Key Points
We report that ruxolitinib reduces murine GVHD via increased Treg numbers.
We demonstrate the potent activity of ruxolitinib treatment in patients with corticosteroid-refractory GVHD.
Abstract
Graft-versus-host-disease (GVHD) is a severe complication of allogeneic hematopoietic cell transplantation (allo-HCT) characterized by the production of high levels of proinflammatory cytokines. Activated Janus kinases (JAKs) are required for T-effector cell responses in different inflammatory diseases, and their blockade could potently reduce acute GVHD. We observed that inhibition of JAK1/2 signaling resulted in reduced proliferation of effector T cells and suppression of proinflammatory cytokine production in response to alloantigen in mice. In vivo JAK 1/2 inhibition improved survival of mice developing acute GVHD and reduced histopathological GVHD grading, serum levels of proinflammatory cytokines, and expansion of alloreactive luc-transgenic T cells. Mechanistically, we could show that ruxolitinib impaired differentiation of CD4+ T cells into IFN-γ– and IL17A-producing cells, and that both T-cell phenotypes are linked to GVHD. Conversely, ruxolitinib treatment in allo-HCT recipients increased FoxP3+ regulatory T cells, which are linked to immunologic tolerance. Based on these results, we treated 6 patients with steroid-refractory GVHD with ruxolitinib. All patients responded with respect to clinical GVHD symptoms and serum levels of proinflammatory cytokines. In summary, ruxolitinib represents a novel targeted approach in GVHD by suppression of proinflammatory signaling that mediates tissue damage and by promotion of tolerogenic Treg cells.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) for many patients with high-risk or relapsed hematologic malignancies constitutes the only potentially curative treatment. However, acute graft-versus-host disease (aGVHD) causes significant morbidity in as much as 50% of recipients after allogeneic allo-HCT and accounts for 15% to 30% of deaths.1 Patients who do not respond to corticosteroid therapy are more likely to die of GVHD than patients with steroid-responsive GVHD.2,3
Ruxolitinib, a selective Janus kinase (JAK) 1/2 inhibitor, has recently been approved for the treatment of myelofibrosis (MF) based on the conception that in comparison with placebo or best available treatment, therapy with ruxolitinib reduced spleen size and constitutional symptoms and improved overall survival.4,5 Of note, clinical responses in MF patients were independent of the JAK2 mutational status, but were linked to suppression of increased serum levels of proinflammatory cytokines such as IL-1, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)-γ.6 Proinflammatory cytokines such as IL-1β,7 IL-6,8 or IFN-γ9 are considered hallmarks of aGVHD and have been linked to inflammation, tissue damage, and fibrosis. Thus suppression of proinflammatory cytokines could potentially reduce disease severity. Moreover, although most conventional immunosuppressive agents target T-cell function, ruxolitinib was shown to impair differentiation, maturation, and cytokine production of dendritic cells (DCs),10 which may further increase its efficacy in GVHD.
Major T-cell activation events via type II cytokine receptors are mediated by JAK 1, 2, and 3 kinases (eg, JAK1 is required for responses to IFN-γ and IL-6).11 When JAK kinases are activated, signal proteins of the STAT family are phosphorylated and act as transcription factors for target genes in the nucleus. In a murine model of aGVHD, STAT1 and STAT3 in CD4+ and CD8+ T cells were shown to be activated in an early stage of disease.12 If STAT1 was missing in donor splenocytes, clinical GVHD signs and the disease-related mortality were significantly impaired, both in the minor and major mismatch setting.13
Here we show that JAK1/2 inhibition by ruxolitinib potently reduced aGVHD in mice and significantly prolonged survival, even in an aggressive major mismatch model. Translating our observation into the clinic, we observed potent reduction of GVHD symptoms and serum cytokines in 6 patients with steroid-refractory aGVHD and chronic GVHD (cGVHD). Based on the well-characterized toxicity profile and the preclinical and clinical efficacy of ruxolitinib in aGVHD, we propose JAK1/2 inhibition as a new concept to interfere with this severe complication after allo-HCT.
Material and methods
Human subjects
Treatment with ruxolitinib, sample collection, and analysis were approved by the institutional review board of the Medical Center, University of Freiburg, Germany (protocol #267/11 and #10024/13). Written informed consent was obtained from each patient. This study was conducted in accordance with the Declaration of Helsinki. Histologic GVHD grading was performed on the basis of histopathology according to a published staging system for histology14 and clinical grading according to criteria for aGVHD15 or cGVHD.16 The patients characteristics, including underlying diagnosis, donor type, conditioning regimen, immunosuppressive regimen, recipient age, and gender, are detailed in supplemental Table 1 available on the Blood Web site.
Inclusion and exclusion criteria
Patients were included if they had aGVHD or cGVHD that was refractory to corticosteroids given for at least 1 month and at least 2 other immunosuppressive approaches. Initial treatment of cGVHD was prednisone at 1 mg/kg per day at our institutions. For cGVHD, the presence of at least 1 diagnostic clinical sign of cGVHD, or presence of at least 1 distinctive manifestation confirmed by pertinent biopsy was used.16 aGVHD was defined according to previous criteria.15 Exclusion criteria included uncontrolled fungal, cytomegalovirus, or varicella zoster virus infection; inability to tolerate oral administration of medications; known hypersensitivity to ruxolitinib; melena, frank gastrointestinal hemorrhage, or ulceration; absolute neutrophil count <1500/μL, and pregnancy or breastfeeding.
Treatment plan and evaluation of response
Six consecutive patients were treated with ruxolitinib at a starting dose of 5 mg orally twice daily, with a dose increase to 10 mg orally twice daily when no side effects were observed after 3 days of treatment. Medications to prevent Pneumocystis pneumonia and infection with cytomegalovirus, herpes simplex virus, varicella zoster virus, and fungal organisms were administered according to institutional practice.
aGVHD.
According to previously defined diagnostic criteria for aGVHD,15 treatment responses were categorized as complete response (CR), partial response (PR), or treatment failure. A CR to ruxolitinib was defined as the absence of any symptoms related to GVHD and no current medication needed to control the disease. A PR was defined as the improvement of at least 1 stage in the severity of aGVHD in 1 organ without deterioration in any other organ. Treatment failure was defined as the absence of improvement of GVHD and deterioration of aGVHD in any organ by at least 1 stage, or the development of GVHD manifestations in an earlier unaffected organ, or the use of any additional agents to control the disease. Patients were scored for their best response at any time after starting the treatment with ruxolitinib, with follow-up censored at the onset of any subsequent systemic immunosuppressive therapy.
cGVHD.
Organ sites considered for cGVHD scoring included skin, mouth, eyes, intestinal tract, liver, lungs, joints and fascia, and the genital tract. Each organ or site was scored according to a 4-point scale (0-3), with 0 representing no involvement and 3 reflecting severe impairment.16 Global scoring included both the number of organs or sites involved and the severity within each affected organ as previously defined.16
Responses in cGVHD were measured according to 2 outcomes. Failure was defined as the use of any additional agents to control GVHD within the time after starting treatment with ruxolitinib, including the resumption of treatment with agents used earlier or the increase of the dose of any immunosuppressive treatment that the patient received. Discontinuation of treatment with study drug because of toxicity was not considered as treatment failure. Response was defined as the discontinuation or durable (4 weeks) reduction of all systemic immunosuppressive therapy. Reduction of the corticosteroid dose by at least 50% for at least 4 weeks was defined as “reduction of corticosteroids” in the response evaluation. Duration of response was calculated from the onset of response after initiation of treatment with ruxolitinib until the end of the follow-up, GVHD relapse, development of new or deterioration of preexisting GVHD signs, or reinstitution of any additional agents to control the disease.
Mice
C57BL/6 (H-2Kb, Thy-1.2, or Thy1.1) and BALB/c (H-2Kd, Thy-1.2) mice were purchased either from Charles River Laboratory (Sulzburg, Germany) or from the local stock of the animal facility at University of Freiburg or Technical University, Munich. Mice between 6 and 12 weeks of age were used, and only female or male donor/recipient pairs were used. Luciferase (luc) transgenic C57BL/6 (Thy1.1) mice have been described previously.17 Animal protocols were approved by the Regierungspräsidium Freiburg, Freiburg, Germany, and Regierungspräsidium Oberbayern, München, Germany.
Bone marrow transplantation model and histopathology scoring
Bone marrow (BM) transplantation experiments were performed as described previously.18 Briefly, recipients were injected IV with 5 × 106 wild-type (WT) BM cells after lethal irradiation with 9 to 10 Gy. To induce GVHD, CD4 and CD8 T cells were isolated from donor spleens and enriched by positive selection with the MACS cell separation system (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Anti-CD4 and anti-CD8 MicroBeads were used. CD4/CD8 T-cell purity was at least 90% as assessed by flow cytometry (data not shown). CD4/CD8+ T cells were given at a dosage of 3 × 105 IV on day 0. Slides of the small bowel, large bowel, liver, and skin specimens collected on different days after allo-HCT were stained with hematoxylin and eosin and scored by an experienced pathologist blinded to the treatment groups. GVHD severity was determined according to a previously published histopathology scoring system.19
Treatment with ruxolitinib (INCB018424)
For in vitro experiments, we used ruxolitinib (INCB018424; Selleck Chemicals, Houston, TX) dissolved in dimethyl sulfoxide (DMSO). For in vivo experiments, we used ruxolitinib (Jakavi, Novartis, Nürnberg, Germany) dissolved in PEG300/dextrose 5% in a ratio of 1:3 (PEG/dex) by oral gavage at a daily dosage of 30 mg/kg 2 times daily starting from day −1 until day 20 after allo-HCT for survival and Beckman Laser Institute studies. The control group received PEG/dex alone.
Flow cytometry
Antibodies were purchased from BD Bioscience (Heidelberg, Germany) BioLegend (San Diego, CA), and eBioscience (San Diego, CA) and used as fluorescein isothiocyanate, phycoerythrin, Alexa647, or Pacific Blue conjugates. The following antibodies were used for flow-cytometric analysis: CD4 (GK 1.5/RM4-5), CD8 (53-6.7), CD44 (IM7), CD62L (MEL-14), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), FoxP3 (MF237-A), and Granzyme B (NGZB). Cells were stained in phosphate-buffered saline and 0.5% bovine serum antigen with fluorochrome-conjugated antibody in a dilution of 1:200 for 20 minutes at 4°C. For FoxP3 staining, permeabilization with the Fix/Perm Kit (ebioscience) was performed according to the manufacturer’s instructions. The FoxP3 antibody was used at a concentration of 1:100. For pSTAT3 expression, an anti-Stat3 (pY705) antibody (BD Biosciences) was used at a concentration of 1:50. Cells were fixed with 2% formalin and then exposed to 90% methanol, before application of the pSTAT3 antibody. After staining, cells were washed twice in phosphate-buffered saline/0.5% bovine serum antigen and acquired on the LSRII (BD Biosciences). Data analysis was performed using Flow Jo software (Tree Star, Ashland, OR).
For cell viability analysis, the live/dead fixable dead cell stain kit from Molecular Probes, (Invitrogen Life Technologies, Grand Island, NY) was used according to the manufacturer`s instructions.
Allogeneic mixed lymphocyte reactions
105 naïve CD4+ CD62L+ T cells extracted from BALB/c mice were cocultured with 25 × 103 BM-derived DCs purified from C57BL/6 (B6) mice. BM-derived DCs were harvested after a 7-day culture of primary BM cells with granulocyte macrophage colony-stimulating factor (10 ng/mL; R&D Systems, Minneapolis, MN). The DCs in the mixed lymphocyte reactions (MLR) were preactivated with 20 ng/mL lipopolysaccharide (LPS) for 24 hours before adding them to the MLR. The JAK1/2 inhibitor ruxolitinib (INCB018424) dissolved in DMSO, was given to the DCs at different concentrations on day 0. On day 1, CD4+ CD62L+ T cells were added and co-cultured with the DCs for an additional 5 days. Supernatants were taken at the end of the co-culturing and cytokine levels were analyzed with the CBA inflammation kit from BD Bioscience according to the manufacturer’s instructions.
Proliferation assay
Proliferation was measured in the MLR by a 3H thymidine uptake proliferation assay. Cells were pulsed on day 4 of the MLR with 1 mCi (3H) thymidine (Amersham, Braunschweig, Germany), incubated for an additional 16 hours and then analyzed within 30 minutes. Exposure to ruxolitinib was 4 days and 16 hours. The 3H thymidine incorporation was analyzed using a β scintillation counter (1450 Microß TriLux; Perkin Elmer, Waltham, MA).
In vivo bioluminescence imaging
In vivo bioluminescence imaging was performed as described previously.20
FoxP3+ Treg and IFNɣ+ T-cell frequencies in vitro
MACS sorted naïve T cells (CD4+ CD62L+) were activated in an in vitro MLR in the presence of allogeneic DC. The percentage of FoxP3+ Tregs was determined by intracellular FoxP3 staining after a 5-day culture. For the intracellular cytokine staining (ICC), T cells were restimulated after a 5-day MLR culture with PMA (1 µM), ionomycin (100 nM), and Brefeldin A for 4 hours. Intracellular fluorescence-activated cell sorting staining was performed for IFN-γ (clone XMG1.2) from eBioscience.
Cytokine measurements
Levels of cytokines in murine sera and cell culture supernatants were analyzed with the CBA Inflammation kit from BD Bioscience according to the manufacturer’s instructions.
Statistical analysis
For the sample size in the murine GVHD survival experiments, power analysis was performed. A sample size of at least 10 per group was determined by 80% power to reach a statistical significance of .05 to detect an effect size of at least 1.06. Differences in animal survival (Kaplan-Meier survival curves) were analyzed by log-rank test. To obtain unbiased data, the histopathologic scoring of the GVHD severity was performed by a pathologist blinded to both the genotype and the treatment group. Only after finalization of the quantitative GVHD severity scores were the samples allocated to their genotypes/treatment group.
For statistical analysis, an unpaired Student t test (2-sided) was applied. If the data did not meet the criteria of normality, the Mann-Whitney U test was applied. Data are presented as mean and standard error of the mean (error bars). Differences were considered significant when the P < .05.
Results
Ruxolitinib treatment reduces GVHD severity in a murine major mismatch model
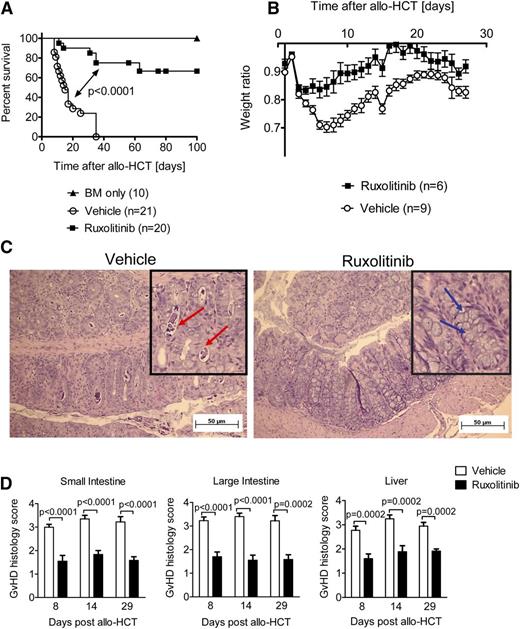
To determine whether ruxolitinib affects established parameters of aGVHD, we used a well-established C57BL/6 (H-2Kb) into a BALB/c (H-2Kd) major MHC mismatch model.18 Mice were treated with vehicle compared with ruxolitinib starting from day −1 to day 20 relative to allo-HCT. Ruxolitinib significantly improved survival of the mice having undergone allo-HCT compared with the group that was treated with vehicle only (Figure 1A). On day 35, all vehicle-treated mice had died, whereas 40% of all ruxolitinib-treated mice were still alive. Mice that had received BM only did not present any signs of GVHD, and their survival rate was 100% until the end point. As an indicator of GVHD, body weight was determined daily and the ratio of actual weight/initial weight was calculated. Ruxolitinib-treated mice had higher weight ratios throughout the experiment as shown for days 1 to 27 after allo-HCT (Figure 1B). On days 8, 14, or 29 after allo-HCT, mice were sacrificed and their small and large intestines and livers were analyzed for histopathologic GVHD signs. Ruxolitinib-treated mice displayed significantly lower GVHD scores and more goblet cells as an indicator of intact intestines21 compared with the vehicle-treated mice as determined by histopathologic analysis (Figure 1C-D). Mice that had received BM only did not present any histologic GVHD signs (data not shown). These data clearly demonstrate the potent reduction of GVHD severity by ruxolitinib treatment in a major MHC mismatch model.
Ruxolitinib treatment reduces GVHD severity in mice. (A) Survival of recipient BALB/c mice, after allo-HCT. Survival was improved in ruxolitinib-treated mice compared with vehicle-treated mice. The experiment was performed twice and the resulting data were pooled. The number of mice is indicated for each group. (B) Weight ratio (actual weight/initial weight) of recipient BALB/c mice, after allo-HCT as described in (A). (C) A representative section of a colon isolated on d8 after allo-HCT from mice treated as described under (A) is shown. Red arrows indicate crypts containing karyorrhectic debris. Blue arrows: goblet cells, absence of apoptotic bodies in the ruxolitinib treated mouse. (D) The organs small intestine, large intestine, and liver were isolated on days 8, 14, and 29 after allo-HCT, and histopathologic changes were scored as described in Material and methods. The data are pooled from 2 independent experiments with at least 6 mice per group.
Ruxolitinib treatment reduces GVHD severity in mice. (A) Survival of recipient BALB/c mice, after allo-HCT. Survival was improved in ruxolitinib-treated mice compared with vehicle-treated mice. The experiment was performed twice and the resulting data were pooled. The number of mice is indicated for each group. (B) Weight ratio (actual weight/initial weight) of recipient BALB/c mice, after allo-HCT as described in (A). (C) A representative section of a colon isolated on d8 after allo-HCT from mice treated as described under (A) is shown. Red arrows indicate crypts containing karyorrhectic debris. Blue arrows: goblet cells, absence of apoptotic bodies in the ruxolitinib treated mouse. (D) The organs small intestine, large intestine, and liver were isolated on days 8, 14, and 29 after allo-HCT, and histopathologic changes were scored as described in Material and methods. The data are pooled from 2 independent experiments with at least 6 mice per group.
Inhibition of proinflammatory cytokine production upon ruxolitinib treatment
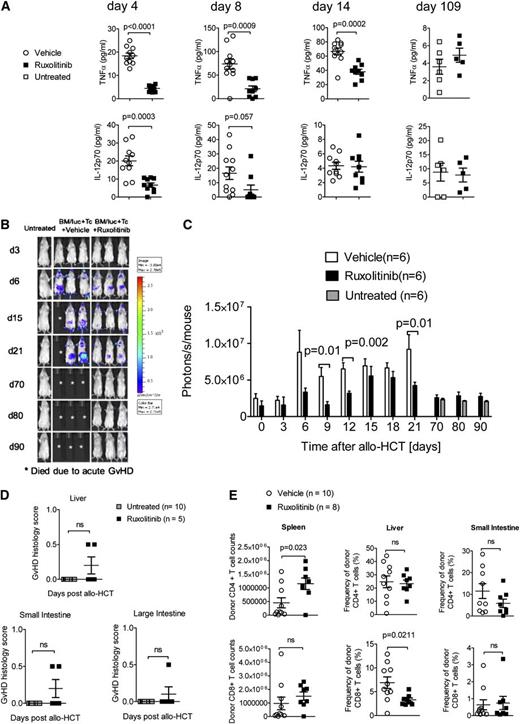
We hypothesized that ruxolitinib reduces GVHD via suppression of inflammatory cytokine production. We therefore measured serum levels of cytokines that are implicated in GVHD. As shown in Figure 2A, TNF-α was significantly reduced in mice treated with ruxolitinib compared with vehicle-treated mice on days 4, 8, and 14 and levels of IL-12p70 in ruxolitinib-treated mice were reduced on day 4 and showed a trend toward lower levels on day 8. On day 109, no vehicle-treated mice in the control group were alive; therefore ruxolitinib- treated mice were compared with untreated mice. We found that for day 109, the surviving ruxolitinib-treated mice had comparable serum levels of TNF-α and IL-12p70 to age-matched untreated controls. Because T-cell expansion is a second hallmark of GVHD, we also analyzed expansion of alloreactive luciferase transgenic T cells. We observed a significantly decreased signal derived from allogeneic T cells in ruxolitinib-treated mice compared with vehicle-treated mice at several time points after allo-HCT and found that at late time points (days 70, 80, and 90) the luc+ T-cell signal was comparable with untreated mice (Figure 2B-C). Ruxolitinib-treated mice analyzed on day 109 after allo-HCT had comparable GVHD scores as untreated mice as determined by histopathologic analysis (Figure 2D). T-cell frequencies in the small intestine were not different in the ruxolitinib-treated mice compared with vehicle-treated mice (Figure 2E). These findings indicate that the protective effect of ruxolitinib in GVHD is mediated by suppression of cytokine production and T-cell expansion after allo-HCT.
Proinflammtory cytokine production is blocked by ruxolitinib treatment. (A) The TNF-α and IL-12 levels determined in the serum on days 4, 8, 14, and 109 after allo-HCT in untreated mice or recipients treated with vehicle or ruxolitinib. The data are pooled from 2 independent experiments with at least 5 mice per group. (B-C) Serial luciferase–specific imaging was performed with BALB/c WT mice that had undergone allo-HCT with WT BM and luc+ CD4/CD8 T cells. The experiment was performed twice and 1 representative experiment is shown in (B). In (C) the respective P values for the individual time points and the number of mice in each group are indicated in the graph. (D) The organs small intestines, large intestines, and liver were isolated on d109 after allo-HCT from ruxolitinib-treated mice or from untreated mice, and histopathologic changes were scored as described in the Material and methods section. (E) The organs, small intestines, spleen, and liver were isolated on day 14 after allo-HCT, and the absolute numbers (spleen) or frequencies (small intestine and liver) of CD4 and CD8 T cells were determined. The number of mice is indicated for each group.
Proinflammtory cytokine production is blocked by ruxolitinib treatment. (A) The TNF-α and IL-12 levels determined in the serum on days 4, 8, 14, and 109 after allo-HCT in untreated mice or recipients treated with vehicle or ruxolitinib. The data are pooled from 2 independent experiments with at least 5 mice per group. (B-C) Serial luciferase–specific imaging was performed with BALB/c WT mice that had undergone allo-HCT with WT BM and luc+ CD4/CD8 T cells. The experiment was performed twice and 1 representative experiment is shown in (B). In (C) the respective P values for the individual time points and the number of mice in each group are indicated in the graph. (D) The organs small intestines, large intestines, and liver were isolated on d109 after allo-HCT from ruxolitinib-treated mice or from untreated mice, and histopathologic changes were scored as described in the Material and methods section. (E) The organs, small intestines, spleen, and liver were isolated on day 14 after allo-HCT, and the absolute numbers (spleen) or frequencies (small intestine and liver) of CD4 and CD8 T cells were determined. The number of mice is indicated for each group.
Ruxolitinib treatment affects the T-cell phenotype
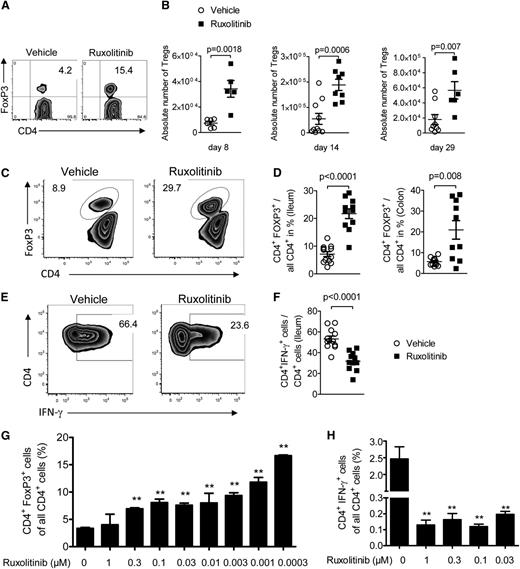
To elucidate the mechanisms by which ruxolitinib reduces T-cell expansion and cytokine production, we first analyzed the donor T-cell phenotype. Intriguingly, we observed higher frequencies of CD4+ FoxP3+ regulatory T cells (Treg) in ruxolitinib-treated mice compared with vehicle-treated mice in the spleen on days 8, 14, and 29 (Figure 3A-B). In addition to the spleen, we also found higher frequencies of Tregs in the ileum and colon of ruxolitinib-treated mice compared with vehicle-treated mice (Figure 3C-D), whereas CD4+IFN-γ+ cells were reduced in the ileum of ruxolitinib-treated mice compared with vehicle-treated mice (Figure 3E-F). Tregs were shown to reduce aGVHD in the applied murine GVHD model22 and promote long-lasting tolerance.20 This could be of clinical relevance given the observation that expansion of Tregs in patients by IL-2 treatment can reduce GVHD severity.23 The observed differences were not caused by myelosuppression, because leukocytes and platelets count analysis on multiple time points revealed no significant reduction in ruxolitinib-treated mice compared with vehicle-treated mice (supplemental Figure 1A-B). Because memory T cells were shown to maintain GVHD,24 we next analyzed this cell population and found lower central memory T-cell frequencies in ruxolitinib-treated mice compared with vehicle-treated mice (supplemental Figure 1C). To determine whether the observed higher Treg numbers in the spleens of ruxolitinib-treated mice were directly caused by the drug, we next incubated CD4+ T cells with allogeneic DCs and added ruxolitinib at increasing concentrations. The proportion of Tregs increased in an inverse concentration-dependent manner when ruxolitinib was added to the cultures (Figure 3G). Conversely, the frequency of IFN-γ producing CD4+ T cells was reduced when ruxolitinib was added to the cultures (Figure 3H).
Treg and T-cell phenotype changes during ruxolitinib treatment. (A-B) Shown are a representative flow cytometry plot and the absolute numbers of Treg cells in the spleens of animals on the indicated time points (days 8, 14, 29) after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (C-D) Shown are a representative flow cytometry plot (ileum) and the percentage of Treg cells in the ileum and colon of animals on the indicated time points after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (E-F) Shown are a representative flow cytometry plot (ileum) and the percentage of CD4+IFN-γ+ cells in the ileum of animals on the indicated time points after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (G) CD4+CD62L+ naïve T cells (BALB/c) were exposed to BM-derived DC (C57BL/6) preactivated with 20 ng/mL LPS. The percentage of CD4+FoxP3+ cells of all CD4+ cells is shown for different concentrations of ruxolitinib. One representative experiment of 3 is shown. (H) CD4+ T cells (BALB/c) were exposed to BM-derived DC (C57BL/6) preactivated with 20 ng/mL LPS. The percentage of CD4+IFN-γ+ cells of all CD4+ cells is shown for different concentrations of ruxolitinib. One representative experiment of 3 is shown.
Treg and T-cell phenotype changes during ruxolitinib treatment. (A-B) Shown are a representative flow cytometry plot and the absolute numbers of Treg cells in the spleens of animals on the indicated time points (days 8, 14, 29) after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (C-D) Shown are a representative flow cytometry plot (ileum) and the percentage of Treg cells in the ileum and colon of animals on the indicated time points after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (E-F) Shown are a representative flow cytometry plot (ileum) and the percentage of CD4+IFN-γ+ cells in the ileum of animals on the indicated time points after allo-HCT from vehicle or ruxolitinib-treated mice. Data from 2 independent experiments are pooled. (G) CD4+CD62L+ naïve T cells (BALB/c) were exposed to BM-derived DC (C57BL/6) preactivated with 20 ng/mL LPS. The percentage of CD4+FoxP3+ cells of all CD4+ cells is shown for different concentrations of ruxolitinib. One representative experiment of 3 is shown. (H) CD4+ T cells (BALB/c) were exposed to BM-derived DC (C57BL/6) preactivated with 20 ng/mL LPS. The percentage of CD4+IFN-γ+ cells of all CD4+ cells is shown for different concentrations of ruxolitinib. One representative experiment of 3 is shown.
Impact of ruxolitinib on T cells and DCs in vitro
To determine the impact of JAK1/2 inhibition on the expansion of CD4+ T cells in response to alloantigen, we exposed CD4+ or CD8+ T cells to allogeneic DCs that were pre-activated with LPS. The proliferation was significantly reduced in ruxolitinib-exposed CD4+ or CD8+ T cells compared with DMSO-exposed CD4+ or CD8+ T cells, respectively (Figure 4A). This was also seen when T cells were stimulated with CD3/CD28 beads in the absence of APCs (Figure 4B) and was not caused by an increased apoptosis rate of T cells becuase viability was comparable in ruxolitinib-exposed T cells compared with DMSO-exposed T cells (Figure 4C). Granzyme B was reduced in CD8 T cells exposed to ruxolitinib (Figure 4D). In addition, the production of IFN-γ, IL-17A, and IL-2 was significantly reduced in the co-cultures (Figure 4E) and there was a trend toward lower IL-6 and TNF production (Figure 4E). Furthermore, STAT3 phosphorylation was significantly reduced in ruxolitinib-exposed CD4+ T cells, thus verifying target inhibition (Figure 4F-G).
Direct impact of ruxolitinib on T cells and DCs function. (A) 3H thymidine uptake as an indicator of proliferation was measured in mixed lymphocyte reactions containing BALB/c spleen–derived CD4+ or CD8+ T cells co-cultured with or without C57BL/6 BM-derived DC preactivated with 20 ng/mL LPS. Different concentrations of ruxolitinib were applied. One representative experiment of 3 is shown. (B) 3H thymidine uptake as an indicator of proliferation was measured in mixed lymphocyte reactions containing BALB/c spleen–derived CD4+ T cells co-cultured with plate-bound CD3 and soluble CD28 beads. Different concentrations of ruxolitinib were applied. One representative experiment of 2 is shown. (C) Viability of CD4+ T cells derived from cultures described under (A) are shown. Live cells were determined by the number of viable cells reacting with the dye used in the live/dead stain kit. One representative experiment of 3 is shown. (D) Intracellular Granzyme B production in CD8+ T cells derived from cultures described under (A) are shown. (E) Proinflammatory cytokines measured in the supernatants of cultures described under (A) are shown. One representative experiment of 3 is shown. (F-G) STAT3 phosphorylation as determined by phospho flow of CD4+ T cells derived from cultures described under (A) are shown. One representative experiment of 3 is shown.
Direct impact of ruxolitinib on T cells and DCs function. (A) 3H thymidine uptake as an indicator of proliferation was measured in mixed lymphocyte reactions containing BALB/c spleen–derived CD4+ or CD8+ T cells co-cultured with or without C57BL/6 BM-derived DC preactivated with 20 ng/mL LPS. Different concentrations of ruxolitinib were applied. One representative experiment of 3 is shown. (B) 3H thymidine uptake as an indicator of proliferation was measured in mixed lymphocyte reactions containing BALB/c spleen–derived CD4+ T cells co-cultured with plate-bound CD3 and soluble CD28 beads. Different concentrations of ruxolitinib were applied. One representative experiment of 2 is shown. (C) Viability of CD4+ T cells derived from cultures described under (A) are shown. Live cells were determined by the number of viable cells reacting with the dye used in the live/dead stain kit. One representative experiment of 3 is shown. (D) Intracellular Granzyme B production in CD8+ T cells derived from cultures described under (A) are shown. (E) Proinflammatory cytokines measured in the supernatants of cultures described under (A) are shown. One representative experiment of 3 is shown. (F-G) STAT3 phosphorylation as determined by phospho flow of CD4+ T cells derived from cultures described under (A) are shown. One representative experiment of 3 is shown.
Ruxolitinib reduced skin, liver, and intestinal GVHD in patients with acute corticosteroid-refractory GVHD
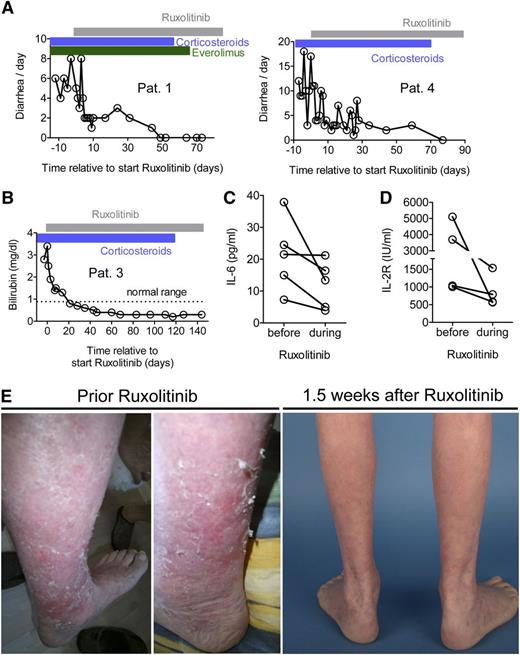
We next intended to evaluate whether the findings derived from our mouse model can be translated into the clinical situation. We treated 6 patients with aGVHD that was refractory to corticosteroids and at least 2 other therapeutic approaches with ruxolitinib at a starting dose of 5 mg twice daily with a dose increase to 10 mg twice daily when no side effects were observed after 3 days. Patients had undergone allo-HCT for various indications (supplemental Table 1) and were heavily pretreated for the current episode of aGVHD (Table 1). Treatment of 2 patients with intestinal GVHD resulted in the reduction of the stool frequency. In both patients, albumin serum levels increased to normal values, and clinical signs of intestinal GVHD resolved completely during treatment with ruxolitinib. The patients had previously not responded to therapy for 5 weeks or 7 weeks, respectively (Figure 5A). In a patient with liver GVHD, the bilirubin level decreased after initiation of ruxolitinib treatment to normal values (Figure 5B). The aGVHD serum parameters IL-6 and soluble IL-2 receptor (IL-2R) decreased in all analyzed patients upon ruxolitinib treatment (Figure 5C-D). All 4 patients with cutaneous GVHD experienced a response to ruxolitinib as shown for 1 representative patient (Figure 5E), and corticosteroids could be reduced (Table 1). Skin GVHD was reduced as monitored by clinical assessment, and reduction of the involved area was from 50% to <25%. Side effects of ruxolitinib such as thrombocytopenia or anemia were not observed. These findings, although being limited by the small number of patients, demonstrate that ruxolitinib exerts a clinically useful therapeutic effect in patients with GVHD refractory to available treatment.
GVHD, previous treatment and response to ruxolitinib
| Patient . | GVHD: organ/grade . | Immunosuppression before ruxolitinib* (duration in weeks) . | Reduction of corticosteroids after ruxolitinib . | Clinical response (PR/CR) . | Time to response (wk) . | Duration of response†/current follow up (wk)‡ . |
|---|---|---|---|---|---|---|
| 1 | Intestines / IV (acute) | Steroids (4) | Yes | CR | 1 | 20/21§ |
| Cyclosporin A (3) | ||||||
| Everolimus (2) | ||||||
| ECP (3) | ||||||
| 2 | Skin / III (acute) | Steroids (18) | Yes | PR | 1.5 | 19.5/21 |
| MMF (4) | ||||||
| Cyclosporin A (4) | ||||||
| Everolimus (9) | ||||||
| ECP (11) | ||||||
| 3 | Skin / IV | Steroids (31) | Yes | PR | 1 | 15/16 |
| liver / III | UVB radiation (5) | |||||
| (acute) | MMF (4) | |||||
| MTX (2) | ||||||
| Cyclosporin A (7) | ||||||
| Everolimus (5) | ||||||
| ECP (9) | ||||||
| 4 | Skin / III | Steroids (29) | Yes | PR | 1.5 | 13.5/15 |
| intestines / IV | Everolimus (8) | |||||
| (acute) | Cyclosporin A (13) | |||||
| ECP (7) | ||||||
| 5 | Skin / III (chronic) | Steroids (44) | Yes | Response | 1 | 36/37 |
| Sirolimus (4) | ||||||
| Cyclosporin A (16) | ||||||
| MMF (61) | ||||||
| MTX (4) | ||||||
| 6 | Skin/ III (chronic) | Steroids (144) | Yes | Response | 1 | 9/10 |
| CyclosporinA (144) | ||||||
| ECP (19) |
| Patient . | GVHD: organ/grade . | Immunosuppression before ruxolitinib* (duration in weeks) . | Reduction of corticosteroids after ruxolitinib . | Clinical response (PR/CR) . | Time to response (wk) . | Duration of response†/current follow up (wk)‡ . |
|---|---|---|---|---|---|---|
| 1 | Intestines / IV (acute) | Steroids (4) | Yes | CR | 1 | 20/21§ |
| Cyclosporin A (3) | ||||||
| Everolimus (2) | ||||||
| ECP (3) | ||||||
| 2 | Skin / III (acute) | Steroids (18) | Yes | PR | 1.5 | 19.5/21 |
| MMF (4) | ||||||
| Cyclosporin A (4) | ||||||
| Everolimus (9) | ||||||
| ECP (11) | ||||||
| 3 | Skin / IV | Steroids (31) | Yes | PR | 1 | 15/16 |
| liver / III | UVB radiation (5) | |||||
| (acute) | MMF (4) | |||||
| MTX (2) | ||||||
| Cyclosporin A (7) | ||||||
| Everolimus (5) | ||||||
| ECP (9) | ||||||
| 4 | Skin / III | Steroids (29) | Yes | PR | 1.5 | 13.5/15 |
| intestines / IV | Everolimus (8) | |||||
| (acute) | Cyclosporin A (13) | |||||
| ECP (7) | ||||||
| 5 | Skin / III (chronic) | Steroids (44) | Yes | Response | 1 | 36/37 |
| Sirolimus (4) | ||||||
| Cyclosporin A (16) | ||||||
| MMF (61) | ||||||
| MTX (4) | ||||||
| 6 | Skin/ III (chronic) | Steroids (144) | Yes | Response | 1 | 9/10 |
| CyclosporinA (144) | ||||||
| ECP (19) |
ECP, extracorporeal photophoresis; MMF, mycophenolate mofetil; MTX, methotrexate; steroids, corticosteroids.
Immunosuppression before ruxolitinib represents the treatment initiated for GVHD, not prophylactic measures. aGVHD15 and cGVHD16 were defined according to National Institutes of Health criteria, which are detailed in the Methods section.
For response definitions, see detailed information in the Methods section. Until last follow-up, none of the patients experienced a relapse of GVHD.
Follow-up was calculated from the time of initiation of ruxolitinib treatment.
In patient 1, ruxolitinib was discontinued at week 16 because of complete resolution of all GVHD signs. The patient did not develop any signs of GVHD after discontinuation of ruxolitinib until last follow-up.
Ruxolitinib reduces GVHD in patients with acute corticosteroid-refractory GVHD. All patients were refractory to steroids and at least 2 other lines of treatment of GVHD (see also Table 1). (A) Two patients with histologically proven intestinal GVHD grade IV were treated with ruxolitinib as described in Methods. The frequency of diarrhea decreased in both patients. No other immunosuppressive therapy was started at the same time point. The patients also had corticosteroids and everolimus when ruxolitinib was started, and corticosteroids could be tapered in both patients during ruxolitinib treatment. (B) One patient with clinically diagnosed liver GVHD grade III was treated with ruxolitinib 5 mg twice per day for the first 3 days and then 10 mg twice per day continuously. The bilirubin level decreased after ruxolitinib treatment. No other immunosuppressive therapy was started at the same time point but corticosteroid treatment (blue area) was reduced by 50% and then discontinued in the observation period. The patient received no additional liver toxic agent that was discontinued during the entire time that is displayed. (C-D) The IL-6 and soluble IL-2R were measured before and after the start of ruxolitinib (range, 1-2 days before at latest 8 days after treatment start). The levels of these serum parameters declined in all analyzed patients. (E) A representative patient with cutaneous GVHD is shown before and 1.5 weeks after ruxolitinib. The patient characteristics and responses are summarized in Table 1 and supplemental Table 1.
Ruxolitinib reduces GVHD in patients with acute corticosteroid-refractory GVHD. All patients were refractory to steroids and at least 2 other lines of treatment of GVHD (see also Table 1). (A) Two patients with histologically proven intestinal GVHD grade IV were treated with ruxolitinib as described in Methods. The frequency of diarrhea decreased in both patients. No other immunosuppressive therapy was started at the same time point. The patients also had corticosteroids and everolimus when ruxolitinib was started, and corticosteroids could be tapered in both patients during ruxolitinib treatment. (B) One patient with clinically diagnosed liver GVHD grade III was treated with ruxolitinib 5 mg twice per day for the first 3 days and then 10 mg twice per day continuously. The bilirubin level decreased after ruxolitinib treatment. No other immunosuppressive therapy was started at the same time point but corticosteroid treatment (blue area) was reduced by 50% and then discontinued in the observation period. The patient received no additional liver toxic agent that was discontinued during the entire time that is displayed. (C-D) The IL-6 and soluble IL-2R were measured before and after the start of ruxolitinib (range, 1-2 days before at latest 8 days after treatment start). The levels of these serum parameters declined in all analyzed patients. (E) A representative patient with cutaneous GVHD is shown before and 1.5 weeks after ruxolitinib. The patient characteristics and responses are summarized in Table 1 and supplemental Table 1.
Discussion
Acute corticosteroid-refractory GVHD occurs in approximately 50% of all patients with GVHD and causes high morbidity and mortality rates, with only approximately 5% to 30% long-term survivors being reported.25 After corticosteroid failure, available second-line therapy approaches such as sirolimus or photopheresis have shown limited activity, and none has been established as a standard salvage therapy for corticosteroid-refractory GVHD. Novel findings on the immunopathology of GVHD have demonstrated the importance of proinflammatory interleukins, which enhance the activation and proliferation of effector T cells as well as their phenotypic commitment toward Th-1 or Th-17 when GVHD ensues.7,26 At the same time, a lack of Tregs with a naïve phenotype in patients developing aGVHD has been recently reported.27
Therefore a targeted therapy that would correct the deregulated cytokine production and promote Treg development in patients with aGVHD could help to improve the clinical outcome of GVHD after allo-HCT. Here we report that ruxolitinib potently impaired production of multiple proinflammatory cytokines and enhanced Treg frequencies, whereas T-cell expansion and GVHD-related mortality were reduced in an aggressive major HLA mismatch mouse model of aGVHD. The encouraging clinical effect in patients with corticosteroid-refractory aGVHD supports the potential therapeutic role of this drug for the treatment of GVHD.28
The protective effect of ruxolitinib observed in our GVHD model was paralleled by increased Treg frequencies in the spleen, ileum, and colon of ruxolitinib-treated mice, and this effect could be reproduced in vitro when murine T cells were exposed to alloantigen. Consistent with the reduced T-cell expansion that we observed when luciferase transgenic T cells were transferred into allogeneic BALB/c recipients, it was previously reported that proliferation of human T cells exposed to allogeneic DCs in vitro was suppressed in the presence of ruxolitinib.10 In addition to this indirect effect of ruxolitinib on T-cell proliferation via impaired DC differentiation and maturation,10 we observed that ruxolitinib in T cells directly suppresses STAT3 phosphorylation during alloantigen-mediated activation. In addition, STAT3 activity critically determines cytokine mediated helper T–cell differentiation and induces production of proinflammatory cytokines,29 several of which were shown to be involved in the pathogenesis of aGVHD.
Our observation that ruxolitinib treatment mediates inhibition of STAT3 phosphorylation and at the same time increases Treg frequencies is consistent with a recent report showing that STAT3 limits Treg cell numbers by causing instability of natural Treg cells and by inhibition of induced Treg-cell polarization from naïve CD4+ T cells.30 Antagonizing these STAT3-mediated effects might well account for our finding of higher Treg-cell frequency in ruxolitinib-treated animals. The second phenotypic change in ruxolitinib-treated animals was the reduction of central memory T cells, which is consistent with the observation that alloreactive memory T cells are responsible for the persistence of GVHD.24 Our findings are consistent with recently reported results showing that JAK2 inhibition causes tolerance toward alloantigen by human DCs in vitro, whereas immunity to recall antigen was preserved.31
Our observation that reduced IFN-γ production after administration of ruxolitinib in a major mismatch mouse model led to lower GVHD severity is consistent with findings of Choi et al demonstrating that pharmacologic inhibition of IFN-R signaling and JAK inhibition resulted in reduced GVHD.32 In addition tofacitinib, a first-generation JAK1/2/3, was used in a different setting. Here, in a semi-allogeneic system, Park and colleagues report that pretreatment of mice with tofacitinib reduced GVHD pathology via the suppression of both proliferation and IFN-γ production by the donor CD4+ T cells.28 Also in a skin-specific GVHD model using expression of chicken ovalbumin in skin and mucosal epithelia under control of the keratin 14 promoter, mucocutaneous GVHD was reduced by tofacitinib.33 Tofacitinib was shown to have efficacy in psoriasis,34 rheumatoid arthritis,35 and ulcerative colitis, the last sharing pathomechanistical features with acute intestinal GVHD.36 However, although tofacitinib is approved for patients with rheumatoid arthritis, it has not been used in patients with hematologic malignancies, and the broad JAK1,2,3 might affect the toxicity profile in those patients.
Our in vitro and in vivo findings encouraged us to initiate a single-center experience for off-label use of ruxolitinib as a salvage therapy in patients with acute corticosteroid-refractory GVHD. All patients had failed at least 2 prior lines of GVHD treatment besides corticosteroids and had mostly cutaneous GVHD. Responses to treatment with improved GVHD grades and reduction of corticosteroids was observed in all patients. Also, the serum levels for IL-68 and soluble IL-2R, both being reported to correlate with GVHD severity, declined in all analyzed patients in the course of the ruxolitinib treatment. These findings, although being preliminary because of the small number of patients, demonstrate that ruxolitinib induces clinically useful responses in GVHD and suggest that therapeutic JAK1/2 inhibition may become an important salvage option in patients with corticosteroid-refractory GVHD.
In summary, we report the efficacy of ruxolitinib in GVHD in a murine model, characterize the drug-specific effects on alloantigen-driven T-cell expansion, cytokine production, and Treg development, and demonstrate its potent activity in patients with corticosteroid-refractory GVHD. Mechanistically we identify inhibition of STAT3 phosphorylation as the key event that is inhibited by ruxolitinib during an allogeneic immune response. Overall our findings demonstrate that targeting JAK1/2 signaling in alloreactive T cells is a powerful approach to inhibition of GVHD.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Franziska Leonhardt for her help with mouse experiments and Lynsey Fairbairn for her assistance with the 3H proliferation assay.
This study was supported by the Deutsche Forschungsgemeinschaft, Germany, Heisenberg Professorship (DFG ZE 872/3-1) and DFG individual grant (DFG ZE 872/1-2) (R.Z.) and PO 1575/3-1 (to H.P.), as well as the Else-Kröner-Fresenius-Stiftung (A61) (to H.P.), Excellence Initiative of the Deutsche Forschungsgemeinschaft (GSC-4, Spemann Graduate School [R.Z.]; and BIOSS II, project no. B13 [R.Z.]), and Deutsche Krebshilfe (109819) (R.Z.).
Authorship
Contribution: S.S., N.R.M., M.B., S.C., M.V., J.F., V.O., M.S., K.M.-B., and T.M. helped to design the experiments and performed experiments; A.S.-G. analyzed GVHD histopathology; J.F., C.P., J.D., and H.P. helped to design the experiments and to treat the patients; N.v.B. developed the overall concept; and R.Z. and N.v.B. designed and supervised the experiments, discussed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikolas von Bubnoff, Department of Hematology, Oncology and Stem Cell Transplantation, University Medical Center Freiburg, Freiburg, D-79106 Freiburg, Germany; e-mail: nikolas.bubnoff@uniklinik-freiburg.de.
References
Author notes
S.S. and N.R.M. are co-first authors. R.Z. and N.v.B. are co-senior authors.