In this issue of Blood, Toubai et al show that sialic acid–binding immunoglobulin-like lectin G (Siglec-G) and CD24, its interaction partner on T cells, can provide tolerizing effects during graft-versus-host disease (GVHD) pathogenesis.1
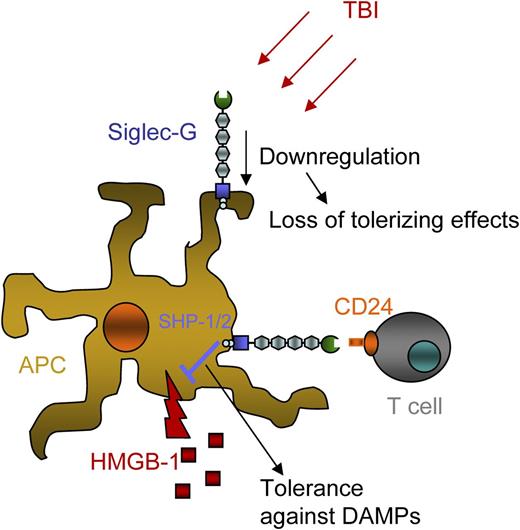
Simplified sketch of the Siglec-G–CD24 axis in GVHD. Host hematopoietic APCs express Siglec-G, and donor T cells express CD24. Total body irradiation (TBI) reduces the expression of Siglec-G, reducing its tolerogenic impact. Siglec-G activation on APCs occurs by binding to CD24 from T cells and leads to Src homology 2-containing inositol 5′ phosphatase 1/2 (SHP-1/2) phosphorylation. This in turn dampens the immune response elicited by the DAMP HMGB-1.
Simplified sketch of the Siglec-G–CD24 axis in GVHD. Host hematopoietic APCs express Siglec-G, and donor T cells express CD24. Total body irradiation (TBI) reduces the expression of Siglec-G, reducing its tolerogenic impact. Siglec-G activation on APCs occurs by binding to CD24 from T cells and leads to Src homology 2-containing inositol 5′ phosphatase 1/2 (SHP-1/2) phosphorylation. This in turn dampens the immune response elicited by the DAMP HMGB-1.
Under pathological conditions such as hypoperfusion, chemotherapy, irradiation, or traumatic tissue damage, levels of molecules that function as damage-associated molecular patterns (DAMPs) become elevated in the extracellular space due to passive leakage from damaged or dying cells. Although this release can induce powerful immune responses that are supposed to prevent infection after tissue damage, negative regulation of these activating stimuli acts as a “brake” on inflammation that is critical for the survival of the injured individual.
Sialic acid–binding immunoglobulin-like lectins (Siglecs) are immune receptors that are widely expressed in mammals and whose main function is to mediate inhibitory signaling in immune cells.2 This signaling functions as a natural antagonist of DAMPs.3 One unique feature of Siglecs is their ability to recognize sialylated carbohydrates. This is in contrast to most other immune receptors, which bind to protein determinants. High-mobility group box 1 (HMGB-1) can activate Siglec-G, and there is also evidence of a role for this molecule in GVHD, because carrying the HMGB-1 2351insT minor allele was shown to reduce the risk of severe GVHD in patients.4
The work of Toubai et al1 indicates that Siglec-G expression is downmodulated by irradiation-induced tissue damage, which reduces its tolerizing effects and renders the host more susceptible to the effects of DAMPs such as HMGB-1 (see figure). The detrimental effect of DAMPs on antigen-presenting cells (APCs) is mitigated when Siglec-G can bind to its ligand, CD24. The authors show for the first time that it is possible to provide CD24 as a Siglec-G ligand either through the presence of donor T cells (see figure) or via the exogenous transfer of a CD24 fusion protein.
An important advance in the understanding of GVHD biology based on this study1 lies in the fact that it can clearly distinguish between the effects of DAMPs and pathogen-associated molecular patterns, because CD24-deficient mice exhibit increased susceptibility to danger- but not pathogen-associated molecular patterns.5 Furthermore, these studies introduce HMGB-1 as a new DAMP found to promote GVHD, in addition to previously reported GVHD-related DAMPs such as adenosine triphosphate6 and uric acid.7 In so doing, they provide an elegant approach in the use of a natural immunoregulatory pathway in preventing uncontrolled immune activation due to the prolonged presence of DAMPs following conditioning therapy.
Previous studies have shown that CD24 associates with and negatively regulates the immune response to HMGB-1.3 The questions as to whether HMGB-1 binds to CD24 on donor T cells and whether this binding is functionally relevant for the protective effects seen in the GVHD model were not directly addressed by Toubai et al1 and could be a subject of further studies.
From a clinical-translational research standpoint, the strategy of harnessing the Siglec-G–CD24 axis with a CD24 fusion protein to interfere with DAMP effects in GVHD is a major observation, because it renders a physiological immunoregulatory mechanism accessible to clinical trials. However, any modification of the allogeneic immune response could potentially lead to a reduction in the desired graft-versus-leukemia (GVL) or graft-versus-infection (GVI) effects. This could be particularly important for GVI effects, because DAMPs are released and required for immune activation during invasive fungal or bacterial infections,8 which are important complications observed in patients who have undergone allogeneic hematopoietic cell transplantation. Therefore, future studies may need to investigate the impact of the Siglec-G–CD24 axis on GVL and GVI effects in order to bring the presented concept closer to a clinical application.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal