In this issue of Blood, McKenzie et al provide further insight into the mechanism of antibody-mediated transfusion-related acute lung injury (TRALI), and Silliman et al demonstrate the potential use of a novel filter to mitigate red blood cell (RBC) transfusion-associated TRALI.1,2 The first manuscript with studies performed in a murine model suggests that HLA class I antibody-mediated TRALI, which requires antibody binding to peripheral blood monocytes producing interleukin-8 (IL-8) which binds chemokine (C-X-C motif) ligand (CXCL), is a chemotactic for neutrophils and induces neutrophil degranulation; the antibody-coated monocytes also result in lung damage.1 The second manuscript shows that prestorage RBC filtration to absorb antibodies and lipids as well as white blood cells and platelets, decreases TRALI-associated antibodies and neutrophil-priming activity of the unit, mitigating TRALI in an animal model.2
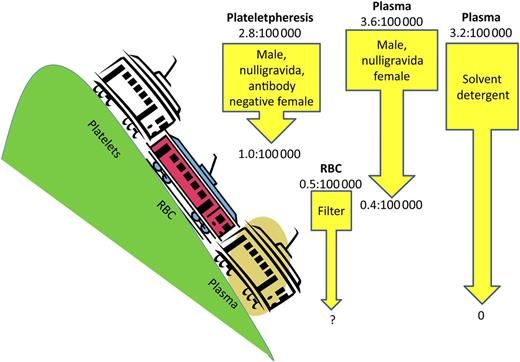
Pre- and post-TRALI mitigation factors: Number of TRALI cases per product distributed/transfused. The plateletpheresis product risk reduction shows pre- and postimplementation of products from males or nulligravida or HLA-antibody-negative parous females per New York Blood Center data.8 The current risk for the RBC product is per New York Blood Center data. The arrow shows potential risk reduction with use of an experimental filter.2,8 The left-most plasma product risk reduction shows pre- and postimplementation of products from males or nulligravida females per New York Blood Center data.8 The right-most plasma product risk reduction shows pre- and postimplementation of solvent detergent products per French Agency for the Safety of Health Products data.3
Pre- and post-TRALI mitigation factors: Number of TRALI cases per product distributed/transfused. The plateletpheresis product risk reduction shows pre- and postimplementation of products from males or nulligravida or HLA-antibody-negative parous females per New York Blood Center data.8 The current risk for the RBC product is per New York Blood Center data. The arrow shows potential risk reduction with use of an experimental filter.2,8 The left-most plasma product risk reduction shows pre- and postimplementation of products from males or nulligravida females per New York Blood Center data.8 The right-most plasma product risk reduction shows pre- and postimplementation of solvent detergent products per French Agency for the Safety of Health Products data.3
TRALI remains the leading cause of transfusion-related fatalities according to the US Food and Drug Administration (FDA) and international hemovigilance systems such as the United Kingdom Serious Hazards of Transfusion system and the French Agency for the Safety of Health Products.3 TRALI accounted for 37% (74 of 198) of transfusion-related fatalities reported to the FDA from 2008 to 2012: the distribution of implicated products was plasma 24%, platelets 22%, red blood cells (RBCs) 37%, and multiple products 22%.4 The incidence of TRALI has decreased through various mitigation strategies (see figure). The use of male and never-pregnant female plasma (which decreased TRALI risk by 68%) and solvent detergent plasma have had no reported cases,3 but additional strategies are needed, particularly for RBC products.
TRALI appears to result from 2 hits: 1 from the product and 1 from the recipient, which together reach an adequate threshold to cause TRALI.5 The product hit, more often, is donor white blood cell antibodies (human leukocyte antigen [HLA] class I or class II, or human neutrophil antigen [HNA] antibodies) directed to recipient antigens and, less often, bioactivation mediators (lipids, cytokines, or CD40L). Patient factors associated with higher TRALI incidence include higher IL-8 levels, liver surgery, chronic alcohol abuse, shock, higher peak airway pressure during mechanical ventilation, current smoking, and positive fluid balance.6
Previously postulated TRALI mechanisms include the following: (1) antibodies bind neutrophils, resulting in neutrophil activation that disturbs pulmonary endothelial barrier function; (2) antibodies directly bind lung endothelium, resulting in neutrophil activation; and (3) bioactive mediators activate neutrophils. The severity of TRALI varies from none or mild to severe, depending on the impact of transfusion-related mediators (based on volume, amount, and strength of mediator) and depending on the patient whose neutrophils and lung endothelial cells are in a resting, primed, or activated state.5
McKenzie et al, by using a low-dose lipopolysaccharide (LPS)–independent HLA class I antibody murine model, question proposed mechanism 1 above by showing that antibodies bind monocytes (instead of neutrophils), resulting in increased IL-8, which results in neutrophilic pulmonary infiltrate with subsequent TRALI.1 The idea that monocytes rather than neutrophils or platelets induce TRALI has been proposed in another study using an LPS-dependent HLA class I antibody murine model. Unlike the study by McKenzie et al, that study suggested that antibodies bind vascular endothelium, which activates complement binding, resulting in activating monocytes that damage the pulmonary endothelium by reactive oxygen species.7 Other murine models have demonstrated direct antibody binding of pulmonary vascular endothelium, such as the anti-HNA-3a LPS-dependent murine model, which suggested that the antibody both binds choline transporter-like protein 2 (CTL2) in the endothelium resulting in reactive oxygen species and primes neutrophils cumulating in TRALI.8 These and other published animal models differ in antibody used, need for LPS for the first hit, mouse genotype, and animal used for the model, which may explain the differences in resultant proposed TRALI mechanisms. Additionally, murine and other animal models may not parallel human pathophysiology. However, animal models have demonstrated potential success of new treatments such as intravenous immunoglobulin and prestorage filtration to remove bioactive mediators.1,2
TRALI mitigation strategies to date have been blunt instruments to remove high-plasma-volume products that may contain HLA or HNA antibodies from the blood supply. Currently, TRALI mitigation is required by the American Association of Blood Banks (AABB) for whole blood and plasma only and not for platelet and RBC products.3 Some US blood suppliers have implemented these TRALI mitigation steps for platelet products, and no significant policies other than deferring donors implicated in TRALI cases have been adopted for RBC products.9 However, the strategies to eliminate plasma and platelet products that may contain HLA or HNA antibodies have resulted in donor and product loss, which could be unsustainable if fully applied to platelet products and if applied to RBC products.9 At this time, AB plasma is in short supply, due in part to TRALI mitigating strategies overlapping with the surge of massive transfusion protocols that entail having thawed plasma available immediately.3,10 The development of a filter that removes the TRALI-causing substances, both antibodies and bioactive mediators, from the blood product is innovative, provides a TRALI mitigation strategy for RBC products, and offers a future alternate means of increasing the safety while maintaining an adequate blood supply.2
A beneficial next step would be to have the filter be usable for platelet products; however, the current configuration traps platelets (as does a standard RBC prestorage leukoreduction filters).2 TRALI mitigation strategies for platelet products have not been studied as much as those for plasma. These strategies include male only plasma to reconstitute buffy coat prepared platelet pools, male only donors, never-pregnant or HLA-antibody-negative female donors, and storage in platelet additive solution.9
Progress has been made in both the understanding of TRALI and its mitigation. However, mitigation strategies decrease product availability and leave room for the need for additional reduction strategies. With further understanding and innovative strategies, more targeted and successful mitigation approaches will be implemented, increasing the safety and availability of the blood supply.
Conflict-of-interest disclosure: The author declares no competing financial interests.