Key Points
TRALI may be induced by antibodies to HLA or HNA antigens or lipids, which accumulate during storage.
Prestorage experimental filtration of RBCs removes HLA and HNA antibodies, decreases lipid priming activity, and mitigates TRALI in an animal model.
Abstract
Transfusion-related acute lung injury (TRALI) remains a significant cause of transfusion-related mortality with red cell transfusion. We hypothesize that prestorage filtration may reduce proinflammatory activity in the red blood cell (RBC) supernatant and prevent TRALI. Filters were manufactured for both small volumes and RBC units. Plasma containing antibodies to human lymphocyte antigen (HLA)-A2 or human neutrophil antigen (HNA)-3a was filtered, and immunoglobulins and specific HNA-3a and HLA-2a neutrophil (PMN) priming activity were measured. Antibodies to OX27 were added to plasma, and filtration was evaluated in a 2-event animal model of TRALI. RBC units from 31 donors known to have antibodies against HLA antigens and from 16 antibody-negative controls were filtered. Furthermore, 4 RBC units were drawn and underwent standard leukoreduction. Immunoglobulins, HLA antibodies, PMN priming activity, and the ability to induce TRALI in an animal model were measured. Small-volume filtration of plasma removed >96% of IgG, antibodies to HLA-A2 and HNA-3a, and their respective priming activity, as well as mitigating antibody-mediated in vivo TRALI. In RBC units, experimental filtration removed antibodies to HLA antigens and inhibited the accumulation of lipid priming activity and lipid-mediated TRALI. We conclude that filtration removes proinflammatory activity and the ability to induce TRALI from RBCs and may represent a TRALI mitigation step.
Introduction
Transfusion-related acute lung injury (TRALI) is a significant cause of transfusion-related mortality.1-4 Despite the implementation of TRALI mitigation strategies for plasma and platelet concentrates (PCs), which employ either male-only and/or “antibody-negative” donors, there are still significant numbers of TRALI-related deaths, especially linked to the transfusion of red blood cell (RBC) units.1,2,4,5 TRALI has been reported to be the result of at least 2 events: the first pertaining to the clinical condition of the patient, and the second to the infusion of antibodies that recognize human lymphocyte antigen (HLA) class I, class II, or granulocyte antigens or the infusion of biological response modifiers, which include both biologically active lipids and soluble CD40 ligand (sCD40L).6-15 Standard prestorage leukoreduction (LR) was reported to have decreased TRALI reactions at 1 medical center; however, LR has not affected TRALI incidence in the United States or worldwide.16,17 To date, there are few RBC mitigation strategies for TRALI.
Standard LR by filtration removes 3 logs of leukocytes and 2 logs of platelets. Removal of leukocytes and platelets has been shown to reduce: (1) HLA antigen exposure; (2) febrile, nonhemolytic transfusion reactions; (3) levels of cytokine accumulation during routine RBC storage; (4) sCD40L accumulation; and (5) cytomegalovirus exposure.8,17-20 The majority of RBC units in most countries are prestorage leukoreduced either by filtration or buffy coat removal.
TRALI in a susceptible host has been related to the infusion of donor antibodies against the HLA antigens of the recipient, which may reside on either the recipient’s leukocytes or endothelial cells, or the infusion of lipids or sCD40L.21-23 These antibodies, lipids, or sCD40L activate neutrophils (PMNs), allowing for PMN-mediated endothelial damage, capillary leak, and acute lung injury (ALI).6-8,13,24 We hypothesize that a prestorage filter that removes antibodies, leukocytes, and platelets and decreases lipid bioactivity would significantly decrease the presence of donor HLA antibodies and the accumulation of biologically active lipids and sCD40L and would abrogate TRALI in a 2-event animal model.
Materials and methods
Materials
All chemicals and reagents were purchased from Sigma Chemical Corporation (St. Louis, MO) unless otherwise delineated. OX27 antibodies were purchased from AbD Serotec (Raleigh, NC) or Abcam (Cambridge, MA). All buffers were made from stock United States Pharmacopeia solutions and filtered with Nalgene MF75 series disposable sterilization filter units (Fisher Scientific Corp., Pittsburgh, PA).25 Enzyme-linked immunosorbent assay (ELISA) kits for arachidonic acid (AA) or 5-hydroxy-eicostetranoic acid (5-HETE) were purchased from Antibodies-online.com (Atlanta, GA).
Filtration
The experimental filters are based on proprietary material, which primarily removes antibodies. Two types of filters were used in the described experiments: (1) a small-volume filter in 2 sizes for 1 to 10 mL or 10 to 50 mL of plasma and (2) filters that are structurally similar to the Pall BPF4 standard LR filters. These RBC filters contain the identical LR filter and additional material that adsorbs antibodies and structurally similar proteins and nonspecifically binds lipids.26 The volume of RBCs that is lost from experimental filtration is 25 mL. With respect to immunoglobulins, the filter removes all 4 classes (data not shown for IgA and IgE) and is preferentially selective for IgG > IgM. The B, P, and M designations are assigned to different methods of packaging the antibody adsorbent material.26
Experiments employing filters for small volumes
To assess the capability to remove IgG and IgM, 5 fresh human plasma samples (100 mL) were obtained. Fifty percent by volume was filtered, and the other 50% left as an unmodified control (volume/volume [v/v]). The amounts of IgG pre- and postfiltration were measured in the clinical laboratory at Children’s Hospital Colorado (Aurora, CO) and confirmed at Pall Corporation.
The ability of the experimental filter to remove immunoglobulins, including HLA-A2 and human neutrophil antigen (HNA)-3a antibodies, was also measured in the small-volume system. Plasma (5 mL) was drawn from 3 multiparous females: 1 with antibodies to HNA-3a who was implicated in 9 TRALI reactions, another with known antibodies to both HLA class I and class II antigens, and a third with known antibodies to HLA-A2. The plasma was divided into equal volumes with 50% filtered and 50% left as an unmodified control. The effects of filtration on PMN priming activity were then completed using matched PMNs (HNA-3a+ PMNs for HNA-3a+ plasma and PMNs from homozygous HLA-A2 donors for HLA-A2+ plasma) and sent to clinical laboratories for HLA or HNA antibody measurements. Lastly, to determine if filtration mitigated TRALI in a rat model of antibody-induced TRALI, heat-treated human plasma (2 mL) (56°C for 30 minutes to destroy human complement and fibrinogen activity) with 50 μg/mL of monoclonal antibodies to OX27, a major histocompatibility complex class I antigen in rats, was divided into equal volumes and was filtered using the smaller (1-10 mL) device. These samples were then used as the second event in a 2-event animal model of TRALI to determine if experimental filtration could remove the antibodies to OX27 and mitigate TRALI.
Collection of RBCs and preparation of RBC supernatants
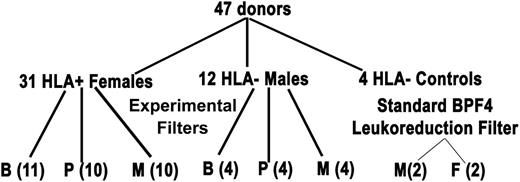
Filtration of entire red cell units in additive solution-5 (AS-5; Optisol) from control and donors known to harbor HLA antibodies was similarly investigated. After obtaining informed consent via a protocol approved by the Western Internal Review Board, 1 unit of whole blood (500 ± 50 mL) was collected from 2 groups of donors. This study was conducted in accordance with the Declaration of Helsinki. The first group consisted of healthy, antibody-negative donors. Specifically, this control group (n = 16) consisted of 4 male donors per experimental filter group (ie, B [4], P [4], and M [4]) and 4 donors (2 males and 2 females) whose RBC units underwent standard LR. The second group was composed of 31 multiparous females who previously tested positive for antibodies to HLA class I antigens, class II antigens, or both by both Luminex bead assays with flow cytometry and via the GTI ELISA (GTI Diagnostics, division of GenProbe, San Diego, CA) within the past 4 years (Figure 1). Briefly, whole blood was collected in citrate-phosphate-dextrose with Optisol collection bag system (Teruflex; Terumo Corporation, Tokyo, Japan). Plasma was separated from RBCs by centrifugation followed by expression, employing an automated closed system, Compomat G4 (Fresenius-Kabi, Schweinfurt, Germany), and AS-5 (Optisol) was added to a final hematocrit of 50% to 60%. The estimated amount of residual plasma per unit was 5 to 10 mL (6.1 ± 1.0 mL).27 A 10-mL sample was drawn from each RBC unit prior to and following experimental filtration with the “B,” “P,” or “M” filters and 4 control units that underwent standard LR. The RBC units were stored at 1°C to 6°C, and samples were obtained through sterile couplers. The supernatant was isolated via centrifugation (5000g for 7 minutes) followed by a second spin at 12 500g for 6 minutes at 4°C to remove contaminating platelets and stored at −80°C.28
Filtration algorithm of HLA antibody-positive donors and antibody-negative controls. The algorithm depicts the experimental design of the donors employed for manufacture of RBC units that underwent experimental filtration using filters B, P, or M or standard LR (Pall BPF4 LR filter). Please note that there are 4 antibody-negative male controls per experimental filter and 4 antibody-negative donors, 2 male and 2 female for the BPF4 LR filter.
Filtration algorithm of HLA antibody-positive donors and antibody-negative controls. The algorithm depicts the experimental design of the donors employed for manufacture of RBC units that underwent experimental filtration using filters B, P, or M or standard LR (Pall BPF4 LR filter). Please note that there are 4 antibody-negative male controls per experimental filter and 4 antibody-negative donors, 2 male and 2 female for the BPF4 LR filter.
Measurement of antibodies to HLA class I and class II antigens
Donor antibodies directed against HLA class I antigens, class II antigens, or both were measured using Luminex beads and flow cytometry at 2 HLA reference laboratories, ClinImmune (Denver, CO) and LABs (Centennial, CO), in a blinded fashion. The mean fluorescence intensity (MFI) data have been included to demonstrate a functional titer/strength of these antibodies.29 These data were confirmed using identical methodology at Pall Corporation and Bonfils Blood Center.
Measurement of antibodies to HNA-3a
Measurement of antibodies to HNA-3a in both prefiltration and postfiltration plasma samples was performed employing standard techniques at the Granulocyte Laboratory, Blood Center of Southeastern Wisconsin (BCW). The pre- and postfiltration samples were tested in a blinded fashion. The antibody titer was not measured, and the plasma came from a donor who was implicated in 9 TRALI reactions.30
Measurement of 2,3-DPG, ATP, pH, IgG, IgM, blood counts, and sCD40L in RBC units
Prior to and following filtration with the experimental filters (B, P, and M) and standard LR, samples were drawn, and IgG, IgM, 2,3 diphosphglycerate (2,3-DPG), and adenosine triphosphate (ATP) were measured. Measurement of 2,3-DPG, ATP, and pH was completed in the clinical laboratories at BCW and confirmed at Pall Corporation. All complete blood counts were performed at BCW, Pall Corporation, and Bonfils Blood Center. The IgG and IgM levels were measured at the Children’s Hospital of Colorado and confirmed at Pall Corporation. sCD40L was measured via commercial ELISA (R&D Systems, Minneapolis, MN).
PMN priming assays
Heparinized whole blood was drawn from healthy donors after obtaining informed consent employing a protocol approved by the Colorado Multiple Institutional Review Board, and the PMNs were isolated by standard techniques.25 PMNs (3.75 × 105) were then incubated with 10% (v/v) fresh plasma (FP) control, plasma containing antibodies to HLA-A2 or HNA-3a, or the supernatant from days 0 (pre- and postfiltration), 21, or 42 for 5 minutes at 37°C. The PMNs were then activated with 1 μM formyl-methionine-leucine-phenylalanine (fMLF), and the maximal rate of superoxide dismutase inhibitable superoxide production (O2−) was measured.25 Lipids were isolated from these samples using a 1:1:1 chloroform:methanol:water 0.2% acetic acid extraction.20,25 The lipids were solubilized with 1.25% fatty-acid-free human albumin, and PMNs were incubated with albumin or the lipids for 5 minutes at 37°C. The PMNs were then activated with 1 μM fMLF, and O2− was measured.20,25
Two-event in vivo model
Male Sprague Dawley rats (Harlan, Indianapolis, IN) underwent a treatment protocol approved by the Animal Care and Use Committee, University of Colorado Denver.6 Briefly, rats were injected intraperitoneally with 2 mg/kg lipopolysaccharide (LPS, Salmonella enteritides), incubated for 2 hours, and anesthetized with 60 mg/kg pentobarbital, and the femoral vessels were cannulated.6 Blood was removed (10 minutes) equal to 10% of the total blood volume31 followed by infusion of an identical volume of heat-treated (56°C for 30 minutes to obviate the effects of fibrinogen and complement) cell-free plasma with 50 μg/mL of OX27 ± filtration or heat-treated supernatant from day 1 or day 42 RBC units that underwent experimental filtration or standard LR. These second events were infused at 4 mL/h followed by 30 mg/kg of Evans blue dye (EBD).6 Six hours later, blood (3 mL) was drawn, and the rats were euthanized followed by a bronchoalveolar lavage.6 Lung leak was measured as the %EBD in the plasma vs the bronchoalveolar lavage fluid.6
Statistics
All data are presented as the mean ± the standard error of the mean. Statistical differences among groups were measured by independent or paired analyses of variance followed by either a Bonferroni’s or Newman-Keuls post hoc test for multiple comparisons depending on the equality of variance. Significance was determined at the P < .05 level.
Results
Filtration of immunoglobulins and HLA antibodies in small volumes of human plasma
FP from 5 donors was employed to determine IgG removal by experimental filtration. The experimental filter for small volumes (10-50 mL) removed 98 ± 2.1% of the IgG from 50 mL of human plasma (P < .05, n = 5). To determine if the experimental filter could remove HLA antibodies, 4 1-mL plasma samples from a female donor with antibodies to both HLA class I and class II antigens were employed. The 1- to 10-mL filter removed 93.5 ± 2.7% of the antibodies to HLA class I antigens and 99.1 ± 0.9% of the HLA class II antibodies, whereas the 10- to 100-mL filter removed 96.4 ± 1.6% and 99.4 ± 0.4% of the antibodies to HLA class I and class II, respectively.
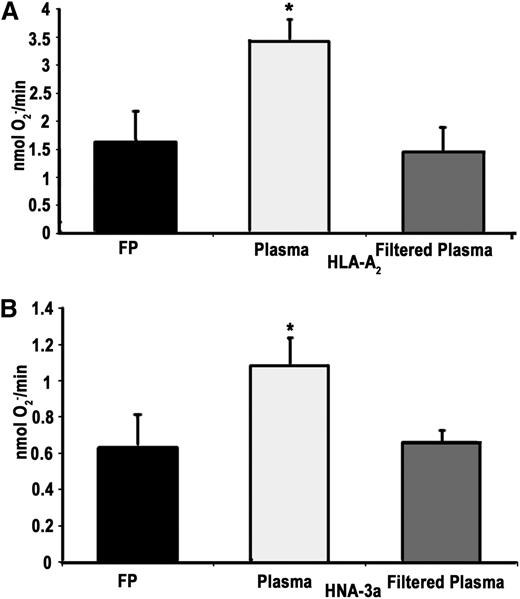
To document removal of antibodies to HLA class I and HNA antigens, plasma samples (3 mL, 50% filtered, v/v) from 2 female donors known to contain these antibodies were employed. Experimental filtration removed antibodies to HLA-A2 and to HNA-3a from the plasma of 2 multiparous female donors, as demonstrated by the presence of these antibodies in the unmodified plasma and its absence in the filtered portion measured by 2 separate reference laboratories. The relative strength/titer, MFI, of the HLA-A2 antibody in the unmodified plasma was 5615 and 4930 against HLA-A2 and below the cutoff of <2000 for both laboratories. With respect to the HNA-3a antibodies, immunoglobulins against HNA-3a were detectable in the unmodified plasma but could not be detected in the filtered plasma. In addition, because antibodies to HLA-A2 and to HNA-3a prime PMNs from homozygous HLA-A2+ donors and HNA-3a+ donors, respectively, the ability of the filters to remove this priming activity was investigated (Figure 2).32,33 Filtration effectively mitigated the priming activity of the HLA-A2+ plasma in PMNs from HLA-A2 homozygous donors (Figure 2A). Furthermore, experimental filtration also removed the priming activity from the HNA-3a+ plasma in PMNs from HNA3a+ donors (Figure 2B).
Experimental filtration mitigates the priming activity of human antibodies against HLA-A2 and HNA-3a. (A) Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst (nmol O2−/min) in PMNs from HLA-A2 homozygotes primed with FP, unmodified plasma with antibodies to HLA-A2, and filtered plasma with antibodies to HLA-A2. Experimental filtration mitigated the priming activity, which was not different from the FP-treated controls. (B) Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst in HNA-3a+ PMNs primed with FP, unmodified plasma that contains antibodies to HNA-3a, and experimentally filtered plasma that contains antibodies to HNA-3a. Experimental filtration mitigated the HNA-3a priming activity. *P < .05 vs all groups, n = 5.
Experimental filtration mitigates the priming activity of human antibodies against HLA-A2 and HNA-3a. (A) Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst (nmol O2−/min) in PMNs from HLA-A2 homozygotes primed with FP, unmodified plasma with antibodies to HLA-A2, and filtered plasma with antibodies to HLA-A2. Experimental filtration mitigated the priming activity, which was not different from the FP-treated controls. (B) Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst in HNA-3a+ PMNs primed with FP, unmodified plasma that contains antibodies to HNA-3a, and experimentally filtered plasma that contains antibodies to HNA-3a. Experimental filtration mitigated the HNA-3a priming activity. *P < .05 vs all groups, n = 5.
Filtration of antibodies from plasma inhibits in vivo TRALI
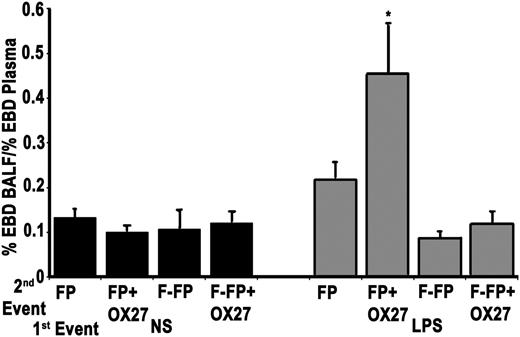
To assess possible in vivo implications of the removal of antibodies from human plasma, 50 μg/mL of OX27 antibody was added to heat-treated human plasma: a total of 8 mL divided into 1-mL aliquots with 4 1-mL aliquots filtered and 4 1-mL aliquots left as unmodified controls, replicated twice. In this 2-event model, rats were incubated with either saline or LPS for 2 hours (first event) and then infused with the filtered or unfiltered heat-treated human plasma (second event). In the saline-treated rodents, no ALI was observed in any treatment group. However, as compared with the saline-treated animals as well as the LPS-treated rats infused with filtered heat-treated plasma, the unfiltered plasma containing 50 μg/mL OX27 demonstrated significant ALI (Figure 3). Filtration of the heat-treated plasma containing 50 μg/mL of OX27 mitigated TRALI in this animal model.
Experimental filtration mitigates ALI in plasma that contains major histocompatibility complex class I antibodies to OX27. The figure illustrates EBD leak, a measure of ALI, as a function of treatment group. Saline-treated rats (first event) did not demonstrate ALI irrespective of the second event: heat-treated (56°C for 30 minutes) FP, FP with OX27 (FP+OX27), filtered FP (F-FP), or filtered FP + OX27 (FP+OX27). In comparison, endotoxin (LPS) pretreatment did evidence ALI with plasma that contained OX27 (LPS/FP+OX27) as the second event, but no ALI was evident in LPS-treated rats infused with plasma (LPS/FP). Experimental filtration of the plasma with OX27 mitigated ALI (LPS/FP+OX27), and there was no ALI produced by filtered FP (LPS/FP). *P < .05 vs all groups, n = 5. BALF, bronchoalveolar lavage fluid.
Experimental filtration mitigates ALI in plasma that contains major histocompatibility complex class I antibodies to OX27. The figure illustrates EBD leak, a measure of ALI, as a function of treatment group. Saline-treated rats (first event) did not demonstrate ALI irrespective of the second event: heat-treated (56°C for 30 minutes) FP, FP with OX27 (FP+OX27), filtered FP (F-FP), or filtered FP + OX27 (FP+OX27). In comparison, endotoxin (LPS) pretreatment did evidence ALI with plasma that contained OX27 (LPS/FP+OX27) as the second event, but no ALI was evident in LPS-treated rats infused with plasma (LPS/FP). Experimental filtration of the plasma with OX27 mitigated ALI (LPS/FP+OX27), and there was no ALI produced by filtered FP (LPS/FP). *P < .05 vs all groups, n = 5. BALF, bronchoalveolar lavage fluid.
Experimental filtration provides LR and maintains ATP and 2,3-DPG concentrations without affecting pH
As expected, the experimental filters demonstrated adequate LR by decreasing leukocyte counts by >3 logs and platelet counts <2 logs similar to standard LR (Table 1). Moreover, experimental filtration did not significantly affect ATP levels throughout the storage interval vs standard LR on any day of storage. Specifically, the ATP levels were not different pre- and postexperimental filtration and standard LR on day 0, although experimental filtration modestly increased the ATP concentrations. The ATP concentrations were also not different on day 21 but were significantly decreased on day 42 for each filter group including standard LR. In addition, the pH range for all units was acceptable, although mildly decreased in the experimental groups (Table 1). Lastly, the experimental filtration did decrease the 2,3-DPG levels similar to standard LR (Table 1). The 2,3-DPG levels decreased during storage for all groups by day 42, which was not statistically different from the concentrations in the standard LR units on day 42.
WBC, Plt, Hct, IgG, IgM, HLA I and II, 2,3-DPG, ATP, and pH in RBC units that underwent experimental filtration or standard LR
| Test . | Filtration and storage day . | Filters . | ||||
|---|---|---|---|---|---|---|
| B (female = 11, male = 4) . | P (female = 10, male = 4) . | M (female = 10, male = 4) . | Standard LR, BPF4 (female = 2, male = 2) . | |||
| WBC (103/μL) | Pre | 6.73 ± 0.54 | 8.32 ± 0.68 | 8.07 ± 0.74 | 9.05 ± 2.05 | |
| Post | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| D21 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| D42 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Plt (103/μL) | Pre | 218.38 ± 26.70 | 196.33 ± 24.54 | 258.93 ± 18.09 | 303.75 ± 87.75 | |
| Post | 0.76 ± 0.20 | 6.13 ± 1.52 | 0.43 ± 0.24 | 1.00 ± 0.00 | ||
| D21 | 0.52 ± 0.16 | 1.00 ± 0.32 | 4.10 ± 2.35 | 0.50 ± 0.00 | ||
| D42 | 3.68 ± 2.55 | 1.30 ± 0.51 | 1.45 ± 0.43 | 1.00 ± 0.00 | ||
| Hct (%) | Pre | 59.30 ± 0.63 | 59.15 ± 0.91 | 59.24 ± 1.00 | 60.13 ± 0.78 | |
| Post | 55.58 ± 0.96 | 55.56 ± 0.87 | 53.04 ± 0.58 | 60.50 ± 0.50 | ||
| D21 | 51.48 ± 5.78 | 54.94 ± 0.57 | 54.70 ± 0.62 | 63.98 ± 1.68 | ||
| D42 | 57.31 ± 0.85 | 55.29 ± 0.91 | 55.32 ± 0.93 | 62.43 ± 0.18 | ||
| Hb (g/dL) | Pre | 19.8 ± 0.19 | 19.77 ± 0.21 | 19.61 ± 0.24 | 20.3 ± 0.47 | |
| Post | 18.56 ± 0.22 | 18.28 ± 0.25 | 17.42 ± 0.21 | 20.35 ± 0.31 | ||
| D21 | 17.11 ± 1.92 | 18.14 ± 0.22 | 18.18 ± 0.30 | 21.50 ± 0.65 | ||
| D42 | 18.89 ± 0.30 | 18.03 ± 0.43 | 18.03 ± 0.33 | 20.60 ± 0.15 | ||
| IgG (mg/dL) | Pre | 121.9 ± 8.1 | 167.1 ± 31.5 | 135.3 ± 10.7 | 110.9 ± 20.5 | |
| Post | 12.4 ± 11.9* | 2.64 ± 2.3* | 2.23 ± 0.9* | 100.5 ± 15.6 | ||
| IgM (mg/dL) | Pre | 8.3 ± 1.1 | 8.69 ± 1.2 | 9.4 ± 1.2 | 10.0 ± 3.7 | |
| Post | 1.3 ± 0.6* | 1.1 ± 0.5* | 1.9 ± 0.5* | 11.9 ± 3.8 | ||
| MFI | HLA I | Pre | 5568.06 ± 938.78 | 3037.52 ± 202.22 | 2682.01 ± 178.84 | N/A |
| Post | <2000 | <2000 | <2000 | N/A | ||
| HLA II | Pre | 2837.67 ± 163.46 | 3163.15 ± 202.09 | 4339.25 ± 583.68 | N/A | |
| Post | <2000 | <2000 | <2000 | N/A | ||
| HLA I (# of donors) | Pre | 2 | 2 | 4† | 0 | |
| Post | 0 | 0 | 0 | 0 | ||
| HLA II (# of donors) | Pre | 2 | 2 | 5† | 0 | |
| Post | 0 | 0 | 0 | 0 | ||
| 2,3-DPG (µmol/gHb) | Pre | 253.8 ± 12.4 | 275.0 ± 12.8 | 259.6 ± 24.2 | 276.5 ± 12.7 | |
| Post | 221.9 ± 6.0 | 229.2 ± 8.3 | 209.6 ± 10.0 | 248.3 ± 10.2 | ||
| D21 | 28.7 ± 3.8 | 35.7 ± 5.3 | 27.7 ± 5.2 | 25.45 ± 3.5 | ||
| D42 | 14.4 ± 1.0* | 13.9 ± 1.6* | 13.0 ± 2.3* | 12.9 ± 0.9* | ||
| ATP (µmol/gHb) | Pre | 4.4 ± 0.3 | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.4 ± 0.3 | |
| Post | 5.1 ± 0.1 | 5.2 ± 0.2 | 5.2 ± 0.1 | 4.5 ± 0.3 | ||
| D21 | 4.7 ± 0.1 | 4.5 ± 0.2 | 4.8 ± 0.1 | 4.4 ± 0.3 | ||
| D42 | 3.1 ± 0.1* | 3.1 ± 0.2* | 3.3 ± 0.2* | 3.3 ± 0.2 | ||
| pH range (D42/D0) | 6.5/7.3 | 6.6/7.4 | 6.4/7.3 | 6.7/7.3 | ||
| Test . | Filtration and storage day . | Filters . | ||||
|---|---|---|---|---|---|---|
| B (female = 11, male = 4) . | P (female = 10, male = 4) . | M (female = 10, male = 4) . | Standard LR, BPF4 (female = 2, male = 2) . | |||
| WBC (103/μL) | Pre | 6.73 ± 0.54 | 8.32 ± 0.68 | 8.07 ± 0.74 | 9.05 ± 2.05 | |
| Post | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| D21 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| D42 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Plt (103/μL) | Pre | 218.38 ± 26.70 | 196.33 ± 24.54 | 258.93 ± 18.09 | 303.75 ± 87.75 | |
| Post | 0.76 ± 0.20 | 6.13 ± 1.52 | 0.43 ± 0.24 | 1.00 ± 0.00 | ||
| D21 | 0.52 ± 0.16 | 1.00 ± 0.32 | 4.10 ± 2.35 | 0.50 ± 0.00 | ||
| D42 | 3.68 ± 2.55 | 1.30 ± 0.51 | 1.45 ± 0.43 | 1.00 ± 0.00 | ||
| Hct (%) | Pre | 59.30 ± 0.63 | 59.15 ± 0.91 | 59.24 ± 1.00 | 60.13 ± 0.78 | |
| Post | 55.58 ± 0.96 | 55.56 ± 0.87 | 53.04 ± 0.58 | 60.50 ± 0.50 | ||
| D21 | 51.48 ± 5.78 | 54.94 ± 0.57 | 54.70 ± 0.62 | 63.98 ± 1.68 | ||
| D42 | 57.31 ± 0.85 | 55.29 ± 0.91 | 55.32 ± 0.93 | 62.43 ± 0.18 | ||
| Hb (g/dL) | Pre | 19.8 ± 0.19 | 19.77 ± 0.21 | 19.61 ± 0.24 | 20.3 ± 0.47 | |
| Post | 18.56 ± 0.22 | 18.28 ± 0.25 | 17.42 ± 0.21 | 20.35 ± 0.31 | ||
| D21 | 17.11 ± 1.92 | 18.14 ± 0.22 | 18.18 ± 0.30 | 21.50 ± 0.65 | ||
| D42 | 18.89 ± 0.30 | 18.03 ± 0.43 | 18.03 ± 0.33 | 20.60 ± 0.15 | ||
| IgG (mg/dL) | Pre | 121.9 ± 8.1 | 167.1 ± 31.5 | 135.3 ± 10.7 | 110.9 ± 20.5 | |
| Post | 12.4 ± 11.9* | 2.64 ± 2.3* | 2.23 ± 0.9* | 100.5 ± 15.6 | ||
| IgM (mg/dL) | Pre | 8.3 ± 1.1 | 8.69 ± 1.2 | 9.4 ± 1.2 | 10.0 ± 3.7 | |
| Post | 1.3 ± 0.6* | 1.1 ± 0.5* | 1.9 ± 0.5* | 11.9 ± 3.8 | ||
| MFI | HLA I | Pre | 5568.06 ± 938.78 | 3037.52 ± 202.22 | 2682.01 ± 178.84 | N/A |
| Post | <2000 | <2000 | <2000 | N/A | ||
| HLA II | Pre | 2837.67 ± 163.46 | 3163.15 ± 202.09 | 4339.25 ± 583.68 | N/A | |
| Post | <2000 | <2000 | <2000 | N/A | ||
| HLA I (# of donors) | Pre | 2 | 2 | 4† | 0 | |
| Post | 0 | 0 | 0 | 0 | ||
| HLA II (# of donors) | Pre | 2 | 2 | 5† | 0 | |
| Post | 0 | 0 | 0 | 0 | ||
| 2,3-DPG (µmol/gHb) | Pre | 253.8 ± 12.4 | 275.0 ± 12.8 | 259.6 ± 24.2 | 276.5 ± 12.7 | |
| Post | 221.9 ± 6.0 | 229.2 ± 8.3 | 209.6 ± 10.0 | 248.3 ± 10.2 | ||
| D21 | 28.7 ± 3.8 | 35.7 ± 5.3 | 27.7 ± 5.2 | 25.45 ± 3.5 | ||
| D42 | 14.4 ± 1.0* | 13.9 ± 1.6* | 13.0 ± 2.3* | 12.9 ± 0.9* | ||
| ATP (µmol/gHb) | Pre | 4.4 ± 0.3 | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.4 ± 0.3 | |
| Post | 5.1 ± 0.1 | 5.2 ± 0.2 | 5.2 ± 0.1 | 4.5 ± 0.3 | ||
| D21 | 4.7 ± 0.1 | 4.5 ± 0.2 | 4.8 ± 0.1 | 4.4 ± 0.3 | ||
| D42 | 3.1 ± 0.1* | 3.1 ± 0.2* | 3.3 ± 0.2* | 3.3 ± 0.2 | ||
| pH range (D42/D0) | 6.5/7.3 | 6.6/7.4 | 6.4/7.3 | 6.7/7.3 | ||
Hb, hemoglobin; Hct, hematocrit; N/A, not available; Plt, platelet; WBC, white blood cell.
Signifies P < .05 from day (D) 0 preexperimental filtration supernatant (Pre) or standard LR.
Indicates that antibodies to both HLA class I and class II were present in the same donor.
Prestorage filtration of RBC units removes IgG and donor antibodies
As compared with the unfiltered samples, filtration removed 2 logs of IgG and >80% of the IgM in all units tested (Table 1). Of the 31 female donors who previously tested positive for antibodies to HLA class I, class II, or both, 13 donors had HLA antibodies in their prefiltration samples: 8 had antibodies to HLA class I antigens, 9 had antibodies to HLA class II antigens, and 4 of these donors had antibodies to both HLA class I and class II antigens (Tables 1 and 2). The strength/titer of these antibodies was calculated as the MFI of the bound Luminex beads (Table 2). In addition, the GTI ELISA values were also positive for each of these antibodies (Table 1). Importantly, the antibodies found in the prefiltration sample were not detected in the filtrate from any of the experimental devices (Table 1).
HLA class I and class II antibodies found in donors used for experimental filtration
| Donor # (filter type) . | HLA class I . | HLA class II . |
|---|---|---|
| MFI (± standard error) | 3784.26 (±405.07) [2044.73-10 653.02] | 3540.38 (±265.75) [2286.94-7938.03] |
| 12 (P) | DQ3,* DQ7, DQ8, DQ9 | |
| 15 (B) | B35 | |
| 19 (M) | DR4 | |
| 21 (M) | Cw5, Cw15, Cw17 | DR13 |
| 24 (M) | B7, B27, B40, B48, B60, B61, B81 | DR1, DR7, DR103, DR51 |
| 25 (M) | Cw6, Cw15 | DR7 |
| 29 (B) | DR4, DR7, DQ7, DQ9 | |
| 30 (B) | DQ1,* DQ5, DQ6 | |
| 37 (P) | B12,* B21,* B40,* B41, B44, B45, B49, B50, B60, B61 | |
| 39 (P) | DR3,* DR5,* DR6,* DR8, DR11, DR12, DR13, DR14, DR17, DR18 | |
| 42 (P) | B35 | |
| 43 (M) | B48, B60, B81 | DR4, DR7, DR9 |
| 48 (B) | A10,* A19,* A25, A26, A29, A30, A31, A32, A33, A34, A43, A66, A74 |
| Donor # (filter type) . | HLA class I . | HLA class II . |
|---|---|---|
| MFI (± standard error) | 3784.26 (±405.07) [2044.73-10 653.02] | 3540.38 (±265.75) [2286.94-7938.03] |
| 12 (P) | DQ3,* DQ7, DQ8, DQ9 | |
| 15 (B) | B35 | |
| 19 (M) | DR4 | |
| 21 (M) | Cw5, Cw15, Cw17 | DR13 |
| 24 (M) | B7, B27, B40, B48, B60, B61, B81 | DR1, DR7, DR103, DR51 |
| 25 (M) | Cw6, Cw15 | DR7 |
| 29 (B) | DR4, DR7, DQ7, DQ9 | |
| 30 (B) | DQ1,* DQ5, DQ6 | |
| 37 (P) | B12,* B21,* B40,* B41, B44, B45, B49, B50, B60, B61 | |
| 39 (P) | DR3,* DR5,* DR6,* DR8, DR11, DR12, DR13, DR14, DR17, DR18 | |
| 42 (P) | B35 | |
| 43 (M) | B48, B60, B81 | DR4, DR7, DR9 |
| 48 (B) | A10,* A19,* A25, A26, A29, A30, A31, A32, A33, A34, A43, A66, A74 |
CREG groups: A10 (A25, A26, A34, A66); A19 (A29, A30, A31); B12 (B44, B45); B21 (B49, B50); B40 (B60, B61); DQ1 (DQ5, DQ6); DQ3 (DQ7, DQ8, DQ9); DR3 (DR17, DR18); DR5 (DR11, DR12); DR6 (DR13, DR14).
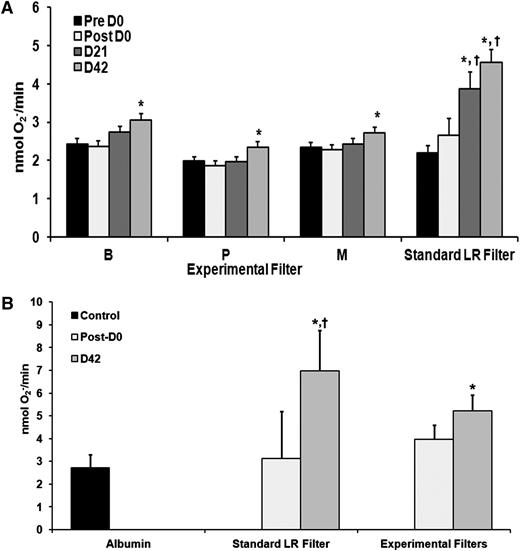
Prestorage experimental filtration significantly inhibits the accumulation of PMN priming activity during RBC storage
Experimental filtration significantly decreased the PMN priming activity that accumulates in the supernatant during routine RBC storage vs standard LR (Figure 4A). There was no difference in priming activity in day 0 RBC supernatants, either pre- or postfiltration in any of the experimental filtration RBC supernatants vs standard LR. At day 21 and day 42, the standard leukoreduced control supernatants exhibited significantly greater priming activity than any of the supernatants from the experimentally filtered RBCs (Figure 4A) (P < .05). There was significant priming activity on day 42 vs day 0 in the RBC supernatants, respectively, in each of the experimentally filtered units (P < .05).
Experimental filtration mitigates the accumulation of PMN priming activity and lipid priming activity in the supernatant of RBC units during routine storage. Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst (nmol O2−/min) is depicted as a function of experimental filtration groups and days of routine storage (A). The standard leukoreduced controls (standard LR filter) demonstrate the accumulation of PMN priming activity as a function of storage with significant activity reached by day 21 with a relative maximum on day 42 of storage (*P < .05 vs day 1 supernatant). In the 3 experimental filters (B, P, and M), there is only an increase in priming activity in the RBC supernatants on day 42 vs day 1 (*P < .05 vs day 1 supernatant). The priming activity from supernatants from RBC units that underwent standard LR was significant from the experimental filtration groups on both day 21 and day 42 (†P < .05 vs day 21 and day 42 supernatants from experimentally filtered RBC units; n ≥ 10 for each group). Experimental filtration inhibited the accumulation of lipid priming activity in the supernatant of RBC units during storage (B) depicted as the maximal rate of superoxide anion production to fMLF (nmol O2−/min) as a function of treatment group. The lipid extracts from the supernatant of the RBCs that underwent standard LR or experimental filtration were not different from albumin-treated controls. However, extracts from the supernatant of RBCs that underwent standard LR demonstrated increased priming activity as compared with albumin-treated controls and extracts from day 0 and day 42 supernatants from experimentally filtered RBCs (*P < .05 vs albumin controls and lipid extracts from day 1 supernatants, †P < .05 vs day 42 extracts or the supernatants of experimentally filtered RBCs; n = 15). Lastly, the extracts from the supernatants of experimentally filtered RBC units showed greater priming activity vs the albumin-treated controls and the extracts from the day 0 experimentally filtered extracts (*P < .05 vs albumin controls and lipid extracts from day 1 supernatants).
Experimental filtration mitigates the accumulation of PMN priming activity and lipid priming activity in the supernatant of RBC units during routine storage. Priming of the fMLF-activated, superoxide dismutase inhibitable respiratory burst (nmol O2−/min) is depicted as a function of experimental filtration groups and days of routine storage (A). The standard leukoreduced controls (standard LR filter) demonstrate the accumulation of PMN priming activity as a function of storage with significant activity reached by day 21 with a relative maximum on day 42 of storage (*P < .05 vs day 1 supernatant). In the 3 experimental filters (B, P, and M), there is only an increase in priming activity in the RBC supernatants on day 42 vs day 1 (*P < .05 vs day 1 supernatant). The priming activity from supernatants from RBC units that underwent standard LR was significant from the experimental filtration groups on both day 21 and day 42 (†P < .05 vs day 21 and day 42 supernatants from experimentally filtered RBC units; n ≥ 10 for each group). Experimental filtration inhibited the accumulation of lipid priming activity in the supernatant of RBC units during storage (B) depicted as the maximal rate of superoxide anion production to fMLF (nmol O2−/min) as a function of treatment group. The lipid extracts from the supernatant of the RBCs that underwent standard LR or experimental filtration were not different from albumin-treated controls. However, extracts from the supernatant of RBCs that underwent standard LR demonstrated increased priming activity as compared with albumin-treated controls and extracts from day 0 and day 42 supernatants from experimentally filtered RBCs (*P < .05 vs albumin controls and lipid extracts from day 1 supernatants, †P < .05 vs day 42 extracts or the supernatants of experimentally filtered RBCs; n = 15). Lastly, the extracts from the supernatants of experimentally filtered RBC units showed greater priming activity vs the albumin-treated controls and the extracts from the day 0 experimentally filtered extracts (*P < .05 vs albumin controls and lipid extracts from day 1 supernatants).
Lipid priming activity was measured on samples from all 3 experimental filters and demonstrated significantly less lipid priming activity compared with standard LR (Figure 4B). In addition, there was a significant decrease in 5-HETE accumulation but not in AA compared with standard LR on day 42 with the pre- and postexperimental filtration having no effect on lipid concentrations on day 42 from standard LR: AA, 1200 ± 250 ng/mL vs experimental filtration: 1321 ± 119 g/mL (n = 20) and 5-HETE, 38 ± 11 ng/mL vs 19 ± 5 ng/mL,* respectively (n = 14, *P < .05 vs 5-HETE standard LR).
Experimental filtration inhibits stored RBC-induced TRALI in a 2-event in vivo model
To determine if the decreased postfiltration priming activity in the RBC supernatants could serve as the second event in a 2-event model of ALI, heat-treated RBC supernatants were used as the second event in a well-described 2-event in vivo model of TRALI.6 In congruence with previous data, no ALI was demonstrated in saline-treated rodents for any second event. However, the supernatants from day 42 LR-RBCs (standard LR) caused ALI as the second event in LPS-treated rats (Figure 5). Importantly, none of the supernatants from day 42 or day 0 RBCs that underwent experimental filtration caused similar ALI in LPS-treated rats (Figure 5).
Experimental filtration inhibits ALI induced by the supernatant from stored day 42 LR-RBCs. EBD leak is depicted as a function of treatment group. Normal saline (NS) treatment of rats as the first event did not result in ALI regardless of the second event: NS or the heat-treated supernatants from day 1 or day 42 plasma from units that underwent experimental filtration of standard LR. In LPS-treated rats (first event), NS did not cause ALI; however, the heat-treated supernatants from day 42 RBCs that underwent standard LR induced significant EBD leak/ALI. Conversely, none of the heat-treated supernatants from the experimentally filtered units on day 42 induced significant EBD leak/ALI (*P < .05 vs LPS/NS and NS/NS controls, n = 5 for each group).
Experimental filtration inhibits ALI induced by the supernatant from stored day 42 LR-RBCs. EBD leak is depicted as a function of treatment group. Normal saline (NS) treatment of rats as the first event did not result in ALI regardless of the second event: NS or the heat-treated supernatants from day 1 or day 42 plasma from units that underwent experimental filtration of standard LR. In LPS-treated rats (first event), NS did not cause ALI; however, the heat-treated supernatants from day 42 RBCs that underwent standard LR induced significant EBD leak/ALI. Conversely, none of the heat-treated supernatants from the experimentally filtered units on day 42 induced significant EBD leak/ALI (*P < .05 vs LPS/NS and NS/NS controls, n = 5 for each group).
Discussion
The presented data demonstrated that the experimental filters developed for small-volume filtration removed >96% of IgG, 93% of antibodies to HLA class I antigens, and 99% of antibodies to HLA class II antigens. The small-volume filters also took away the in vitro priming activity of 2 antibodies implicated in TRALI (eg, antibodies to HNA-3a and HLA-A2).32,33 In addition, such removal of antibodies inhibited ALI in a 2-event in vivo animal model of TRALI because removal of antibodies to OX27 resulted in a second event that did not induce TRALI in LPS pretreated rats. In contrast, the unfiltered heat-treated plasma that contained antibodies to OX27 did elicit TRALI as the second event in LPS-treated rats.
When the experimental filters were used in the preparation of RBC units in comparison with standard LR, experimental filtration decreased the amount of 2,3-DPG by day 42 of storage; however, there was not a difference between experimentally filtered units and the standard leukoreduced controls. Surprisingly, the ATP levels modestly increased postexperimental filtration, but the ATP levels at day 42, the end of storage for AS-5 stored units, were not different from the leukoreduced controls. Prestorage filtration with the experimental filters decreased leukocyte counts by 3.5 logs, platelets by 2 logs, and sCD40L by >90%, identical to standard LR.8 Moreover, experimental filtration decreased the accumulation of proinflammatory priming activity in the RBC supernatant, specifically lipid priming activity, as compared with RBC units that underwent standard LR. Measurement of 2 lipid moieties, specifically AA and 5-HETE, demonstrated no change in AA and a 50% decrease in 5-HETE. This decrease in lipid proinflammatory priming activity mitigated TRALI in a 2-event animal model, when day 42 supernatants from experimentally filtered RBC units were used as the second event vs day 42 supernatants from leukoreduced RBC units. Lastly, the amount of residual plasma may differ in RBC units, and the amount of antibody adsorbent material in the B, P, and M filters would remove >96% of all IgG from 50 mL of plasma; therefore, these experimental filters could be employed for RBC units with larger amounts of residual plasma. However, these filters are not designed for whole blood and would likely require more antibody adsorbent material.
Prestorage LR is done differently depending on the locale either by buffy coat removal or by filtration. There has been a recent controversy as to whether lipids accumulate in RBC units as a result of plasma contamination.34 However, because these LR techniques are not synonymous, the techniques used by these investigators to identify lipids and detect PMN priming are dissimilar to previous studies, and the amount of contaminating plasma is small (5-10 mL, mean of 6 ± 1 mL), direct examination of LR methodology and RBC storage needs to be completed using identical donors at the same institution.28,34
The in vivo model previously demonstrated that EBD leak was synonymous with ALI employing 5 other measurements including histology, increased protein concentration and cytokine-induced neutrophil chemoattractant-1 in the bronchoalveolar lavage fluid, myeloperoxidase in the lung, and histologic examination of the lung tissue.6 In the reported data, lung leak was used because it is the most consistent marker of ALI in this model.6 With respect to OX27, concentration-response data were completed previously in rats as a second event in this in vivo model.6 OX27 concentrations of <50 μg, for example, 25 μg (0.075 mg/kg), did not cause ALI in this model, and the filter for small volumes employed removes 93% of all IgG regardless of the species of origin, human or murine.6
Experimental filtration decreased (1) the accumulation of PMN priming activity in the day 42 RBC supernatant, (2) the lipid priming activity in the day 42 RBC supernatant, and (3) the concentration of 5-HETE in comparison with leukoreduced control RBCs.8,20 These filters were constructed to remove immunoglobulins and do passively bind lipophilic compounds and a number of enzymes and other cofactors. The decrease in 5-HETE accumulation without any decrease in AA, the most common precursor, is likely attributable to removal of the enzyme(s) responsible for its conversion.35 We have previously reported that both 5-lipoxygenase and 5-lipoxygenase activating protein are present in RBC units, and their activity likely results in the accumulation of 5-HETE.36 In addition, there is 12% to 17% sequence homology between human IgG and 5-lipoxygenase activating protein or human IgG and 5-lipoxygenase, which may account for clearance of one of these enzymes by experimental filtration.37 The PMN priming activity that accumulates in the supernatant during LR-RBC storage is almost totally composed of lipophilic compounds, which were significantly decreased by experimental filtration.8,20
Experimental filtration for RBC units represents a possible method for TRALI mitigation via antibody removal, which to date has not been implemented in most blood centers. Washing of RBC units removes proinflammatory activity, including both antibodies and lipids, and ALI may be mitigated in high-risk populations by washing of cellular components.38-40 TRALI secondary to RBC infusion results in a number of reported fatalities, and that number has likely increased with antibody-negative plasma transfusion strategies.1-4,41,42 Moreover, removal of the lipophilic compounds may also diminish the clinical effects of stored RBCs especially in the critically ill and in the injured. Although never associated with RBC storage age, TRALI did significantly correlate with PC age and, more importantly, with the amount of proinflammatory lipid priming activity present in the implicated PCs.13 Unfortunately, many investigators equate proinflammatory bioactivity with storage age because the bioactivity reaches a relative maximum at product outdate. However, TRALI was associated with stored PCs that contained increased bioactivity as compared with similar PCs with the same storage age, which did not cause adverse events when transfused.13 A recent prospective study of TRALI did not demonstrate an association with lysophosphatidylcholines; however, lysophosphatidylcholines do not accumulate during storage of leukoreduced RBCs, and there were enough LR-RBC units to mar these analyses.20,42 Furthermore, there are a number of clinical trials investigating the role of stored vs fresh RBC transfusion in the critically ill in whom TRALI may be as common as 5% to 8% of transfused patients.43,44 In a series of injured patients controlled for the number of units transfused, the older units were associated with the development of multiple organ failure, which invariably is initiated by ALI and is a significant cause of patient morbidity and mortality.45 One may hypothesize that such filtration with removal of antibodies, leukocytes, and sCD40L may make transfusions safer with diminution of the proinflammatory activity of RBC units.
Further work is certainly required including the safety and efficacy of the clinical effects of experimental filtration including the introduction of organic compounds, which filtration imparts, and possible adverse events, as well as RBC survival studies in vivo. These filters are designed for prestorage LR of RBC units and have nothing to do with bedside filtration or any of the adverse reactions that such a process may impart.46 One may question why prestorage filtration should be employed if lipids accumulate during routine storage reaching a relative maximum at outdate. The observed diminution of the lipid bioactivity, specifically 5-HETE, was not anticipated and may be attributable to binding of the enzymes required for its generation from AA. Lung inflammation has also been linked to the RBCs themselves, especially those without the Duffy antigen, and such filtration would not mitigate such effects related to stored RBCs.39,47 In conclusion, the presented data describe a method for TRALI mitigation for RBCs that may ultimately make transfusions safer, especially for the critically ill and traumatically injured.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Plasma that contained antibodies to HNA-3a was the kind gift of Dr Brian Curtis, Blood Center of Wisconsin (Milwaukee, WI). The authors would like to acknowledge Travis Berry, Division of Blood Components, Bonfils Blood Center (Denver, CO); Kadi Schroeder, Specialized Donations, Bonfils Blood Center; and Drs Tuan Le and D. Joseph Chaffin, Medical Affairs, Bonfils Blood Center. The authors also wish to acknowledge Linda Cagle of Clinimmune (Denver, CO) for help with the antibody assays and antigen specificities.
This work was supported by Bonfils Blood Center, grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL59355) and National Institute of General Medical Sciences (GM049222), and a grant from Pall Corporation to complete all of the described studies.
Authorship
Contribution: C.C.S. designed the bulk of the experiments and wrote the manuscript; M.R.K. performed priming and all of the animal work and contributed to writing the manuscript; S.Y.K. performed priming experiments and contributed to writing the manuscript; M.L. was responsible for all data sent to the reference laboratories and its compilation; F.B.W. ensured that all RBC components were manufactured properly and contributed to writing the manuscript; K.J.L. helped with the experimental design, organized the roles of Bonfils Blood Center, and contributed to writing the manuscript; B.M. and L.C. were vital to the manufacture of all the experimental filters, performed all of the experiments completed by Pall Corporation, and compiled all data; and S.S.-C. contributed significantly to the experimental design, designed and supervised experimental filter manufacture, and helped to write the manuscript.
Conflict-of-interest disclosure: All experiments were paid for by a grant from Pall Corporation. B.M., L.C., and S.S.-C. were paid employees of Pall Corporation. S.S.-C. is currently a paid employee of Haemonetics Corporation. C.C.S. received a one-time honorarium in 2009 for a presentation at Pall Corporation. The remaining authors declare no competing financial interests.
Correspondence: Christopher C. Silliman, Research Laboratories, Bonfils Blood Center, 717 Yosemite Circle, Denver, CO 80123; e-mail: christopher.silliman@ucdenver.edu.