karyotyping data [ie, t(4;14), t(14;16), and 17p deletion]. All patients received VMP as previously described1 (nine 6-week cycles of oral melphalan 9 mg/m2 and oral prednisone 60 mg/m2, days 1 to 4, in combination with intravenous bortezomib [1.3 mg/m2: days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1 to 4; days 1, 8, 22, and 29 during cycles 5 to 9]). Siltuximab was given at 11 mg/kg every 3 weeks by intravenous infusion over 1 hour. Treatment was discontinued upon consent withdrawal, disease progression, or unacceptable toxicity. Dose reductions of melphalan, bortezomib, and prednisone were allowed as previously described.1 Siltuximab administration could be delayed in case of toxicity. Antiviral prophylaxis for herpes zoster reactivation and bisphosphonate treatment in case of myeloma-related bone disease was recommended. No prophylaxis was required for infusion reactions.
Study objectives and assessments
The primary objective of the part 1 run-in was to assess the safety of S+VMP. The primary objective of part 2 was to assess the efficacy by CR rate of S+VMP. Secondary end points included OR rate, PFS, duration of response (DOR), 1-year survival, safety, siltuximab pharmacokinetics, and biomarker analysis, including analyses of serum CRP concentrations. Response and progression were determined using a validated computer algorithm implementing the European Group for Blood and Marrow Transplantation (EBMT) criteria.1,23 Minimal residual disease (MRD) was monitored centrally in a University of Salamanca laboratory by exploratory multiparametric flow cytometry in bone marrow aspirates collected at baseline and at the time CR was suspected. The MRD analysis was performed using a 7-color antibody panel consisting of CD38/CD138/CD56/CD19/CD45/cyIgKAPPA/cyIgLAMBDA markers to identify myelomatous plasma cells with a sensitivity range of 10−4 to 10−5 cells. Investigator-assessed response based on International Myeloma Working Group (IMWG) criteria (in particular the category of very good PR [VGPR]) was also collected and analyzed.24 Blood and 24-hour urine samples were collected every 3 weeks during the 54-week treatment and then every 9 weeks until disease progression; all M-protein analyses were performed by a central laboratory. Other efficacy assessments included bone marrow examination and skeletal survey and measurements of extramedullary plasmacytomas and corrected serum calcium. In part 2, the EORTC QLQ C-30 questionnaire was collected on day 1 of every cycle. Safety was evaluated throughout the study and until 30 days after the last dose of study drug. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria version 3.0.
Statistical considerations
The study was based on a Simon’s randomized phase 2 design.25 Assuming that addition of siltuximab to VMP could improve CR rate from 30%1 to 40%, 50 response-evaluable subjects per arm (100 in total) would provide an 85% probability of observing this improvement. Approximately 104 subjects were planned to be randomized. Descriptive statistics were used to summarize data. For continuous parameters, number of observations, mean, standard deviation, median, and range were used. For discrete parameters, frequency was summarized. For time-to-event parameters, Kaplan-Meier estimates were generated. Post hoc comparisons using a χ2 test were performed where required for interpretation of results. For part 2, the response-evaluable population (defined as patients with measurable disease treated with ≥1 administration of study agent and with ≥1 postbaseline response assessment) was used for the analysis of the primary end point and the secondary response rate and DOR end points. The analysis of PFS and OS was based on the intent-to-treat population. A safety evaluation team, consisting of principal investigators and study-sponsor representatives, conducted the safety review for part 1 after all subjects were treated for 1 cycle. Primary analysis for part 2 was conducted as planned 12 months after the last subject had been randomized; PFS and OS results were updated 6 months later, after which the sponsor decided to terminate the study. Final results for all end points are provided in this article.
Results
Patient characteristics
From June 2009 to May 2011, 12 patients were enrolled in part 1 and 106 patients were randomized to the S+VMP arm (n = 52) or the VMP arm (n = 54) in part 2. Baseline patient characteristics were well balanced between the 2 arms (Table 1). The median age was 71 years (range, 48-90 years) with 91.5% ≥65 years of age. Thirty-eight percent in the S+VMP arm and 41% in the VMP arm had ISS stage II disease, whereas 54% in both arms had ISS stage III disease. High-risk cytogenetic abnormalities were present in 17% in the S+VMP arm and 10% in the VMP arm, including 17p deletion in 15% and 4%, respectively (this difference was statistically nonsignificant). Other baseline characteristics were well balanced, with the exception of myeloma subtype (immunoglobulin A [IgA] in 41% in S+VMP and 18.5% in VMP) and Easter Cooperative Oncology Group (ECOG) performance status (PS 0 in 9% in S+VMP and 24% in VMP). Patients in part 1 had similar disease characteristics to part 2. Four noneligible patients were included in the study: 1 in the VMP arm (prior dexamethasone) and 3 in the S+VMP arm (elevated serum alanine aminotransferase level, nonmeasurable disease, and creatinine clearance <20 mL/min).
Summary of patient baseline demographics
| . | VMP . | S+VMP . |
|---|---|---|
| Patients randomized | 54 | 52 |
| Age, y | ||
| Median | 70.0 | 71.0 |
| Range | (48; 90) | (59; 83) |
| ECOG performance score | ||
| 0 | 13 (24%) | 5 (9%) |
| 1 | 28 (52%) | 30 (58%) |
| 2 | 13 (24%) | 17 (33%) |
| Type of myeloma | ||
| IgG | 37 (68.5%) | 22 (42%) |
| IgA | 10 (18.5%) | 21 (41%) |
| Light chain | 6 (11%) | 8 (15%) |
| Biclonal | 1 (2%) | 1 (2%) |
| ISS staging | ||
| I | 3 (5%) | 4 (8%) |
| II | 22 (41%) | 20 (38%) |
| III | 29 (54%) | 28 (54%) |
| Cytogenetic abnormality | ||
| High-risk* | 5 (10%) | 8 (17%) |
| Intermediate: del13q by karyotype | 2 (4%) | 1 (2%) |
| Intermediate: amp1q | 1 (2%) | 0 |
| % Plasma cells, bone marrow biopsy/aspirate | ||
| >30 | 37 (68.5%) | 34 (65%) |
| Hemoglobin (g/L) | ||
| Median | 101.50 | 103.50 |
| Range | (75.0; 132.0) | (75.0; 141.0) |
| Platelet (×109/L) | ||
| Median | 225.5 | 236.5 |
| Range | (101; 435) | (81; 579) |
| Creatinine clearance (mL/min) | ||
| Median | 56.40 | 58.38 |
| Range | (26.6; 114.1) | (18.7; 107.2) |
| . | VMP . | S+VMP . |
|---|---|---|
| Patients randomized | 54 | 52 |
| Age, y | ||
| Median | 70.0 | 71.0 |
| Range | (48; 90) | (59; 83) |
| ECOG performance score | ||
| 0 | 13 (24%) | 5 (9%) |
| 1 | 28 (52%) | 30 (58%) |
| 2 | 13 (24%) | 17 (33%) |
| Type of myeloma | ||
| IgG | 37 (68.5%) | 22 (42%) |
| IgA | 10 (18.5%) | 21 (41%) |
| Light chain | 6 (11%) | 8 (15%) |
| Biclonal | 1 (2%) | 1 (2%) |
| ISS staging | ||
| I | 3 (5%) | 4 (8%) |
| II | 22 (41%) | 20 (38%) |
| III | 29 (54%) | 28 (54%) |
| Cytogenetic abnormality | ||
| High-risk* | 5 (10%) | 8 (17%) |
| Intermediate: del13q by karyotype | 2 (4%) | 1 (2%) |
| Intermediate: amp1q | 1 (2%) | 0 |
| % Plasma cells, bone marrow biopsy/aspirate | ||
| >30 | 37 (68.5%) | 34 (65%) |
| Hemoglobin (g/L) | ||
| Median | 101.50 | 103.50 |
| Range | (75.0; 132.0) | (75.0; 141.0) |
| Platelet (×109/L) | ||
| Median | 225.5 | 236.5 |
| Range | (101; 435) | (81; 579) |
| Creatinine clearance (mL/min) | ||
| Median | 56.40 | 58.38 |
| Range | (26.6; 114.1) | (18.7; 107.2) |
High-risk abnormality is defined as t(4;14), t(14;16), and del17p.
Study treatment exposure
One patient in the VMP arm was randomized but never treated. During induction treatment cycles 1 to 9, median treatment duration was 12.5 months for the S+VMP arm and 12.9 months for the VMP arm. A total of 67% in the S+VMP arm and 81% in the VMP arm completed cycles 1 to 4, and 52% in the S+VMP arm and 60% in the VMP arm completed cycles 5 to 9. The most frequent reasons for discontinuation were AEs (14% in S+VMP, 6% in VMP) and progressive disease (10% in S+VMP, 13% in VMP). Twenty-one patients (40%) in the S+VMP arm went on to receive maintenance siltuximab, with a median maintenance treatment duration of 6.25 months at the time of study termination by the sponsor. At that moment, 9 patients (43%) had discontinued maintenance therapy due to progression and none discontinued due to AEs.
Exposure to bortezomib, melphalan, and prednisone was similar in the 2 arms during induction treatment cycles 1 to 9. The median cumulative dose for bortezomib was 45 mg/m2 in the S+VMP arm and 42 mg/m2 in the VMP arm, and the median cumulative dose of melphalan and prednisone was 257 mg/m2 and 2122 mg/m2 in the S+VMP arm and 297 mg/m2 and 2052 mg/m2 in the VMP arm, respectively. The median cumulative siltuximab dose was 151 mg/kg during induction, and 110 mg/kg during maintenance for the 21 patients receiving maintenance therapy.
Efficacy
In part 2, the CR rate was 27% in the S+VMP arm and 22% in the VMP arm; therefore, the study did not meet the prespecified hypothesis of a 10% increase in CR rate. The overall response rate (CR plus PR) was 88% with S+VMP and 80% with VMP, whereas at least VGPR rate by IMWG criteria was achieved in 71% of patients with S+VMP and 51% with VMP (post hoc analysis P = .0382; Table 2).
Efficacy analysis
| . | VMP . | S+VMP . |
|---|---|---|
| Evaluable patients in part 2 | 49 | 49 |
| Overall response (CR or PR) per EBMT criteria (95% CI) | 80% (66, 90) | 88% (75, 95) |
| CR (95% CI) | 22% (12, 37) | 27% (15, 41) |
| PR | 57% | 61% |
| ≥VGPR (IMWG) | 51% | 71% |
| MR | 8% | 2% |
| No change | 12% | 10% |
| PD | 0% | 0% |
| . | VMP . | S+VMP . |
|---|---|---|
| Evaluable patients in part 2 | 49 | 49 |
| Overall response (CR or PR) per EBMT criteria (95% CI) | 80% (66, 90) | 88% (75, 95) |
| CR (95% CI) | 22% (12, 37) | 27% (15, 41) |
| PR | 57% | 61% |
| ≥VGPR (IMWG) | 51% | 71% |
| MR | 8% | 2% |
| No change | 12% | 10% |
| PD | 0% | 0% |
MR, minimal response; PD, progressive disease.
Of the subjects with measurable heavy-chain disease at baseline, 61% in the S+VMP arm and 38% in the VMP arm had 100% reduction in serum M-protein. Of the patients with only measurable Bence Jones protein at baseline, all (100%) on S+VMP and 57% on VMP had 100% reduction in urine M-protein (Table 3).
Overall M-protein response
| . | VMP (n = 49) . | S+VMP (n = 49) . |
|---|---|---|
| Best M-protein response in serum N (heavy chain) | 42 | 41 |
| 100% reduction | 16 (38%) | 25 (61%) |
| ≥90% reduction | 21 (50%) | 28 (68%) |
| ≥50% reduction | 40 (95%) | 38 (93%) |
| Best M-protein response in urine N (light chain) | 7 | 8 |
| 100% reduction | 4 (57%) | 8 (100%) |
| ≥90% reduction | 5 (71%) | 8 (100%) |
| ≥50% reduction | 7 (100%) | 8 (100%) |
| . | VMP (n = 49) . | S+VMP (n = 49) . |
|---|---|---|
| Best M-protein response in serum N (heavy chain) | 42 | 41 |
| 100% reduction | 16 (38%) | 25 (61%) |
| ≥90% reduction | 21 (50%) | 28 (68%) |
| ≥50% reduction | 40 (95%) | 38 (93%) |
| Best M-protein response in urine N (light chain) | 7 | 8 |
| 100% reduction | 4 (57%) | 8 (100%) |
| ≥90% reduction | 5 (71%) | 8 (100%) |
| ≥50% reduction | 7 (100%) | 8 (100%) |
For 11 patients on S+VMP (10 with CR) and 7 patients on VMP (5 with CR), a bone marrow sample for MRD analysis was provided; 5 patients on S+VMP (4 with CR, 1 with immofixation-positive/near-CR) and 5 patients on VMP (all CR) were MRD negative.
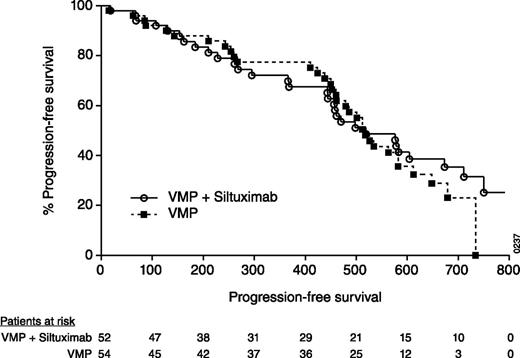
The median time to first response was numerically shorter for the S+VMP arm (0.8 months) than the VMP arm (1.4 months). The median time to CR was also shorter for S+VMP (3 months) compared with VMP (5.6 months). The median DOR by the Kaplan-Meier method was 19.2 months on S+VMP and 16.3 months on VMP. The median PFS based on stringent implementation of EBMT criteria through a validated computer algorithm was 17 months in both arms (Figure 1). The median PFS, as determined by the investigator, was 19 months with S+VMP and 17.2 months with VMP.
Kaplan-Meier plot of PFS in the randomized intention-to-treat population. Numbers on the x-axis represent days.
Kaplan-Meier plot of PFS in the randomized intention-to-treat population. Numbers on the x-axis represent days.
After a median follow-up of 23.3 months in the S+VMP arm and 21.9 months in the VMP arm, the median survival was not reached in either treatment arm. The 1-year survival rate was 88% in both arms. Of the 12 subjects in part 1, 5 subjects (42%) had CR and 5 subjects (42%) had PR.
Safety
During cycles 1 to 9, there was a moderate increase in incidence in different AE categories in the S+VMP arm compared with the VMP arm. At least 1 grade ≥3 AE was reported in 92% of patients with S+VMP and in 81% of patients with VMP in this 54-week treatment period (P = .09). There was a 7% higher incidence of serious AEs (SAEs) (S+VMP 58% vs VMP: 51%; P = .49). Five patients in the S+VMP arm (10%) and 4 patients in VMP arm (7.5%) died due to AEs (for 1 subject on VMP, this was considered treatment related [pneumonia]; Table 4).
AEs
| . | VMP (n = 53) . | S+VMP (n = 52) . |
|---|---|---|
| Any AE grade ≥3 | 81% | 92% |
| Most common AEs grade ≥3 | ||
| Neutropenia | 43% | 62% |
| Thrombocytopenia | 25% | 44% |
| Bronchopneumonia/pneumonia | 17% | 17% |
| Anemia | 13% | 13% |
| Hypokalemia | 2% | 11.5% |
| Diarrhea | 9% | 4% |
| Peripheral sensory neuropathy | 9% | 6% |
| SAEs | 51% | 58% |
| Most frequently reported SAEs | ||
| Infections | 17% | 23% |
| Gastrointestinal disorders | 11% | 11.5% |
| Death due to an AE | 4 (7%)* | 5 (10%)* |
| . | VMP (n = 53) . | S+VMP (n = 52) . |
|---|---|---|
| Any AE grade ≥3 | 81% | 92% |
| Most common AEs grade ≥3 | ||
| Neutropenia | 43% | 62% |
| Thrombocytopenia | 25% | 44% |
| Bronchopneumonia/pneumonia | 17% | 17% |
| Anemia | 13% | 13% |
| Hypokalemia | 2% | 11.5% |
| Diarrhea | 9% | 4% |
| Peripheral sensory neuropathy | 9% | 6% |
| SAEs | 51% | 58% |
| Most frequently reported SAEs | ||
| Infections | 17% | 23% |
| Gastrointestinal disorders | 11% | 11.5% |
| Death due to an AE | 4 (7%)* | 5 (10%)* |
Death was considered drug related in 1 patient in the VMP arm. Causes of death in the VMP arm were pneumonia/bronchopneumonia (n = 3) and cardiorespiratory arrest (n = 1). Causes of death in the S+VMP arm were pneumonia/bronchopneumonia (n = 3), respiratory failure (n = 1), and suicide (n = 1).
The most frequent grade ≥3 AEs were neutropenia (S+VMP: 62% vs VMP: 43%; P = .06) and thrombocytopenia (S+VMP: 44% vs VMP: 25%; P = .034). However, there were no major bleeding events in either treatment arm. Platelet transfusions were given to 23% in the S+VMP arm and 11% in the VMP arm, and colony-stimulating factors were used in 46% and 34%, respectively. There was a trend toward higher incidence of grade ≥3 infections in the S+VMP arm (29%) compared with the VMP arm (17%; P = .15) consistent with the trend toward more high-grade neutropenia. The most frequent grade ≥3 infections were pneumonia and bronchopneumonia (17% in each arm).
A similar incidence of any grade and grade ≥2 peripheral neuropathy was reported in both arms (S+VMP 60% and 31%, VMP: 65% and 30%, respectively). Grade ≥3 peripheral sensory neuropathy was seen in 6% with S+VMP and 9% with VMP. The incidence of neuralgia (neuropathic pain) appeared lower on S+VMP (19%) than on VMP (30%; P = .193), which contributed to a higher incidence of bortezomib dose reductions in the VMP arm (S+VMP: 54%, 14% due to neuralgia, and VMP: 63%, 28% due to neuralgia). Two patients on S+VMP had a low-grade infusion-related reaction (1 grade 1 anxiety and 1 grade 2 leukocytoclastic vasculitis).
The maintenance therapy with siltuximab was very well tolerated. Of the 21 patients, 1 patient (5%) had an unrelated SAE of radius fracture due to disease progression, and no patient died or discontinued due to AEs. Grade ≥3 AEs were reported in 6 patients (2 neutropenia, 2 thrombocytopenia, 1 hypokalemia, and 1 radius fracture).
In part 1, 11 of the 12 patients treated with S+VMP had at least 1 grade ≥3 AE, and 8 patients had at least 1 SAE. One patient died due to septic shock. The types of AEs in this part were similar to those observed in the S+VMP arm in part 2.
Pharmacokinetics, pharmacodynamics, and patient-reportedoutcomes
Siltuximab pharmacokinetic analysis was performed on all patients of part 1 and in 40 evaluable patients in part 2 (n = 52). The pharmacokinetic profile of siltuximab was similar to previous observations in single-agent studies (data not shown).17,26 None of the 54 evaluable patients treated with S+VMP tested positive for an immune response to siltuximab.
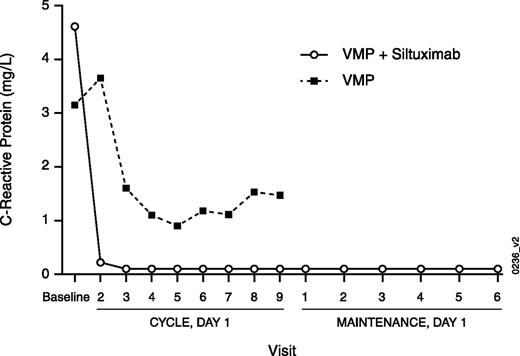
Systemic levels of IL-6 concentrations in serum during treatment cannot be used as a pharmacodynamic marker, because siltuximab-neutralized antibody-IL-6 complexes interfere with current immunologic-based IL-6 quantification methods. However, because IL-6 stimulates the acute-phase expression of CRP,12 posttreatment CRP serum concentrations can be used as a pharmacodynamic marker of neutralization of IL-6 bioactivity. Suppression of CRP was observed in both arms but was more pronounced and occurred earlier with S+VMP (Figure 2). At day 1 of cycle 2, median CRP levels decreased by 97% in part 1 and by 92% in the S+VMP arm (part 2) compared with only a 5% decrease in the VMP arm. A maximum median CRP decrease of 56% in the VMP arm was observed at day 1 of cycle 6 compared with 99% and 93% median CRP decrease in parts 1 and 2 of the S+VMP arm, respectively, at the same time point. Siltuximab maintenance therapy continued to provide sustained CRP suppression (Figure 2).
Median serum concentration for CRP by study visit in the randomized intention-to-treat population. C, cycle; D, day.
Median serum concentration for CRP by study visit in the randomized intention-to-treat population. C, cycle; D, day.
For the EORTC QLQ-C30 global health status scale collected in part 2, baseline mean scores were numerically higher for VMP (mean = 46.83) than for S+VMP (mean = 43.14). This is consistent with the more favorable distribution of ECOG performance scores on VMP, as indicated in Table 1. Mean scores increased from baseline to cycle 9 in both treatment arms, indicating a positive impact of treatment on the patients’ perception of their global health. A larger increase from baseline was observed on VMP (mean change at cycle 9 = 14.78) than on S+VMP (mean change at cycle 9 = 8.33), indicating less improvement on S+VMP. In both treatment arms, mean scores showed a larger increase after cycle 5 (weekly bortezomib treatment cycles).
Discussion
In this randomized phase 2 study in patients with newly diagnosed MM ineligible for high-dose therapy, the addition of siltuximab to the approved regimen of VMP resulted in a CR rate of 27%. This increase was not sufficient to confirm the study hypothesis of a 10% increase in CR by addition of siltuximab to VMP. However, for several other response parameters, a more pronounced difference with S+VMP was noted: the ≥VGPR rate was 71% on S+VMP and 51% on VMP, and a 100% decrease in serum M-protein was noted in 61% on S+VMP vs 38% on VMP.
It is striking that these differences in tumor response data did not translate into a difference in PFS or OS. Median PFS, based on stringent implementation of EBMT criteria through a validated computer algorithm, was 17 months in both treatment groups. In addition, investigator-determined PFS did not show a meaningful difference between the treatment groups. Several factors may have contributed to the absence of improvement in long-term outcomes. First, treatment with VMP alone may indirectly inhibit the IL-6 pathway and may eventually overlap with IL-6 blockade by siltuximab. This hypothesis is supported by prior observations that nuclear factor κB inhibition can reduce both IL-6 and CRP27 and by findings in this study that VMP also suppressed CRP, albeit to a lesser degree and with a slower time course than S+VMP. Second, it is possible that the siltuximab effect on an IL-6–dependent subclone results in initially fast responses but fails to control an IL-6–independent subclone ultimately responsible for disease relapse. This is supported by the observation in this study that many of the VGPR responses and 100% of the M-protein responses ultimately did not convert to a true CR upon more prolonged therapy. This study seems to confirm the importance of stringently assessed CR and of MRD negativity as robust predictors of long-term outcome. Third, there appears to be an imbalance in favor of the VMP treatment group of some disease characteristics with negative prognostic value, such as del17p (4% vs 15%) and the incidence of IgA myeloma (18.5% vs 41%).22,28 The high incidence of some adverse prognostic factors, such as IgA myeloma and ISS stage III disease, may also have contributed to the lower CR rate and investigator-determined PFS on the VMP arm as compared with the VMP results of the original VISTA study (CR 22% vs 30%, PFS 17.2 months vs 21.7 months).1
At the same time, the study data also allow a few potential explanations for the absence of long-term improvements with S+VMP to be excluded. First, the siltuximab dose schedule of 11 mg/kg every 3 weeks was able to provide sustained suppression of systemic IL-6 activity, as measured by suppression of CRP, during both combination therapy and single-agent maintenance. In a randomized study in MCD, an IL-6–driven lymphoproliferative disorder, siltuximab at this dose and schedule was recently shown to provide statistically significant improvements in disease symptoms, lymphadenopathy, and inflammatory parameters.18 Because the level of CRP suppression with S+VMP in the current study was similar to the level of CRP suppression by siltuximab alone in this MCD study, a paradoxical pharmacodynamic effect of VMP on siltuximab-based IL-6 inhibition appears unlikely. Second, the pharmacokinetic characteristics of siltuximab in this trial were consistent with previous observations at this dose and schedule in single-agent studies, which renders pharmacokinetic drug-drug interactions between any of the components of VMP and siltuximab unlikely. Third, there was no evidence in this study of development of neutralizing antibodies against siltuximab. And fourth, despite a moderate increase in AEs with the combination, the exposure to the components of the VMP regimen, as measured by their cumulative dose, was similar in both treatment groups, indicating that dose modifications to VMP were not counterbalancing any positive effect with siltuximab.
The addition of siltuximab resulted in a moderate increase in the AE profile of VMP, in particular in terms of neutropenia, thrombocytopenia, and infections. Maintenance therapy with siltuximab was very well tolerated, with no AE-related discontinuation and only 1 myeloma-related SAE reported that did not delay disease recurrence or progression. The safety observations of the maintenance therapy are consistent with other observations of the tolerability of single-agent siltuximab, including a low rate of low-grade infusion reactions, enabling prolonged treatment up to 7 years.18,29 This tolerability profile, together with the potential of siltuximab to affect pathways traditionally targeted by standard combination therapies as apparent from this study and, particularly, the existing information on the role of IL-6 in the initial development of MM,30,31 supports the idea to study siltuximab as monotherapy and in smoldering myeloma, where currently no standard therapy is given and a long-term low-toxicity approach would be relevant. A randomized placebo-controlled trial with single-agent siltuximab in high-risk smoldering myeloma is ongoing.
Presented in abstract form at the 18th annual meeting of the European Hematology Association, Stockholm, Sweden, June 13, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and staff members at the study sites and acknowledge An Van Eyken from Janssen Research & Development for excellent editorial assistance. This study was funded by Janssen Research & Development.
Research support was provided by Janssen Pharmaceutical Research & Development.
Authorship
Contribution: J.S.M., C.-K.M., S.P., A.P., T.P., H.V., H.X., and R.O. were involved in conception and design of the study; J.S.M., C.-K.M., M.-V.M., T.P., M.R., H.V., and H.X. were involved in development of methodology; J.S.M., J.B., O.S., F.M., C.-K.M., S.G., M.P.Z., S.P., Y.G.T., T.R., A.S., M.R., C.U., H.X., and R.O. collected and assembled the data; J.S.M., J.B., O.S., F.M., C.-K.M, S.G., M.P.Z., S.P., Y.G.T, T.R., A.S., M.-V.M, T.P., M.R., C.U., X.Q., H.V., H.X., and R.O. were involved in analysis and interpretation of data; J.L. was involved in patient care; and all authors contributed to the content of the manuscript and approved the final version to be published.
Conflict-of-interest disclosure: J.S.M. is member of the advisory committees for Millennium, Celgene, Novartis, Onyx, Janssen, Bristol-Myers Squibb, and MSD; J.B. receives research funding from Janssen and Celgene and receives honoraria from Janssen, Celgene, Onyx, GlaxoSmithKline, and Bristol-Myers Squibb; O.S. receives research funding from Janssen; F.M. consults/advises for Novartis, receives research funding from Hospira and Amgen, and receives honoraria from Pfizer; T.R. receives research funding from Janssen; G.Y.T. receives research funding from Novartis and Janssen, receives honoraria from Novartis, Janssen, Gilead, Hospira, and Sanofi-Aventis, and is member of speakers bureau of Novartis, Onyx, Janssen, Alexion, and Sanofi-Aventis; A.S. receives honoraria from Janssen and is member of Advisory committee for Janssen; M.-V.M. receives honoraria from Janssen; A.P. consults/advises for and receives honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium, and Onyx; R.O. consults/advises for Centocor and Millennium, receives research funding and honoraria from Centocor and Millennium, and is member of the advisory committees for Centocor and Millennium; T.P., M.R., C.U., X.Q., H.V., and H.X. disclose employment with Janssen R&D.; T.P., M.R., H.V., and H.X. hold stock with Johnson and Johnson. The remaining authors declare no competing financial interests.
Correspondence: Jesus San-Miguel, Clinica Universidad de Navarra, Universidad de Navarra, Navarra 31009, Spain; e-mail: sanmiguel@unav.es.