Key Points
Transcription factor KLF2 is a critical determinant of vascular thrombosis.
The antithrombotic effect noted with bortezomib is KLF2 dependent.
Abstract
Multiple myeloma confers a high risk for vascular thrombosis, a risk that is increased by treatment with immunomodulatory agents. Strikingly, inclusion of the proteasome inhibitor bortezomib reduces thrombotic risk, yet the molecular basis for this observation remains unknown. Here, we show that bortezomib prolongs thrombosis times in the carotid artery photochemical injury assay in normal mice. Cell-based studies show that bortezomib increases expression of the transcription factor Kruppel-like factor 2 (KLF2) in multiple cell types. Global postnatal overexpression of KLF2 (GL-K2-TG) increased time to thrombosis, and global postnatal deletion of KLF2 (GL-K2-KO) conferred an antiparallel effect. Finally, studies in GL-K2-KO mice showed that the thromboprotective effect of bortezomib is KLF2 dependent. These findings identify a transcriptional basis for the antithrombotic effects of bortezomib.
Introduction
Cancer is associated with a high risk of thrombosis.1 Among hematologic malignancies, multiple myeloma (MM) has an especially high rate of both arterial and venous thrombotic events (VTEs).2 Further, patients treated with the immunomodulatory drugs (IMiDs) thalidomide and lenalidomide experience a substantially elevated VTE risk (15% to 30%).3 In sharp contrast, MM patients receiving proteasome inhibitor bortezomib (BZ) have a VTE risk that is less than 4%.4,5 BZ treatment in hypertensive rat models consistently decreases arterial thrombosis.6 Although in vitro and ex vivo studies show that BZ inhibits platelet aggregation, the magnitude of this effect is not sufficient for explaining the profound decrease in VTEs noted with BZ.7-9 Consequently, alternative mechanisms are likely operative and account for the antithrombotic effects of BZ.
Kruppel-like factor 2 (KLF2) is a member of the zinc finger family of transcription factors, which are highly expressed in endothelial and hematopoietic cells.10-13 Studies in our laboratory identified KLF2 as a key regulator of endothelial14-16 and myeloid inflammation with favorable antithrombotic properties.17,18 Further, studies conducted by us and others reveal that BZ induces KLF2 messenger RNA (mRNA) in endothelial19 and hematopoietic cells. Because endothelial and hematopoietic cells are centrally involved in thrombotic events, we hypothesized that the antithrombotic effect noted with BZ may be KLF2 dependent.
Methods
CAG-cre/ERT2 (control) mice (Jackson Laboratory) were crossed with floxed KLF2 mice or floxed stop KLF2 mice to generate global KLF2-knockout (GL-K2-KO) or global KLF2-overexpressing (GL-K2-TG) mice (previously described20 ), respectively. Indicated mice and cells were treated with BZ at the mentioned dose and schedule.
All the mouse colonies were maintained in a clean animal facility, and all animal experimentation was approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Further details are provided in the supplemental Methods available on the Blood Web site.
Results and discussion
BZ prolongs time to occlusion in a carotid artery thrombosis assay
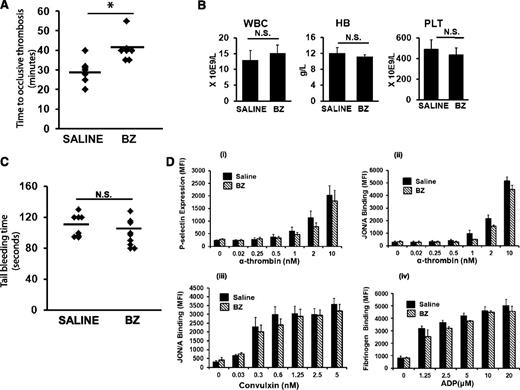
To determine whether BZ confers an antithrombotic effect, C57BL/6J mice treated with a nonmyelosuppressive dose of BZ (0.3 mg/kg intraperitoneally 3 times per week for 2 weeks) were subjected to carotid artery thrombosis by using the photochemical injury model.21 Wild-type mice treated with BZ demonstrated a significantly prolonged time to occlusive carotid artery thrombosis compared with mice treated with saline (40.8 ± 6 vs 29.4 ± 6 minutes; P = .006; n = 7 mice per group) (Figure 1A). There was no difference between white blood cell (WBC) counts and platelet counts and red blood cell (RBC) hemoglobin in saline- and BZ-treated animals (Figure 1B). Further, the antithrombotic effect was not associated with concomitant changes in the tail-bleeding (Figure 1C) or in platelet activation assays (Figure 1D).
BZ has an antithrombotic effect. (A) C57BL/6J mice at age 8 to 12 weeks treated with intraperitoneal BZ injections (0.3 mg/kg) 3 times per week for 2 weeks. Carotid artery thrombosis21 was performed 24 to 30 hours after the last dose of BZ. *P < .05. (B) WBC count, hemoglobin (HB), and platelet (PLT) counts in C57BL/6J mice treated with BZ. (C) C57BL/6J mice treated with BZ as described in (A) were subjected to tail bleeding assay 24 hours after last dose of BZ. (D) The assay used flow cytometry to examine (i) JON/A binding to platelets stimulated with alpha-thrombin, (ii) P-selectin expression of platelets stimulated with alpha-thrombin, (iii) JON/A binding to platelets stimulated with convulxin, and (iv) fibrinogen binding to platelets after stimulation of platelets with adenosine 5′-diphosphate (ADP). The concentration-dependent results are the mean ± standard error of the mean of 5 individual experiments. N.S. no significant difference. *P < .05
BZ has an antithrombotic effect. (A) C57BL/6J mice at age 8 to 12 weeks treated with intraperitoneal BZ injections (0.3 mg/kg) 3 times per week for 2 weeks. Carotid artery thrombosis21 was performed 24 to 30 hours after the last dose of BZ. *P < .05. (B) WBC count, hemoglobin (HB), and platelet (PLT) counts in C57BL/6J mice treated with BZ. (C) C57BL/6J mice treated with BZ as described in (A) were subjected to tail bleeding assay 24 hours after last dose of BZ. (D) The assay used flow cytometry to examine (i) JON/A binding to platelets stimulated with alpha-thrombin, (ii) P-selectin expression of platelets stimulated with alpha-thrombin, (iii) JON/A binding to platelets stimulated with convulxin, and (iv) fibrinogen binding to platelets after stimulation of platelets with adenosine 5′-diphosphate (ADP). The concentration-dependent results are the mean ± standard error of the mean of 5 individual experiments. N.S. no significant difference. *P < .05
BZ induces KLF2
A large body of work suggests that interaction between endothelial and hematopoietic cells (eg, platelets and myeloid cells) regulates thrombosis.22 As shown in Figure 2A, BZ induced KLF2 mRNA in both endothelial (human umbilical vein endothelial cells) and hematopoietic (RAW264.7 and MEG-01) cell lines. This induction is specific to KLF2 in the myeloid and megakaryocytic cell lines. Although KLF4 is concomitantly induced in endothelial cells (human umbilical vein endothelial cells), it was not as robust as the increase in KLF2 levels (supplemental Figure 1). Treatment of C57BL/6J mice with BZ consistently induced KLF2 mRNA in peripheral WBCs (Figure 2B). To further understand how KLF2 levels are induced, we examined the effect of BZ on a KLF2 promoter luciferase construct and found that BZ induced KLF2 promoter activity (supplemental Figure 2).
Antithrombotic effect of BZ is KLF2 dependent. (A) Endothelial (human umbilical vein endothelial cells [HUVECs]), monocytic (RAW 264.7), and megakaryocyte (MEG-01) cell lines treated with BZ at 5 µM for 24 hours (*P < .05; n = 3 to 5 mice per group). Total RNA was isolated, and KLF2 mRNA expression was analyzed by quantitative polymerase chain reaction (qPCR) and was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) C57BL/6J mice were treated with BZ, and WBCs were isolated for KLF2 mRNA expression and were normalized to GAPDH (*P < .05; n = 5). (C) Carotid artery thrombosis assay (photochemical injury model) was performed in CAG-Cre-ERT2 (CRE), GL-K2-KO, and GL-K2-TG mice. (D) CRE and GL-K2-KO mice were treated with intraperitoneal BZ (0.3 mg/kg) vs saline 3 times per week for 2 weeks followed by carotid artery thrombosis assay (photochemical injury model) performed 24 to 30 hours after the last dose of BZ. (E) Total RNA was isolated from primary KLF2 knockout and overexpressed macrophages (peritoneal macrophages) and endothelial cells (cardiac and pulmonary), and mRNA expression was analyzed as indicated by qPCR and normalized to GAPDH. eNOS, endothelial nitric oxide synthase; TF, tissue factor; WT, wild-type.
Antithrombotic effect of BZ is KLF2 dependent. (A) Endothelial (human umbilical vein endothelial cells [HUVECs]), monocytic (RAW 264.7), and megakaryocyte (MEG-01) cell lines treated with BZ at 5 µM for 24 hours (*P < .05; n = 3 to 5 mice per group). Total RNA was isolated, and KLF2 mRNA expression was analyzed by quantitative polymerase chain reaction (qPCR) and was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) C57BL/6J mice were treated with BZ, and WBCs were isolated for KLF2 mRNA expression and were normalized to GAPDH (*P < .05; n = 5). (C) Carotid artery thrombosis assay (photochemical injury model) was performed in CAG-Cre-ERT2 (CRE), GL-K2-KO, and GL-K2-TG mice. (D) CRE and GL-K2-KO mice were treated with intraperitoneal BZ (0.3 mg/kg) vs saline 3 times per week for 2 weeks followed by carotid artery thrombosis assay (photochemical injury model) performed 24 to 30 hours after the last dose of BZ. (E) Total RNA was isolated from primary KLF2 knockout and overexpressed macrophages (peritoneal macrophages) and endothelial cells (cardiac and pulmonary), and mRNA expression was analyzed as indicated by qPCR and normalized to GAPDH. eNOS, endothelial nitric oxide synthase; TF, tissue factor; WT, wild-type.
KLF2 is a critical determinant of the thrombotic phenotype
Cell-based gene expression studies in endothelial and myeloid cells by our group suggest that KLF2 likely confers an antithrombotic effect.13,15 However, the significance of these observations has not been studied in vivo. We performed carotid artery thrombosis assays in mice with global postnatal KLF2 deletion (GL-K2-KO) or overexpression (GL-K2-TG). Compared with the controls, GL-K2-KO mice have a significantly shortened time to occlusive thrombosis (Figure 2C; 20.6 ± 2 vs 32.8 ± 4 minutes; P = .0001; n = 6 to 7). Conversely, GL-K2-TG mice demonstrated significantly prolonged times to occlusive thrombosis (44.8 ± 1 vs 32.8 ± 4 minutes; P = .0003; n = 5 to 7). These results identify KLF2 as a critical determinant of thrombosis in vivo.
The antithrombotic effect of BZ is KLF2 dependent
To determine whether the antithrombotic effect of BZ is KLF2 dependent, we treated GL-K2-KO and control mice with BZ. Although occlusive carotid artery thrombosis times were significantly prolonged in the control mice treated with BZ (36 ± 5 vs 51.2 ± 8 minutes; P = .009; n = 5 to 7) (Figure 2D), no prolongation was noted in the GL-K2-KO mice (26.1 ± 4 vs 25.6 ± 4 minutes; P = .8; n = 5 to 7) after BZ treatment demonstrating that the thromboprotective effect of BZ is dependent on KLF2.
KLF2 alters the expression of thrombotic targets
We analyzed primary endothelial cells and peritoneal macrophages obtained from mice with KLF2 knockout or overexpression. KLF2 overexpression was associated with a significant decrease in inducible nitric oxide synthase (iNOS) and monocyte chemotactic protein-1 (MCP-1) in peritoneal macrophages and protease-activated receptor-1 (PAR-1) and thrombomodulin (TM) in endothelial cells. Conversely, KLF2 knockout demonstrated a significant increase in iNOS levels in peritoneal macrophages and plasminogen activator inhibitor 1 (PAI-1) in endothelial cells (Figure 2E).
Our findings are the first in vivo studies to implicate a transcription factor, namely KLF2, as a key orchestrator of the thromboprotective effect observed with BZ. Previous in vitro studies demonstrated that BZ treatment is associated with increased TM expression that was dependent on KLF2.19 Furthermore, studies identified that BZ induces endothelial NOS (eNOS) and decreases secondary thrombotic events and inflammatory responses.23,24 Given that KLF2 is known to confer potent anti-inflammatory effects and induce eNOS, it is possible that these observations are secondary to increased KLF2 levels, as suggested by our studies.
An additional mechanism implicated in the antithrombotic effect of BZ is inhibition of platelet aggregation.7-9 However, Avcu et al7 described their observations as a mild platelet defect that was insufficient to explain the significant antithrombotic effect. Consistent with this view, we did not observe a significant effect on platelet activation after treatment with BZ. Hence, our finding that the antithrombotic effects of BZ are abrogated in the absence of KLF2 implicates a more fundamental underlying mechanism. Interestingly, BZ treatment in hematopoietic cells (myeloid and megakaryocyte) specifically induces only one member of the KLF family (ie, KLF2). Although both KLF2 and KLF4 are induced in the endothelium, the effect on KLF2 transcript levels is more robust. Since KLF4, a closely related member of the Kruppel family of transcription factors also has thromboprotective properties,25 the demonstration that the antithrombotic effect of BZ is essentially lost in the absence of KLF2 compels us to postulate that in the context of BZ treatment, KLF2 must provide a more dominant antithrombotic effect, one that cannot be compensated for by endothelial induction of KLF4, which can occur with BZ treatment. We also demonstrated that KLF2 alters key thrombotic targets such as TM, PAI-1, and PAR-1. Although there is significant overlap in the number of downstream targets altered by both KLF2 and KLF4, it is likely that genes more critical to the thrombotic process are affected by KLF2 alone and reflect the dependency of BZ on this transcription factor for the antithrombotic benefit.
In summary, this study implicates the transcription factor KLF2 as a critical determinant of thrombosis. Our study also demonstrates that BZ induces KLF2 in both hematopoietic and endothelial cells and provides in vivo genetic evidence that the antithrombotic benefit noted with BZ is KLF2 dependent. Hence, KLF2 may be a novel target amenable to pharmacologic manipulation in the management of thrombosis. Further studies are being pursued to elucidate the relative contribution of each cell type in the thromboprotective effect and will provide useful insights into the molecular mechanisms and potential targets for amelioration of thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Mentored Research Award granted by the Hemostasis and Thrombosis Research Society (2011-2013) (L.N.); Visconsi Scholarship (G.B.A.); National Heart, Lung and Blood Institute grants HL087595, HL117759 (Z.L.), HL052279-18, HL112666-02 (A.H.S.), R01 HL075427, HL097593, HL112486, HL119195, and HL086548; and an American Heart Association Established Investigator Award (M.K.J.).
Authorship
Contribution: L.N. and H.S. performed experiments; L.N., G.B.A., and Z.L. analyzed and interpreted the data; L.N. and M.K.J. designed the research; G.B.A., Z.L., and A.H.S. provided critical advice on research design and experiments; L.N. drafted the manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mukesh K. Jain, Case Cardiovascular Research Institute, Department of Medicine, Case Western Reserve University School of Medicine, Iris S. and Bert Wolstein Research Bldg, 2103 Cornell Rd, Cleveland, OH 44106; e-mail: mxj84@case.edu.

![Figure 2. Antithrombotic effect of BZ is KLF2 dependent. (A) Endothelial (human umbilical vein endothelial cells [HUVECs]), monocytic (RAW 264.7), and megakaryocyte (MEG-01) cell lines treated with BZ at 5 µM for 24 hours (*P < .05; n = 3 to 5 mice per group). Total RNA was isolated, and KLF2 mRNA expression was analyzed by quantitative polymerase chain reaction (qPCR) and was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) C57BL/6J mice were treated with BZ, and WBCs were isolated for KLF2 mRNA expression and were normalized to GAPDH (*P < .05; n = 5). (C) Carotid artery thrombosis assay (photochemical injury model) was performed in CAG-Cre-ERT2 (CRE), GL-K2-KO, and GL-K2-TG mice. (D) CRE and GL-K2-KO mice were treated with intraperitoneal BZ (0.3 mg/kg) vs saline 3 times per week for 2 weeks followed by carotid artery thrombosis assay (photochemical injury model) performed 24 to 30 hours after the last dose of BZ. (E) Total RNA was isolated from primary KLF2 knockout and overexpressed macrophages (peritoneal macrophages) and endothelial cells (cardiac and pulmonary), and mRNA expression was analyzed as indicated by qPCR and normalized to GAPDH. eNOS, endothelial nitric oxide synthase; TF, tissue factor; WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/24/10.1182_blood-2014-01-547448/4/m_3828f2.jpeg?Expires=1769224260&Signature=dlVAldEr8dEPdzY6LG0WqLfApvuDxOw~RqAtt2Qm6ql68fj75o-o-MFUK6Tqm1stH1uRlbNjtDPpilvL4tDyPwhLZ3zKlitnhFUadEre2Ll6vtXULt8WxZzRdHwtMIyEQ4znI80K9fPxh66rvglPxdiXsY9goI3WEnkpqhjw4yHSsVXeh3jVhNIs7G9UnazrXsrRxcDjT79Fmm1rQGG4hvE1DHiedNLMbXJ~tHOyhorsURvd-VbywqwaVQd2AkXeFWRbh6p2YUFto-USJEAx-4SMQc4hJ4MedjdGuCwCcUmcp6RtXhpBJLta6aZi2YtvmmHM99O5hPEf-ukvASiWvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)