Key Points
Demonstrates a role for negative regulator of innate immunity, Siglec-G, in controlling GVHD.
Shows that enhancing the interaction between host Siglec-G and CD24 on donor T cells with a novel CD24 fusion protein mitigates GVHD.
Abstract
Activation of sialic-acid–binding immunoglobulin-like lectin-G (Siglec-G) by noninfectious damage-associated molecular patterns controls innate immune responses. However, whether it also regulates T-cell–mediated adaptive immune responses is not known. Graft-versus-host reaction is a robust adaptive immune response caused by allogeneic hematopoietic cell transplantation that have been activated by antigen-presenting cells (APCs) in the context of damaged host tissues following allogeneic hematopoietic cell transplantation. The role of infectious and noninfectious pattern recognition receptor–mediated activation in the induction and aggravation of graft-versus-host disease (GVHD) is being increasingly appreciated. But the role of pathways that control innate immune responses to noninfectious stimuli in modulating GVHD has heretofore not been recognized. We report that Siglec-G expression on host APCs, specifically on hematopoietic cells, negatively regulates GVHD in multiple clinically relevant murine models. Mechanistic studies with various relevant Siglec-G and CD24 knockout mice and chimeric animals, along with rescue experiments with novel CD24 fusion protein demonstrate that enhancing the interaction between Siglec-G on host APCs with CD24 on donor T cells attenuates GVHD. Taken together, our data demonstrate that Siglec-G–CD24 axis, controls the severity of GVHD and suggest that enhancing this interaction may represent a novel strategy for mitigating GVHD.
Introduction
Innate immune response is initiated by the evolutionarily conserved Toll-like receptors (TLRs), nod-like receptors, and other pattern recognition receptors that respond to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). Immune activation by these pathways initiates and/or accentuates T-cell–mediated immunity.1 However, it is less clear whether a dedicated recognition pathway serves as a negative regulator for innate immune responses and may also attenuate T-cell–mediated responses.
Members of the family of sialic-acid–binding immunoglobulin-like lectins (Siglecs) have emerged as potential negative regulators of innate immunity.2 A number of homologous members of the Siglec family have been identified in humans and mice.2 Most Siglec family members have immunoreceptor tyrosine-based inhibitory motifs (ITIMs) or ITIM-like regions in their intracellular domains.2,3 Recent data demonstrated that Siglec-G deletion in mice (Siglec-G−/−) exacerbated the production of inflammatory cytokines and acute organ failure in response to DAMPs, such as high-mobility group box 1 (HMGB-1) in acetaminophen-induced liver necrosis,4 and cecal ligation and puncture models.5 In contrast to their exacerbated inflammatory response to DAMPs, Siglec-G−/− mice showed similar inflammatory responses to PAMPs, such as lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid, which are agonists of TLR 4 and 3, respectively.4,5 Thus, recent data have identified Siglec-G expression on antigen-presenting cells (APCs), such as dendritic cells (DCs), as an important negative regulator of innate immunity. However, whether Siglec-G expression on APCs has any impact on the modulation of T-cell–mediated disease processes has heretofore not been appreciated.
Host tissue injuries caused by hematopoietic cell transplantation (HCT) conditioning regimens, including high-dose chemotherapy and/or total body irradiation (TBI), is considered to be the first step in the development of acute graft-versus-host disease (GVHD),6 a life-threatening complication of allogeneic HCT (allo-HCT).7 Host tissue injuries caused by the conditioning regimen leads to the release of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, as well as the release of DAMPs and PAMPs.6,8-11 Both DAMPs and PAMPs can activate APCs, such as DCs,6,8-11 which are critical for the development of acute GVHD.12-14 Recent experimental data have demonstrated that targeting certain DAMPs and the activation of APCs induced by them, can lead to aggravation of acute GVHD.15-17 Alloreactive donor T lymphocytes, activated by both donor and host APCs, are absolutely critical for induction and perpetuation of GVHD.7 Activation of the innate immune system, such as the APCs, plays a key role in enhancing the severity of donor T-cell–mediated GVHD. However, the role of negative regulators of innate immunity in regulating the severity of GVHD has not been recognized. This is particularly critical in light of the recent observations, which demonstrate that the absence of any one subset of professional host-derived hematopoietic APCs, such as DCs or macrophages, contrary to the expectations of reducing donor T-cell responses, are either irrelevant, or actually enhance donor T-cell responses and accentuate GVHD severity.18-22 These newer observations suggest that pathways that mitigate the hematopoietic APCs may be as critical for attenuating GVHD.
Following conditioning for allo-HCT, several DAMPs are released, including uric acid and adenosine triphosphate (ATP) that have been demonstrated to contribute to activation of host APCs and enhance GVHD. However, it is less clear whether a dedicated DAMP recognition pathway may serve as a negative regulator for innate immune responses, and control the responses of donor T cells and the severity of GVHD. Herein, utilizing a multimodal approach in several well-defined, clinically relevant murine models of allo-HCT, we determined the role of a defined negative regulator of responses to DAMPs and Siglec-G, in modulating T-cell responses and GVHD severity. We used Siglec-G–deficient animals and its ligand knockouts, the CD24 deficient donor T cells, along with rescue experiments with novel CD24 fusion protein in multiple animals and chimeras, and found that Siglec-G expression on host APCs controls GVHD severity by regulating CD24-dependent donor T-cell responses. Our data therefore, demonstrates a novel role for Sigleg-G–CD24 axis in controlling GVHD and suggests that enhancing this interaction may represent a novel pathway for mitigating GVHD.
Materials and methods
Mice
C57BL/6 C3H.sw BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and Charles River Laboratories (Wilmington, MA). B6 Ly5.2 mice were purchased from NCI-Frederick (Frederick, MD). B6-background Siglec-G−/−GFP+/+ and CD24−/− have been previously described.23,24 All animals were cared for according to regulations reviewed and approved by the University Committee on the Use and Care of Animals per University Laboratory Animal Medicine guidelines of the University of Michigan. Bone marrow (BM) chimeras were generated as previously described and detailed in the supplemental Methods on the Blood Web site.14
BM transplantation (BMT)
BMTs were performed as in the supplemental Methods and as previously described.14,25 Wild-type (WT) B6 and Siglec-G−/− animals received either 8 Gy or 13 Gy (137Cs source) on day −1 and 2.5 × 106 CD90+ T cells, along with 5 × 106 T-cell–depleted BM (TCD-BM) cells from either syngeneic B6 or allogeneic BALB/c donors. For studies with BM chimeras, they received 9 Gy TBI on day −1 and were injected with 5 × 106 BM and 2.5 × 106 CD90+ T cells from syngeneic or allogeneic donors on day 0 as previously described.14,26 Survival was monitored daily, and the recipients’ body weight and GVHD clinical scores were measured weekly as in the supplemental Methods and as previously described.27 Histopathologic analysis of the liver and gastrointestinal (GI) tract, the primary GVHD target organs, was performed as described.28 For experiments with CD24 fusion protein, comprising of the extracellular domain of CD24 and IgG Fc (5 mg/kg) (OncoImmune, Ann Arbor, MI), an equivalent dose of nonspecific IgG were administered IP on day −1 of allo-HCT.29
In vitro cultures and fluorescence-activated cell sorter (FACS) analysis
BMDCs were obtained from WT-B6, Siglec-G−/−, and CD24−/− B6 animals as detailed in the supplemental Methods. FACS analyses, including carboxyfluorescein diacetate succinimidyl ester (CFSE) and apoptosis analysis with Annexin staining, were performed as previously described.21,26 For mixed leukocyte reaction (MLR) cultures, splenic T cells were magnetically separated and cultured with BMDCs from WT-B6, Siglec-G−/−, or B6-background CD24−/− mice. The incorporation of 3H-thymidine (1 µCi/well) by proliferating T cells during the final 16 hours was measured (see the supplemental Methods).
Cytokine enzyme-linked immunosorbent assay (ELISA)
Concentrations of IL-1β, IL-2, IL-6, IL-10, IL-12, TNF-α, IL-17A, IFN-γ, and HMGB-1 were measured in the serum and culture supernatants by ELISA, performed according to the manufacturer’s protocol (see the supplemental Methods for details), and read at 450 nm using a microplate reader (Model 3550; Bio-Rad Laboratories, Hercules, CA). All samples and standards were run in duplicate.
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of in vitro data, and the Wilcoxon signed-rank test was used to analyze survival data. A P value <.05 was considered to be statistically significant.
Results
Expression of Siglec-G after radiation
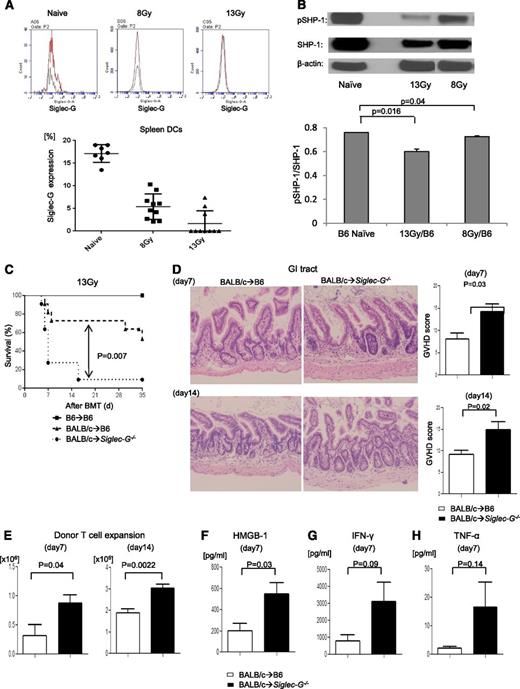
Because radiation induces tissue damage and Siglec-G is critical for negatively regulating inflammatory responses to DAMPs,4,5 we first explored whether conditioning with radiation affected the expression of Siglec-G on APCs. B6 animals were irradiated with 8 and 13 Gy, and splenic DCs were analyzed 8 and 24 hours later for expression of Siglec-G. We observed a significant reduction in the expression of Siglec-G (Figure 1A) in a radiation dose-dependent manner. We next determined whether the reduction in Siglec-G is associated with increased inflammation following high-dose irradiation. We analyzed the secretion of proinflammatory cytokines, such as TNF-α, following high-intensity (13 Gy) and low-intensity (8 Gy) conditioning. Consistent with previous reports,30 radiation caused a dose-dependent increase in the release of proinflammatory cytokines TNF-α (supplemental Figure 1). Siglec-G negatively regulates the secretion of proinflammatory cytokine release through its signaling via intracellular ITIM domains that are associated with SHP-1.4 Therefore, to determine whether the reduction in the expression of Siglec-G had functional consequences, we analyzed the expression of intracellular markers of Siglec-G–dependent ITIM signaling. As shown in Figure 1B, irradiation caused a significant reduction in the expression of pSHP-1 expression, demonstrating a significant reduction in the Siglec-G ITIM signaling after radiation. These data show that irradiation reduced the expression of negative regulator of DAMP responses, Siglec-G and its signaling in APCs.
The exacerbation of GVHD in Siglec-G–deficient hosts is dependent on the intensity of conditioning in experimental MHC-mismatched allo-HCT. (A) Siglec-G expression on splenic DCs tested by FACS with anti–Siglec-G antibody in naïve mouse and at 24 hours after TBI; the representative histogram (upper) and summarized data from 3 experiments (lower) (n = 2 to 4 per group). (B) The absence of Siglec-G decreased phosphorylated and total of SHP-1. Immunoblot analysis of phosphorylated(p)- and total SHP-1 protein in lysates of splenocytes from WT-B6 or Siglec-G−/− animals after irradiation. The bar graph shows the ratio pSHP-1/total SHP-1. (C-H) Siglec-G deficiency exacerbates GVHD. WT-B6 and Siglec-G−/− mice were lethally irradiated with 13 Gy and infused with 2 to 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. (C) Survival following 13 Gy TBI (n = 6 to 11 per group, pooled from 2 experiments); P = .007 when B6 vs Siglec-G−/− B6 mice were used as recipients. (D) Histopathologic analysis of GI tracts (small and large intestine) by hematoxylin and eosin stain (left) and GVHD score on days 7 and 14 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). Original magnification ×200. (E) Donor T-cell (H-2kd+CD4+CD8+) expansion in the spleen on days 7 and 14 after allo-HCT (n = 4 to 6 per group, pooled from 2 experiments). (F-H) Serum HMGB-1, IFN-γ, and TNF-α levels on day 7 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). The bar shows the mean ± standard error of the mean (SEM).
The exacerbation of GVHD in Siglec-G–deficient hosts is dependent on the intensity of conditioning in experimental MHC-mismatched allo-HCT. (A) Siglec-G expression on splenic DCs tested by FACS with anti–Siglec-G antibody in naïve mouse and at 24 hours after TBI; the representative histogram (upper) and summarized data from 3 experiments (lower) (n = 2 to 4 per group). (B) The absence of Siglec-G decreased phosphorylated and total of SHP-1. Immunoblot analysis of phosphorylated(p)- and total SHP-1 protein in lysates of splenocytes from WT-B6 or Siglec-G−/− animals after irradiation. The bar graph shows the ratio pSHP-1/total SHP-1. (C-H) Siglec-G deficiency exacerbates GVHD. WT-B6 and Siglec-G−/− mice were lethally irradiated with 13 Gy and infused with 2 to 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. (C) Survival following 13 Gy TBI (n = 6 to 11 per group, pooled from 2 experiments); P = .007 when B6 vs Siglec-G−/− B6 mice were used as recipients. (D) Histopathologic analysis of GI tracts (small and large intestine) by hematoxylin and eosin stain (left) and GVHD score on days 7 and 14 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). Original magnification ×200. (E) Donor T-cell (H-2kd+CD4+CD8+) expansion in the spleen on days 7 and 14 after allo-HCT (n = 4 to 6 per group, pooled from 2 experiments). (F-H) Serum HMGB-1, IFN-γ, and TNF-α levels on day 7 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). The bar shows the mean ± standard error of the mean (SEM).
Siglec-G–deficient hosts show greater severity of GVHD
We hypothesized that the absence of Siglec-G in the irradiated recipients of allo-HCT would lead to an increased inflammatory response resulting in more severe GVHD. We used the well-established major histocompatibility complex (MHC)-mismatched (BALB/c→B6) model of allo-HCT, and conditioned the WT and Siglec-G−/− B6 recipients with 13 Gy radiation. They were then transplanted with TCD-BM and splenic T cells from syngeneic B6 or allogeneic BALB/c donors. Compared with WT allogeneic B6 animals, the allogeneic Siglec-G−/− mice demonstrated significantly greater mortality from GVHD after allo-HCT (Figure 1C; P = .007). The enhanced mortality of the allogeneic Siglec-G−/− mice was associated with a more severe histopathologic GVHD of the GI tract on days +7 and +14 than the WT-B6 allogeneic mice (Figure 1D). The increase in GVHD severity and mortality was also associated with greater donor T-cell expansion and serum levels of HMGB-1, and proinflammatory cytokines IFN-γ and TNF-α in the allogeneic Siglec-G−/− mice, compared with the allogeneic WT-B6 mice (Figure 1E-H).
To rule out strain- and model-dependent artifacts, we next evaluated the role of Siglec-G in another well-established, MHC-matched, minor histocompatibility antigen (MiHA)-mismatched GVHD model (C3H.SW→B6),12 and found that Siglec-G deficiency in the hosts enhanced GVHD severity (supplemental Figure 2). Because the increase in DAMPs was dependent on the radiation dose, we reasoned that reduced intensity of conditioning might not enhance GVHD despite the absence of Siglec-G. Consistent with this notion, when recipient animals were conditioned with a reduced dose of irradiation (8 Gy), Siglec-G−/− mice demonstrated a propensity toward higher mortality and severity of GVHD that did not reach statistical significance when compared with WT-B6 mice (supplemental Figure 3A-B).
Siglec-G expression on host hematopoietic cells is crucial for regulating GVHD
We next evaluated the role of Siglec-G expression on host hematopoietic- and nonhematopoietic-derived APCs in enhancing the severity of GVHD. WT-B6 Ly5.2 or the Siglec-G−/− B6 animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic Ly5.1 WT-B6 or Siglec-G−/− donors, such that Siglec-G deficiency was confined to only the hematopoietic or the nonhematopoietic cell compartments, respectively. Chimerism analyses of the donor hematopoietic cells were complete donor types (>95%), 3 months after first transplantation. The [B6→B6Ly5.2], [B6 Ly5.2→Siglec-G−/−], and the [Siglec-G−/−→B6Ly5.2] animals were then used as recipients in an allo-HCT about 3 to 4 months later. The chimeric mice received 9 Gy and were injected IV with 2.5 × 106 CD90+ T cells along with 5 × 106 BM cells from either syngeneic B6 or MHC-mismatched allogeneic BALB/c donors. All chimeras that received syngeneic T cells and BM cells survived the duration of the observation period with no signs of GVHD (Figure 2A). By contrast, the allogeneic [B6→B6Ly5.2] and the [B6Ly5.2→Siglec-G−/−] chimeras showed similar GVHD, while the [Siglec-G−/−→B6Ly5.2] chimeras showed more severe GVHD (P = .03).
The absence of Siglec-G expression on host APCs enhances GVHD. To make BM chimeras, WT-B6 Ly5.1, WT-B6 Ly5.2, and Siglec-G−/− animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic WT-B6 Ly5.2, WT-B6 Ly5.1, or Siglec-G−/− donors. Four months later, [B6 Ly5.2→B6 Ly5.1], [B6 Ly5.1→B6Ly5.2], [Siglec-G−/−→B6Ly5.2], or [B6 Ly5.2→Siglec-G−/−] animals were irradiated with 9 Gy and received 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors, or received 1.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple MiHA-mismatched, C3H.sw donors. (A) Survival (left) and clinical GVHD score (right) (n = 6 to 18 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (B) Survival (left) and clinical GVHD score (right) (n = 8 to 12 per group, pooled from 2 experiments) in MHC-matched, multiple MiHA-mismatched C3H.sw→B6 model. (C) Donor T-cell (H-2kd+CD4+CD8+) expansion in the spleen on day 7 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (D) Donor T-cell (CD229.1+CD4+CD8+) expansion in the spleen on day 14 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (E) Donor T-cell (H-2d+CD3+) expansion in the spleen and MLNs on day 1 after allo-HCT (n = 3 per group, pooled from 2 experiments). (F) Serum TNF-α levels on day 1 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (G) Donor T-cell (H-2d+CD3+) expansion in the spleen and intraepitherial lymphocytes on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (H) Donor IFN-γ+CD3+ T-cell expansion in the spleen on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (I) Serum IFN-γ levels on day 3 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (D-I) The bars show the mean ± SEM.
The absence of Siglec-G expression on host APCs enhances GVHD. To make BM chimeras, WT-B6 Ly5.1, WT-B6 Ly5.2, and Siglec-G−/− animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic WT-B6 Ly5.2, WT-B6 Ly5.1, or Siglec-G−/− donors. Four months later, [B6 Ly5.2→B6 Ly5.1], [B6 Ly5.1→B6Ly5.2], [Siglec-G−/−→B6Ly5.2], or [B6 Ly5.2→Siglec-G−/−] animals were irradiated with 9 Gy and received 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors, or received 1.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple MiHA-mismatched, C3H.sw donors. (A) Survival (left) and clinical GVHD score (right) (n = 6 to 18 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (B) Survival (left) and clinical GVHD score (right) (n = 8 to 12 per group, pooled from 2 experiments) in MHC-matched, multiple MiHA-mismatched C3H.sw→B6 model. (C) Donor T-cell (H-2kd+CD4+CD8+) expansion in the spleen on day 7 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (D) Donor T-cell (CD229.1+CD4+CD8+) expansion in the spleen on day 14 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (E) Donor T-cell (H-2d+CD3+) expansion in the spleen and MLNs on day 1 after allo-HCT (n = 3 per group, pooled from 2 experiments). (F) Serum TNF-α levels on day 1 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (G) Donor T-cell (H-2d+CD3+) expansion in the spleen and intraepitherial lymphocytes on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (H) Donor IFN-γ+CD3+ T-cell expansion in the spleen on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (I) Serum IFN-γ levels on day 3 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (D-I) The bars show the mean ± SEM.
To preclude strain-specific responses, we also used the [B6→B6Ly5.2], [B6Ly5.2→Siglec-G−/−], and [Siglec-G−/−→B6Ly5.2] chimeras as recipients in an MHC-matched, multiple MiHA-mismatched C3H.sw→B6 model of allo-HCT. The [B6→B6Ly5.2], [B6Ly5.2→Siglec-G−/−], and [Siglec-G−/−→B6Ly5.2] chimeras were irradiated and transplanted with TCD-BM and splenic T cells from B6 or allogeneic C3H.sw donors. As expected, all of the syngeneic recipients survived. Once again, the allogeneic [Siglec-G−/−→B6Ly5.2] animals that lacked Siglec-G expression on the host hematopoietic-derived APCs, showed greater mortality and more severe GVHD than the allogeneic [B6→B6Ly5.2] or the [B6Ly5.2→Siglec-G−/−] recipients after HCT (Figure 2B; P = .02). The enhanced mortality and severity of GVHD in the allogeneic [Siglec-G−/−→B6Ly5.2] animals was associated with greater donor T-cell expansion on days 7 and 14 in both MHC-mismatched and MHC-matched minor disparate systems (Figure 2C-D), and was also associated with an increase in serum levels of IFN-γ on day 14 after allo-HCT (supplemental Figure 4).
The enhanced mortality in these multiple models was apparent early, after BMT. To gain further insight into the enhancement of early mortality, we next determined whether the presence of Siglec-G on host APCs led to the regulation of only inflammation, early after BMT, and/or if it also caused a retention or reduced the expansion of donor T cells in the secondary lymphoid organs (eg, spleen and regional lymph nodes [LN] such as mesenteric LN [MLN]), preventing T-cell egress into target tissues. Even very early after BMT, on day +1 (Figure 2E-F), the absence of Siglec-G on host cells led to increased recovery of donor T cells in secondary LN such as MLN, and also increased the levels of proinflammatory cytokine TNFα. To further determine the role and kinetics of the early increase in donor T cells and inflammatory cytokines, we also assessed the donor T-cell expansion and recovery in the secondary LN, and additionally in the GVHD target tissues and GI tract on day +3 after BMT. In the absence of Siglec-G in the host APCs, allogeneic donor T cells showed greater expansion and recovery in both the secondary LNs (Figure 2G) and the GVHD target tissues, as well as the GI tract (Figure 2H); and also showed greater levels of T-cell proinflammatory cytokines such as INF-γ (Figure 2I). These data suggest that host Siglec-G attenuates early mortality after allo-HCT, both by regulating enhanced donor T-cell expansion and proinflammatory cytokine milieu early after HCT. There were no detectable levels of serum LPS early after BMT on day 1 and no increase on day 3 (data not shown). This, when taken in light of the absence of early mortality in syngeneic animals despite increased conditioning, suggests that Siglec-G–mediated regulation in early GVHD mortality is not solely due to condition-related inflammation.
Siglec-G deficiency enhances DAMP-induced inflammatory responses from hematopoietic APCs
To evaluate the mechanisms of enhanced GVHD in the absence of Siglec-G on hematopoietic-derived cells, we focused on professional hematopoietic APC subsets, namely DCs25,26 and macrophages. We first determined the effect of Siglec-G deficiency on the number and phenotype of DCs. No significant differences were observed between the WT or Siglec-G−/− animals in either the percent or the absolute numbers of CD11c+ DCs, or the absolute numbers and percent expression of co-stimulatory molecules such as CD80, CD86, CD40, and PD-L1 (supplemental Figure 5A-F). The expression of MHC class II and the number of plasmacytoid DCs were also similar in WT-B6 and Siglec-G−/− mice (supplemental Figure 5G-H). Because granulocyte macrophage–colony stimulating factor-cultured BMDCs are considered to be a model of in vivo inflammatory monocyte-derived DCs that differentiate from circulating monocytes in response to inflammation,31 we next examined the impact of Siglec-G on the generation of DCs from BM. The phenotype (CD80, CD86, and I-Ab) of granulocyte macrophage–colony stimulating factor-induced BMDCs generated from Siglec-G−/− mice was similar to the phenotype of WT BMDCs (supplemental Figure 6A).
We next evaluated the functional responses of conventional DCs from WT and Siglec-G−/− mice. We tested the effect of Siglec-G deficiency on the innate immune responses of DCs that are triggered through TLRs. BMDCs from either WT or Siglec-G−/− mice were stimulated with LPS, a potent stimulator of the innate immune response via TLR4. Siglec-G–deficient DCs and WT DCs responded in a comparable manner, as determined by the secretion of IL-6, TNF-α, IL-10, IL-1β, and IL-12, as shown in a previous report4 (supplemental Figure 6B-F), and also found that Siglec-G–deficient DCs showed similar expression of co-stimulatory molecules, CD80, CD86, and MHC class II, as WT DCs after TLR4 stimulation (supplemental Figure 6A). Taken together, these data demonstrate that intrinsic Siglec-G deficiency did not alter conventional DC responses that are mediated through TLR4 stimulation. We next tested the response of DCs to DAMPs. Both WT and Siglec-G−/− DCs were stimulated with HMGB-1 (5 μg/mL). In contrast to PAMP stimulation, DAMP stimulation led to a significantly greater production of IL-6 and TNF-α by Siglec-G−/− DCs than by WT DCs (Figure 3A-B). We also tested the impact of Siglec-G expression on macrophages and the GI tract DCs (purity >70%; supplemental Figure 7). Similar to the effects on BMDCs, stimulation with the DAMPs caused greater release of proinflammatory cytokines such as TNFα from the Siglec-G−/− macrophages when compared with WT macrophages and the GI tract DCs (Figure 3C-D). Absence of Siglec-G did not alter the expression of TLR4 on the cell surface (Figure 3E). This data indicated that the expression of Siglec-G on professional hematopoietic APCs is critical for controlling DAMP-induced inflammatory responses.
Siglec-G deficiency enhances DAMP-induced inflammatory responses from hematopoietic APCs. (A-B) Both B6 and Siglec-G−/− BMDCs, (C) peritoneal macrophages, and the GI tract DCs were harvested and stimulated with HMGB-1 (5 μg/ml) for 16 hours. The supernatants were analyzed for TNF-α (A,C-D), and IL-6 (B). (E) TLR-4 expression on BMDCs. The data are representative of 3 independent experiments and show the mean ± SEM.
Siglec-G deficiency enhances DAMP-induced inflammatory responses from hematopoietic APCs. (A-B) Both B6 and Siglec-G−/− BMDCs, (C) peritoneal macrophages, and the GI tract DCs were harvested and stimulated with HMGB-1 (5 μg/ml) for 16 hours. The supernatants were analyzed for TNF-α (A,C-D), and IL-6 (B). (E) TLR-4 expression on BMDCs. The data are representative of 3 independent experiments and show the mean ± SEM.
Siglec-G regulates allogeneic T-cell responses in a CD24-dependent manner
Because we observed significantly greater donor T-cell expansion in the Siglec-G–deficient hosts, we next examined whether Siglec-G expression on DCs also directly modulated their ability to stimulate allogeneic T cells. Absence of Siglec-G in BMDCs significantly augmented allogeneic T-cell expansion (Figure 4A; P = .0005) and IFN-γ secretion (Figure 4B; P = .0077). Similar expansion of allogeneic T cells was also observed following stimulation by irradiated splenocytes from Siglec-G−/− mice (supplemental Figure 8). The increase in expansion of allogeneic T cells in the absence of Siglec-G in DCs was due to increased proliferation (CFSE staining; Figure 4C) without a significant change in the rate of apoptosis compared with the rate in WT DCs (Figure 4D). Collectively, these data indicate that the absence of Siglec-G in DCs directly enhances allogeneic T-cell proliferation.
Siglec-G–deficient DCs show enhanced stimulation of allogeneic T cells. BMDCs from WT-B6 and Siglec-G−/− mice were used as stimulators in an MLR with T cells from either BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for (A) T-cell proliferation based on 3H-thymidine incorporation at 96 hours. The data are the mean ± SEM of quadruplicate cultures and are representative of 3 independent experiments. (B) Supernatants from the cultures were collected at 80 hours and analyzed for IFN-γ by ELISA. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (C) Representative figures for CFSE, and (D) Annexin-V staining of cells gated on CD90.2+ T cells after the MLR. CFSE-labeled splenic CD90+ T cells from BALB/c mice were cultured for 96 hours with BMDCs from either WT-B6 or Siglec-G−/− mice. The cells were then harvested and analyzed. The right column shows the collective data of 3 independent experiments. N.S., no significant differences between B6 and Siglec-G−/− DCs.
Siglec-G–deficient DCs show enhanced stimulation of allogeneic T cells. BMDCs from WT-B6 and Siglec-G−/− mice were used as stimulators in an MLR with T cells from either BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for (A) T-cell proliferation based on 3H-thymidine incorporation at 96 hours. The data are the mean ± SEM of quadruplicate cultures and are representative of 3 independent experiments. (B) Supernatants from the cultures were collected at 80 hours and analyzed for IFN-γ by ELISA. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (C) Representative figures for CFSE, and (D) Annexin-V staining of cells gated on CD90.2+ T cells after the MLR. CFSE-labeled splenic CD90+ T cells from BALB/c mice were cultured for 96 hours with BMDCs from either WT-B6 or Siglec-G−/− mice. The cells were then harvested and analyzed. The right column shows the collective data of 3 independent experiments. N.S., no significant differences between B6 and Siglec-G−/− DCs.
Recent data demonstrated that the interaction between CD24 and Siglec-G is critical for Siglec-G–mediated immune regulation following stimulation by the DAMPs.4 Because CD24 is expressed on T cells, to explore the mechanisms of enhanced allogeneic responses by the Siglec-G−/− DCs, we focused on the relevance of T-cell–CD24 interaction with Siglec-G on DCs by disrupting the CD24–Siglec-G interaction. We first used BALB/c CD24−/− T cells as responders and cultured these cells with allogeneic B6 WT or Siglec-G−/− DCs in an MLR. As shown in Figure 5A, CD24−/− T cells stimulated by Siglec-G−/− DCs proliferated significantly more than those stimulated with WT DCs. The increase in proliferation was associated with enhanced secretion of IFN-γ and IL-2 (Figure 5B-C).
Disruption of the CD24–Siglec-G interaction enhances allogeneic T-cell responses. BMDCs from WT-B6 and Siglec-G−/− mice were used as stimulators in an MLR with T cells from either CD24−/− BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for (A) T-cell proliferation based on 3H-thymidine incorporation at 96 hours. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (B-C) Supernatants from the cultures were collected at 80 hours and analyzed for IFN-γ (B) and IL-2 (C) by ELISA. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (D) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c or CD24−/− donors (n = 4 to 15 per group, pooled from 2 experiments). The bar shows the mean ± SEM. (E) Clinical GVHD score. For ▲ vs ●, **P < .01 and *P < .05. (F) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched CD24−/− donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 10 per group, pooled from 2 experiments). For ♦ vs ◊, P = .02. The bar shows the mean ± SEM.
Disruption of the CD24–Siglec-G interaction enhances allogeneic T-cell responses. BMDCs from WT-B6 and Siglec-G−/− mice were used as stimulators in an MLR with T cells from either CD24−/− BALB/c (allogeneic) or C57BL/6 (syngeneic) mice and analyzed for (A) T-cell proliferation based on 3H-thymidine incorporation at 96 hours. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (B-C) Supernatants from the cultures were collected at 80 hours and analyzed for IFN-γ (B) and IL-2 (C) by ELISA. The data are the mean ± SEM of quadruplicate cultures and are from 1 of 3 similar experiments. (D) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c or CD24−/− donors (n = 4 to 15 per group, pooled from 2 experiments). The bar shows the mean ± SEM. (E) Clinical GVHD score. For ▲ vs ●, **P < .01 and *P < .05. (F) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched CD24−/− donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 10 per group, pooled from 2 experiments). For ♦ vs ◊, P = .02. The bar shows the mean ± SEM.
CD24 expression on donor T cells mitigates GVHD
CD24 is a ligand required for Siglec-G–mediated immunoregulation. Because its expression by T cells attenuates their proliferative responses in vitro, we next investigated the functional relevance of the CD24–Siglec-G interaction during the development of GVHD in vivo. We first determined whether the absence of the ligand, CD24, on donor T cells enhanced GVHD severity similar to the lack of its receptor, Siglec-G, on host APCs. The B6 recipients were lethally irradiated and transplanted with TCD- BM and splenic T cells from the syngeneic B6 animals, or TCD-BM from allogeneic WT BALB/c, along with splenic T cells from either WT or CD24−/− BALB/c donors. As shown in Figure 5D-E, all of the syngeneic animals survived with no signs of GVHD. The allogeneic recipients that were transplanted with WT T cells demonstrated GVHD, which was significantly less severe than in those animals that were transplanted with CD24−/− T cells (P = .03).
Next, to confirm that CD24 is critical for controlling GVHD, we used a novel CD24 fusion protein that served as an agonist and enhanced CD24-mediated function. When the recipients of the CD24−/− T cells were injected with the CD24 Fc protein on day −1 (5 mg/kg) before allo-HCT, it reduced the enhanced severity of GVHD that was observed in the control-treated animals (Figure 5F). Furthermore, the administration of CD24 fusion protein significantly reduced GVHD severity even in the recipients of WT T cells (Figure 6A). These data collectively demonstrates that the expression of ligand CD24 on donor T cells controls GVHD severity in WT hosts that express Siglec-G. Importantly, these data further suggests that accentuating CD24-mediated immunoregulation, even in WT animals with the novel CD24 fusion protein, can further mitigate GVHD.
Enhanced Siglec-G interaction with the CD24 fusion protein mitigates GVHD. (A) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 25 per group, pooled from 3 experiments). For ▲ vs △, P = .03. The bar shows the mean ± SEM. (B) Siglec-G−/− mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 12 per group, pooled from 2 experiments). The bar shows the mean ± SEM.
Enhanced Siglec-G interaction with the CD24 fusion protein mitigates GVHD. (A) Survival. WT-B6 mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 25 per group, pooled from 3 experiments). For ▲ vs △, P = .03. The bar shows the mean ± SEM. (B) Siglec-G−/− mice were lethally irradiated with 13 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 4 to 12 per group, pooled from 2 experiments). The bar shows the mean ± SEM.
CD24 fusion protein mitigates GVHD by interacting with Siglec-G on host APCs
We next investigated whether donor T-cell CD24-mediated regulation of GVHD is directly dependent on the expression of GVHD on host APCs. We treated the recipients with CD24 fusion protein on day −1 (5 mg/kg) before allo-HCT to enhance the CD24–Siglec-G interaction on the host APCs. Both B6 and Siglec-G−/− mice received 13 Gy on day −1 and were injected IV with 2.5 × 106 CD90+ T cells, along with 5 × 106 TCD-BM cells from either syngeneic B6 or MHC-mismatched allogeneic BALB/c donors on day 0. Although WT-B6 mice treated with CD24 fusion protein showed significant amelioration in GVHD severity with complete donor chimerism of hematopoietic-derived cells (supplemental Figure 9) and improvement in survival, treated Siglec-G−/−-B6 mice demonstrated enhanced GVHD severity (Figure 6B). Because we were interested in the expression of Siglec-G specifically on host APCs, we generated [B6→B6Ly5.2] and [Siglec-G−/−→B6 Ly5.2] BM chimeras and then used these chimeras as hosts in allogeneic HCT 4 months later. In this case, allogeneic [B6→B6Ly5.2] chimeras treated with CD24 fusion protein also showed prolonged survival (Figure 7A). However, allogeneic [Siglec-G−/−→B6 Ly5.2] chimeras treated with CD24 protein showed equivalent survival as that of the group without treatment (Figure 7B). Taken together, our data demonstrates a critical role for CD24–Siglec-G interactions in regulating GVHD and suggests that the administration of the novel CD24 fusion protein may be an innovative strategy for mitigating GVHD.
Enhanced Siglec-G interaction with the CD24 fusion protein mitigates GVHD in WT but not Siglec-G−/− mice. (A-B) Survival. [WT-B6→B6Ly5.2] and [Siglec-G−/−→B6 Ly5.2] chimeras were lethally irradiated with 9 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 5 to 17 per group, pooled from 2 experiments). For ▲ vs △, P = .001. (B). The bar shows the mean ± SEM.
Enhanced Siglec-G interaction with the CD24 fusion protein mitigates GVHD in WT but not Siglec-G−/− mice. (A-B) Survival. [WT-B6→B6Ly5.2] and [Siglec-G−/−→B6 Ly5.2] chimeras were lethally irradiated with 9 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 5 to 17 per group, pooled from 2 experiments). For ▲ vs △, P = .001. (B). The bar shows the mean ± SEM.
Discussion
Our data collectively demonstrates that GVHD is controlled by the host hematopoietic Siglec-G and donor T-cell–CD24 axis. Host tissue damage after conditioning is known to lead to the release of not only proinflammatory cytokines, but also DAMPs and PAMPs.6,15,27,32-34 Both DAMPs and PAMPs can activate APCs and enhance T-cell responses.7 The role of PAMPs in exacerbating GVHD may be model/context dependent. However, recent experimental studies suggests a role for DAMPs such as ATP, heparan sulfate (HS), and uric acid, in enhancing allogeneic T-cell responses and in exacerbating GVHD.15,17,32 Clinically, the data also suggests that response to DAMPs such as HMGB-1,35 heat shock protein 70 (HSP70),36,37 and HSP9038 may be associated with GVHD severity in humans. In line with this notion, several pattern recognition receptors that are known to activate innate immunity in response to DAMP-mediated stimulation of APCs have been shown to exacerbate GVHD. However, to date, the impact on GVHD by the pathways that suppress an innate immune response to DAMPs has not been explored. Siglec-G, with the human homolog Siglec10, is one of the Siglec family members. Nearly all Siglec family members have ITIMs and ITIM-like regions that are associated with SHP-1 and/or SHP-2 phosphatase in their intracellular domains, and can negatively regulate cellular activation induced by DAMPs during immune recognition by cell-to-cell interaction.2,4,5,10,11 The DAMPs are presented to Siglecs by binding to their ligand CD24 leading to ITIM activation, and thus, restraining the innate immune response. But their role in regulating adaptive immune responses, including alloreactivity that is activated by innate APCs has been unrecognized. We made a surprising observation that irradiation reduced the expression of Siglec-G, despite increasing the release of DAMPs and secretion of proinflammatory cytokines in a dose-dependent manner. While the impact of increasing intensity of chemotherapy or other damage on the expression of Siglec-G is not known and will be explored in future studies, our data nonetheless demonstrates that the deficiency of Siglec-G, specifically on host hematopoietic-derived APCs but not on nonhematopoietic APCs, aggravated the severity of GVHD, depending on the intensity of irradiation. We found that, consistent with recent observations,4 in the absence of Siglec-G, hematopoietic-derived professional APCs such as DCs and macrophages are comparable PAMP responses to innate TLR4 responses. However, Siglec-G−/− DCs showed significantly increased secretion of inflammatory cytokines following stimulation by DAMPs, such as ATP and HMGB-1. These data are consistent with the findings of previous reports.4,5 Additionally, we demonstrated that Siglec-G also negatively regulates the allostimulatory functions of APCs. Thus, our data indicates that Siglec-G expression is required in a host hematopoietic APC-autonomous fashion for the regulation of GVHD, and suggests that enhancing Siglec-G–dependent pathways might allow for enhancing dose intensity of preparative regimens without increasing transplant-related toxicity.
To explore the exact mechanisms by which a deficiency in Siglec-G expression on host hematopoietic APCs aggravates GVHD in high-intensity conditioning, we focused on CD24–Siglec-G pathways. CD24 is expressed as a glycosyl-phosphatidylinositol–anchored molecule at the surface of most immune cells, including T cells.39,40 CD24 is also the high-affinity ligand for Siglec-G.4 CD24 is associated with a broad range of DAMPs, such as via HSP70, HSP90, and HMGB-1, binds to Siglec-G and then represses the responses to these DAMPs via the activation of ITIMs.4,5,11 We found that allogeneic CD24-deficient T cells proliferated more and exhibited increased secretion of IL-2 and IFN-γ following stimulation by Siglec-G−/− DCs compared with WT DCs. These data suggested that the CD24-Siglec-G interaction negatively regulates DAMP dependent allogeneic responses. Our data also demonstrates that the interaction between Siglec-G on host hematopoietic-derived APCs and CD24 on donor T cells is critical for controlling the severity of GVHD. Our data suggests that the increased amounts of DAMPs that were released from damaged tissues during severe conditioning, stimulated host APCs and then enhanced allogeneic T-cell expansion. This enhancement of GVHD can be mitigated by the Siglec-G–CD24 axis. This notion is consistent with the clinical observation that myeloablative conditioning, particularly those involving high dose radiation, causes earlier and more severe GVHD development.41-43 Moreover, previous studies of HSP70 polymorphism and expression in target tissues demonstrated decreased survival only when utilizing myeloablative conditioning.36,37
Recent data have indicated that both host hematopoietic or nonhematopoietic APCs, in the absence of one or another, can activate donor T cells and induce GVHD.18,19,21 Furthermore, the elimination of specific hematopoietic APC subsets, such as DCs or macrophages, may aggravate GVHD, suggesting that these cells may play a role in controlling GVHD.18,20,22,44 These observations, taken in light of our data, suggest that the negative regulatory pathways of professional APCs, DCs, or macrophages, might be critical for reducing GVHD severity following its induction by the activation of donor T cells by the DAMP/PAMP activated host APCs.
To confirm the mechanistic link and also to explore the potential clinical relevance of regulating the CD24–Siglec-G interaction in GVHD, we used CD24 fusion protein in our models. We found that treatment with the CD24 fusion protein in WT-B6 allogeneic animals mitigated GVHD severity and improved overall survival, but not in the absence of Siglec-G−/− in the recipients. Therefore, the administration of novel CD24 fusion protein may represent a novel strategy for immunoprophylaxis against GVHD. Because CD24–Siglec-G interaction has only been associated in regulating DAMP but not PAMP responses, it is tempting to speculate that utilization of CD24 fusion protein in the context of allo-HCT may not increase the infectious risk, while reducing DAMPs-associated aggravation of GVHD. CD24 fusion protein treatment is entering phase 1 and 2 studies, with multiple sclerosis as the therapeutic target. However, it is worth noting that CD24 may be a promiscuous ligand for the adhesion molecules,45 and therefore, may cause unexpected toxicities. This will need to be evaluated carefully in well-designed clinical trials.
In conclusion, our data demonstrates (1) a novel role for Siglec-G on hematopoietic-derived APCs in negative regulation of adaptive T-cell responses, namely, alloreactivity, in a CD24-dependent manner; (2) an important role for negative regulator of DAMP-mediated innate immune pathways in controlling GVHD; and (3) that enhancement of the Siglec-G–CD24 interaction by the novel CD24 fusion protein may represent a novel strategy for mitigating GVHD.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Evelyn Nieves and Steven Kapples for technical assistance.
This work was supported by grants from the National Institutes of Health (National Cancer Institute, CA-0173878, CA143379, and National Heart, Lung and Blood Institute, HL-090775) (P.R.).
Authorship
Contribution: T.T. performed experiments, analyzed the data, and wrote the paper; G.H. and N.M. performed experiments; C.L. performed experiments and histopathologic analysis; Y.W., K.O.W., E.C., C.R., R.E., Y.S., and J.W. performed experiments; S.W.C. and D.F. contributed reagents; P.Z. contributed reagents and helped to write the paper; Y.L. analyzed the data, contributed reagents, and helped to write the paper; and P.R. designed experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, Department of Internal Medicine, Division of Hematology and Oncology, Blood and Marrow Transplantation Program, University of Michigan Comprehensive Cancer Center, 3312 CCGC, 1500 East Medical Center Dr, Ann Arbor, MI 48105-1942; e-mail: reddypr@med.umich.edu.

![Figure 2. The absence of Siglec-G expression on host APCs enhances GVHD. To make BM chimeras, WT-B6 Ly5.1, WT-B6 Ly5.2, and Siglec-G−/− animals were lethally irradiated with 11 Gy and infused with 5 × 106 BM cells and 5 × 106 splenocytes from syngeneic WT-B6 Ly5.2, WT-B6 Ly5.1, or Siglec-G−/− donors. Four months later, [B6 Ly5.2→B6 Ly5.1], [B6 Ly5.1→B6Ly5.2], [Siglec-G−/−→B6Ly5.2], or [B6 Ly5.2→Siglec-G−/−] animals were irradiated with 9 Gy and received 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors, or received 1.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-matched, multiple MiHA-mismatched, C3H.sw donors. (A) Survival (left) and clinical GVHD score (right) (n = 6 to 18 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (B) Survival (left) and clinical GVHD score (right) (n = 8 to 12 per group, pooled from 2 experiments) in MHC-matched, multiple MiHA-mismatched C3H.sw→B6 model. (C) Donor T-cell (H-2kd+CD4+CD8+) expansion in the spleen on day 7 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments) in MHC-mismatched BALB/c→B6 model. (D) Donor T-cell (CD229.1+CD4+CD8+) expansion in the spleen on day 14 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (E) Donor T-cell (H-2d+CD3+) expansion in the spleen and MLNs on day 1 after allo-HCT (n = 3 per group, pooled from 2 experiments). (F) Serum TNF-α levels on day 1 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (G) Donor T-cell (H-2d+CD3+) expansion in the spleen and intraepitherial lymphocytes on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (H) Donor IFN-γ+CD3+ T-cell expansion in the spleen on day 3 after allo-HCT (n = 3 per group, pooled from 2 experiments). (I) Serum IFN-γ levels on day 3 after allo-HCT (n = 3 to 6 per group, pooled from 2 experiments). (D-I) The bars show the mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/22/10.1182_blood-2013-12-545335/4/m_3512f2.jpeg?Expires=1767707389&Signature=fBHEGA9JeH1n26tFfV2-IJr7KP5llv-AcyoyPLiLNcgCxRO07lBQKpEMU6KoWp~iXuFLe40xMCT6CSYOVWwUwtPlr1MPxOqE2dZHYgLF7bNnpLXduCyGBXS4EZ-O1FOmywWPQ8Tu~DCKufMDOEWm~tm38pSHC6UUO9rIRykQDgbehtvVvYIDsuQ8ZLR1Yguw2E4z5yjznA4nxTXAkSWefN9t14~hZOSXMqJtPKBVx~d1rxnHBl037WqQorxjANySaVcPcIlp-yRZNB~55R2Ftd6h8lftd68SBhxCa7-q60JBe3VM06fILseZWQD-JNPIgaVoyJpjm6HgPi9x1q3k6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 7. Enhanced Siglec-G interaction with the CD24 fusion protein mitigates GVHD in WT but not Siglec-G−/− mice. (A-B) Survival. [WT-B6→B6Ly5.2] and [Siglec-G−/−→B6 Ly5.2] chimeras were lethally irradiated with 9 Gy and infused with 2.5 × 106 CD90+ T cells along with 5 × 106 TCD-BM cells from either syngeneic B6 or allogeneic MHC-mismatched BALB/c donors. The recipients were injected with the CD24 Fc protein on day −1 (5 mg/kg) or diluent control on day −1 before allo-HCT (n = 5 to 17 per group, pooled from 2 experiments). For ▲ vs △, P = .001. (B). The bar shows the mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/22/10.1182_blood-2013-12-545335/4/m_3512f7.jpeg?Expires=1767707389&Signature=RTSbddRe9ZjK6PbVWd5qONNMEeXt8ALLv-a5WG0OQygR3VDhkOdu4le~2uKRnjPb~Cm2JzhgPlq0A-3BTWv3x~2S8oMD9Y2BzcgOpS4se8X3D2Kom2XXXEBSO3MkdcPJ7UXosRcya9gwZTYhTEEe-sZ0RGFLa7OBm6V5sAWsbKMLF0-CxoeA40GuiECPJgvz0uuQS0U0PA7JzJT9Dki3DHomIlKEJJ6FG3YCOA75ttNPu3YCYGOC4Tr5QXPem9I0T4vTb-qHRqjmy5-cyy794KZo9KIZ5ovRILus3LrWrR2w1Z5i39CKOy0eZXzWmWhYtN3W0DB9wzT4MCndSdqV1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)