Key Points
Anti-MHC antibodies that induce TRALI in a murine model first bind their cognate antigen and stimulate blood monocytes to secrete chemokines.
Full TRALI induction (lung damage) requires a subsequent monocyte Fc-dependent process.
Abstract
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related mortality and can occur with any type of transfusion. TRALI is thought to be primarily mediated by donor antibodies activating recipient neutrophils resulting in pulmonary endothelial damage. Nonetheless, details regarding the interactions between donor antibodies and recipient factors are unknown. A murine antibody-mediated TRALI model was used to elucidate the roles of the F(ab′)2 and Fc regions of a TRALI-inducing immunoglobulin G anti–major histocompatibility complex (MHC) class I antibody (34.1.2s). Compared with intact antibody, F(ab′)2 fragments significantly increased serum levels of the neutrophil chemoattractant macrophage inflammatory protein 2 (MIP-2); however, pulmonary neutrophil levels were only moderately increased, and no pulmonary edema or mortality occurred. Fc fragments did not modulate any of these parameters. TRALI induction by intact antibody was completely abrogated by in vivo peripheral blood monocyte depletion by gadolinium chloride (GdCl3) or chemokine blockade with a MIP-2 receptor antagonist but was restored upon repletion with purified monocytes. The results suggest a two-step process for antibody-mediated TRALI induction: the first step involves antibody binding its cognate antigen on blood monocytes, which generates MIP-2 chemokine production that is correlated with pulmonary neutrophil recruitment; the second step occurs when antibody-coated monocytes increase Fc-dependent lung damage.
Introduction
Transfusion-related acute lung injury (TRALI) is presently considered one of the leading causes of mortality associated with transfusion.1,2 However, the pathogenesis of the disease is poorly understood, and there is still debate regarding the incidence of TRALI.3 With regard to the pathophysiology of TRALI, it has previously been reported that the majority of TRALI reactions are caused by donor antibodies within the transfused blood product.4-7 It appears, however, that not all leukocyte antibodies cause TRALI in patients exhibiting the cognate antigen and antibodies, such as anti-human neutrophil antigen 3a (HNA-3a) and anti-human leukocyte antigen A2 (HLA-A2), which are associated with more clinically severe TRALI reactions.8-10 Conversely, TRALI can occur in the absence of any identifiable antibodies in the transfusion product.11,12 Because of this, two principle theories have evolved to explain TRALI pathogenesis. The first is that anti-leukocyte antibodies stimulate pulmonary neutrophils to produce reactive oxygen species (ROS), which subsequently damage the endothelium of the pulmonary vessels.6,7,9-14 Second, it has been postulated that, independently of antibody, the storage of blood leads to increases in vasoactive biologic response modifiers that either directly increase pulmonary vascular permeability or interact with leukocytes to instigate the release of mediators that result in TRALI.13,14 Both theories are part of a two-hit mechanism of TRALI induction in which the first hit is the transfusion recipient’s morbidity and the second is the presence of either antibody and/or bioactive markers.6
Several animal models of human TRALI have been developed such as ex vivo lung models, which have shown the importance of lung damage associated with human antibodies, and in vivo models, in which biological response modifiers such as platelet-derived CD40L and oxidized lipids are used to induce TRALI in the recipient animal and antibody-mediated models.15-22
In 2006, an in vivo murine model of antibody-mediated TRALI that demonstrated similarities with human TRALI induction was developed. Transfusion of the anti–major histocompatibility complex (MHC) class 1 monoclonal antibody against H2Kd (34.1.2s) into healthy BALB/c mice expressing the cognate antigen resulted in acute lung injury with increased lung epithelial and vascular permeability, excess water on the lung, and a 50% mortality within 2 hours.20 It was established that TRALI induction was due to Fcγ receptor (FcγR)-mediated activation of neutrophil ROS production, and it was subsequently shown that the recipient’s platelets may also have a role in antibody-mediated TRALI.21,23,24 However, more recent work by Strait et al have shown that 34.1.2s, which may interact with monocytes or endothelial cells rather than neutrophils, and the C5a fragment of complement, rather than FcγR,25 are the critical factors of antibody-mediated TRALI induction. These apparently contradictory findings suggest that there are perhaps multiple immune pathways of antibody-mediated TRALI induction. Nonetheless, the contrasting results suggest that both the antigen-binding region and the Fc domain of TRALI-inducing antibodies are required for full TRALI induction.
To better understand how 34.1.2s mediates TRALI on its own, we prepared F(ab′)2 and Fc fragments of the antibody, and we report that although the F(ab′)2 portion can affect chemokine responses and pulmonary neutrophil accumulation, only the intact form of the antibody can stimulate full induction of TRALI. It appears that the first essential step occurs when antibody binds to its cognate antigen on peripheral blood monocytes, and this gives rise to significant chemokine (macrophage inflammatory protein 2 [MIP-2]) production and the accumulation of pulmonary neutrophils. The second step, however, is dependent on the Fc portion of the antibody, which ultimately induces significant lung damage. Thus, targeting peripheral blood monocytes and their chemokine production may be an effective way to alleviate TRALI.
Methods
Mice
Male CB.17 (H-2d, CB17/Icr-Prkdcscid/IcrCrl) severe combined immunodeficient (SCID) mice, 6 to 12 weeks of age, were obtained from Charles River Laboratories (Montreal, QC, Canada). All animal studies were approved by the St. Michael’s Hospital Animal Care Committee. SCID mice were tested for serum levels of immunoglobulin G (IgG) by a murine IgG enzyme-linked immunosorbent assay (ELISA) (Cedarlane Laboratories, Mississauga, ON, Canada). Any mouse found to be “leaky” (serum IgG levels >30 µg/mL) was excluded from the study.
34.1.2s Antibody
The hybridoma 34.1.2s (ATCC, Manassas, VA) produces an IgG2a(κ) monoclonal antibody against MHC class I H-2Kd and H-2Dd.26 Cells were grown in Protein Free Hybridoma Medium II (PFHM II; Invitrogen, Burlington, ON, Canada) at 37°C and 5% CO2 in CELLine flasks (BD Biosciences, Burlington, MA). The antibody in the cell supernatants was purified by Protein G adsorption (Thermo Scientific Pierce, Rockford, IL).
F(ab′)2 and Fc fragment preparation
F(ab′)2 and Fc fragments were generated according to the Pierce F(ab′)2 Preparation Kit (Thermo Scientific, catalog #44988) and the Pierce Fab Preparation Kits (Thermo Scientific, catalog #44985), respectively. Briefly, 0.5 mL of 9.96 mg/mL 34.1.2s was added to immobilized pepsin or papain and incubated at 37°C with constant mixing to digest the antibody into the corresponding F(ab′)2 and Fc fragments. After digestion, the digested antibody was washed in 1× phosphate-buffered saline (PBS) and separated from the column by centrifugation. The digested antibody was then passed through a Protein A column, and the runoff was collected and centrifuged with a 100-kDa Centrifugal Filter tube (Millipore) to separate the F(ab′)2 fragments, Fc fragments, and intact 34.1.2s molecules to further purify the samples. Protein concentration was determined by using a Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc. Hercules, CA). Purity of the Fc and F(ab′)2 fragments was found to be greater than 95% by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
34.1.2s (antibody-mediated) TRALI model
The TRALI model was performed as previously described.22 SCID mice were prebled, weighed, and administered either an intravenous (100 μL) tail-vein injection of (50 µg total) intact 34.1.2s antibody (2 mg/kg) or the molar equivalents of the antibody’s F(ab′)2 fragments, Fc fragments, or both F(ab′)2 and Fc fragments together with the same molar equivalents as the single-agent doses. After the antibody or antibody fragment infusions, physiological measurements, including core rectal temperature, pulmonary edema, neutrophil chemokine (MIP-2) levels, pulmonary neutrophil accumulation, and survival were taken. A depiction of the expected outcomes of the various treatments is provided in supplemental Table 1, available at the Blood Web site. Previous work has described a “first hit” model using 34.1.2s in conjunction with low-dose lipopolysaccharide (LPS), to mimic infection.21,23,24,27 However, both 34.1.2s alone and LPS alone are able to induce lung damage, MIP-2 production, and pulmonary neutrophil recruitment20,22,28,29 and therefore would confound each other’s contribution to TRALI induction.
Flow cytometry
The in vitro and in vivo binding of 34.1.2s to spleen cells and monocytes was determined by flow cytometry. Briefly, to determine the ability of 34.1.2s to bind circulating leukocytes in vivo, SCID mice either were not treated or were treated with 50 μg of either 34.1.2s or control IgG, and 2 hours after administration, they were euthanized and their spleen cells were stained with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Fc specific; Cedarlane Laboratories). To confirm that 34.1.2s binds to monocytes, spleen cells from either naive or 34.1.2s–treated SCID mice were stained with phycoerythrin-conjugated anti-F4/80 (Cedarlane Laboratories) and then 34.1.2s and an FITC-conjugated goat anti-mouse IgG (Fc specific; Cedarlane Laboratories), and FITC fluorescence was detected in an F4/80 gate of the spleen cells.
In vivo gadolinium chloride depletion of peripheral monocytes
To deplete peripheral monocytes, a 100-µL volume of gadolinium chloride (GdCl3) (Sigma-Aldrich, Oakville, ON, Canada; catalog #439770-5G) at 45 mg/kg was injected intravenously via the tail vein and intraperitoneally into SCID mice 24 hours prior to the induction of TRALI. Monocyte depletion was measured in the peripheral blood and lung by flow cytometry, and the procedure resulted in a >98% depletion of peripheral CD14+F4/80+ monocytes but no depletion of pulmonary monocytes (supplemental Figure 1).
Monocyte sorting and repletion into GdCl3-depleted SCID mice
To determine whether purified monocytes could restore TRALI induction in GdCl3-depleted mice, spleen cells from BALB/c mice were first sorted by using FITC/CD14 and phycoerythrin/F4/80 selection and a BD Canto cell sorter (BD, Mississauga, ON, Canada). The sorted monocytes were counted, and 3.5 × 105 cells were injected intravenously into SCID mice pretreated with GdCl3 24 hours earlier. Immediately after monocyte injection, mice were injected with 50 µg 34.1.2s, as described earlier.
Body temperature measurements
Body temperatures indicative of systemic shock were measured at 30-minute intervals post infusion with intact 34.1.2s monoclonal antibody (mAb), F(ab′)2, and/or Fc fragment, up to 120 minutes. Measurements were taken by using an RET-3 rectal probe for mice (VWR International, Mississauga, ON, Canada) connected to a Traceable Digital Thermometer (Model 77776-726; Physitemp Instruments, Inc., Clifton, NJ).
Wet:dry lung weight ratio
Wet:dry lung weight ratios, used as a measure of pulmonary edema, were determined as described previously.22 At 2 hours post infusion with intact 34.1.2s mAb, F(ab′)2, and/or Fc fragments, mice were anesthetized with Avertin (2% final in PBS, intraperitoneally), and the chest cavity was exposed. The left lung of each mouse was removed, weighed to determine the wet weight, dried in an oven for ≥48 hours at 60°C, and re-weighed to obtain the dry weight. The wet:dry weight ratio was subsequently determined as the net wet weight/net dry weight.
MIP-2 measurements and in vivo inhibition
Serum MIP-2 levels were measured as previously described.28 Two hours after the infusion of 34.1.2s mAb, F(ab′)2, and/or Fc fragments, blood was collected from mice, and sera were obtained on ice to measure the levels of the CXCL2 chemokine. Sera were stored at −86°C and analyzed in batch. An ultrasensitive commercial solid-phase ELISA kit (mouse CXCL2/MIP-2 Quantikine ELISA Kit; R&D Systems, Minneapolis, MN) was used to measure the serum levels of MIP-2. The sensitivity of the MIP-2 assay was >1.5 pg/mL.
To suppress the activity of MIP-2 in vivo, mice were injected intraperitoneally 2 hours prior to TRALI induction with 1 mg of the hexapeptide antileukinate (Ac-RRWWCR-NH; AnaSpec, Fremont, CA), a potent inhibitor of MIP-2 binding to CXCR1 and CXCR2.29 The peptide has been shown to inhibit bleomycin-induced acute lung injury by suppressing neutrophil mobilization and activation.31
Pulmonary neutrophil accumulation
The percentage of pulmonary neutrophils was determined as described previously.22 Briefly, mice were anesthetized with Avertin (2% final in PBS, intraperitoneally), and the chest cavity was exposed. Next, the right lung was removed, homogenized, and filtered through a 40-µm cell strainer (BD Biosciences, Bedford, MA) with PBS. The cells were then centrifuged for 7 minutes at 400g. The supernatant was removed, and the cells were resuspended. Red blood cells were removed by the addition of ammonium chloride/potassium carbonate lysis solution (0.15 M NH4Cl, 10 mM KHCO3, Na2EDTA, pH 7.2 to 7.4) for 2 minutes, and then cold PBS was added prior to further centrifugation. The supernatant was again removed, and the cells were resuspended in 500 µL PBS. Cells were then mounted on microscope slides by using a Shandon Cytospin 4 (ThermoFisher Scientific, Nepean, ON, Canada) and stained by using a hematoxylin and eosin kit (Harleco–Hemacolor; EMD Chemicals, Inc., Darmstadt, Germany). The slides were then examined by light microscopy (Olympus Canada, Markham, ON, Canada), with a ×40 objective. Polymorphonuclear cells were determined by counting the total number of nucleated cells in four fields of view, and neutrophils were enumerated by using ImageJ software to mark cells. The percentage of neutrophils was determined by using the following formula: total number of neutrophils/total number of nucleated cells × 100. Typical cytospins of pulmonary homogenates are shown in supplemental Figure 2.
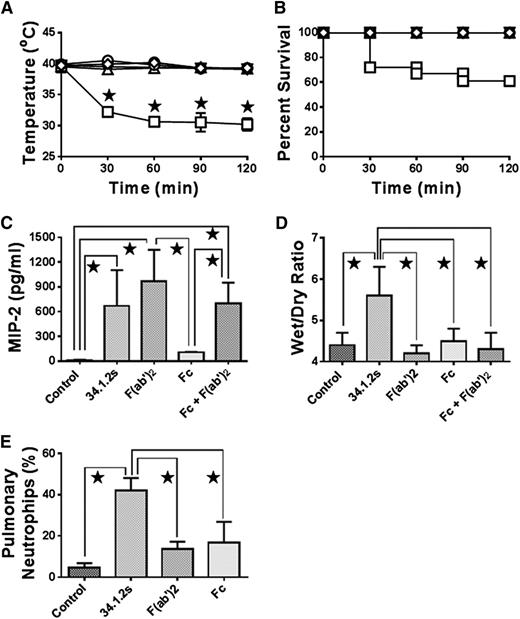
F(ab′)2 fragments of 34.1.2s induce MIP-2 production and neutrophil accumulation, but not hypothermia, edema, or mortality in SCID mice. Time course of (A) rectal temperatures and (B) survival of SCID mice either not injected (○) or injected with 50 µg 34.1.2s (□), 75 µg F(ab′)2 fragments (♢), or 75 µg Fc fragments (△). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± SD of the indicated parameters (y-axes) in A and C-E and as means in B (n = 6 to 21 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA. All other comparisons were not significant except for control vs Fc (C, P < .05) and control vs F(ab′)2 and Fc (E, P < .05).
F(ab′)2 fragments of 34.1.2s induce MIP-2 production and neutrophil accumulation, but not hypothermia, edema, or mortality in SCID mice. Time course of (A) rectal temperatures and (B) survival of SCID mice either not injected (○) or injected with 50 µg 34.1.2s (□), 75 µg F(ab′)2 fragments (♢), or 75 µg Fc fragments (△). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± SD of the indicated parameters (y-axes) in A and C-E and as means in B (n = 6 to 21 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA. All other comparisons were not significant except for control vs Fc (C, P < .05) and control vs F(ab′)2 and Fc (E, P < .05).
Statistical analysis
Data are expressed as mean ± standard deviation. Data were analyzed with one-way analysis of variance, comparing the mean of each value with the mean of the control value. Significance was determined as a P value <.05.
Results
34.1.2s binds splenic monocytes in vitro and in vivo
To determine the binding of 34.1.2s to monocytes, either naive mouse spleen cells or spleen cells from 34.1.2s–treated mice were analyzed for antibody binding. The 34.1.2s antibody bound F4/80-positive monocytes in vitro with high affinity, and significant 34.1.2s binding to spleen cells was observed 2 hours after infusion of 34.1.2s compared with mice infused with control IgG (supplemental Figure 3).
34.1.2s, F(ab′)2, and Fc fragments do not induce hypothermia or mortality in SCID mice
Rectal temperatures of mice were measured as an indication of systemic shock after administration of 34.1.2s, F(ab′)2, and Fc fragments. Compared with mice injected with the intact antibody, which resulted in a significant and sustained drop in body temperature, SCID mice injected with F(ab′)2 or Fc fragments showed no drop in rectal temperature at any of the experimental time points (Figure 1A). In addition, compared with SCID mice injected with intact 34.1.2s, which caused 40% mortality, SCID mice injected with F(ab′)2 or Fc fragments of 34.1.2s showed no mortality (Figure 1B). Similar results were observed if both Fc and F(ab′)2 fragments were injected together at equimolar concentrations (Figure 1A-B).
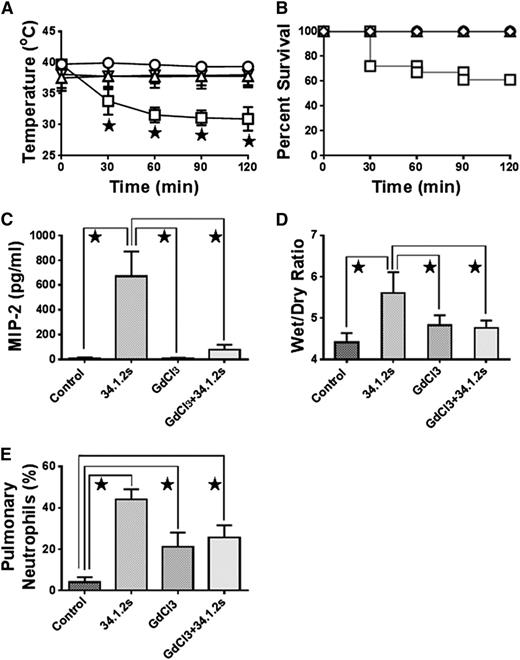
Monocyte depletion with GdCl3 treatment abrogates TRALI in SCID mice. Time course of (A) rectal temperatures and (B) survival of SCID mice either not injected (control, ○) or injected with 50 µg 34.1.2s (□), 90 mg/kg GdCl3 (△), or 90 mg/kg GdCl3 plus 50 µg 34.1.2s (▿). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± standard deviation (SD) of the indicated parameters (y-axes) in A and C-E and as means in B (n = 6 to 11 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way analysis of variance (ANOVA). All other comparisons were not significant except for control vs GdCl3 + 34.1.2s (C, P < .05), and control vs GdCl3 and GdCl3 + 34.1.2s (E, P < .05).
Monocyte depletion with GdCl3 treatment abrogates TRALI in SCID mice. Time course of (A) rectal temperatures and (B) survival of SCID mice either not injected (control, ○) or injected with 50 µg 34.1.2s (□), 90 mg/kg GdCl3 (△), or 90 mg/kg GdCl3 plus 50 µg 34.1.2s (▿). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± standard deviation (SD) of the indicated parameters (y-axes) in A and C-E and as means in B (n = 6 to 11 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way analysis of variance (ANOVA). All other comparisons were not significant except for control vs GdCl3 + 34.1.2s (C, P < .05), and control vs GdCl3 and GdCl3 + 34.1.2s (E, P < .05).
Intact 34.1.2s and its F(ab′)2 fragment induced significant in vivo MIP-2 production and neutrophil accumulation
Production of MIP-2, the murine equivalent of human interleukin-8, was measured in the sera of antibody- and/or fragment-treated mice. Compared with naive SCID mice, those treated with either intact antibody, F(ab′)2 fragments, or F(ab′)2 fragments mixed with Fc fragments had significantly elevated serum levels of MIP-2 (Figure 1C). In contrast, Fc fragments alone did not induce any MIP-2 production (Figure 1C). SCID mice treated with F(ab′)2 fragments or Fc fragments showed elevated pulmonary neutrophil accumulation compared with control mice, although this accumulation was not as considerable as that in intact 34.1.2s–treated mice (Figure 1E).
34.1.2s F(ab′)2 and Fc fragments do not induce lung injury in SCID mice
Measurements of postmortem wet:dry lung weight ratios were used to determine extravascular pulmonary fluid, which was used as an indicator of pulmonary edema. Pulmonary wet:dry lung weight ratios of SCID mice infused with intact 34.1.2s were found to be significantly higher than the values found for control mice (Figure 1D). In contrast, mice infused with F(ab′)2 fragments, Fc fragments, or mixtures of F(ab′)2 and Fc fragments had wet:dry lung weight values that were comparable to control values (Figure 1D).
GdCl3 treatment protects mice from 34.1.2s-mediated TRALI
We analyzed whether the depletion of peripheral monocytes with GdCl3 would attenuate intact antibody–mediated TRALI. Compared with control mice, mice treated with GdCl3 showed attenuated ability of intact 34.1.2s to decrease rectal temperatures (Figure 2A), increase wet:dry lung weight ratios (Figure 2D), and induce mortality (Figure 2B). In addition, GdCl3 treatment significantly reduced the ability of intact 34.1.2s to raise serum MIP-2 levels (Figure 2C); similar MIP-2 results were observed with F(ab′)2 fragments of 34.1.2s (not shown). Pulmonary neutrophil accumulation was also abrogated when compared with that in controls (Figure 2E). The ability of 34.1.2s to cause TRALI is independent of complement, because DBA mice deficient in C5 still developed TRALI when challenged with 34.1.2s (J.W.S., unpublished data, July 2010).
Blockade of MIP-2 CXC receptors inhibits 34.1.2s-mediated TRALI
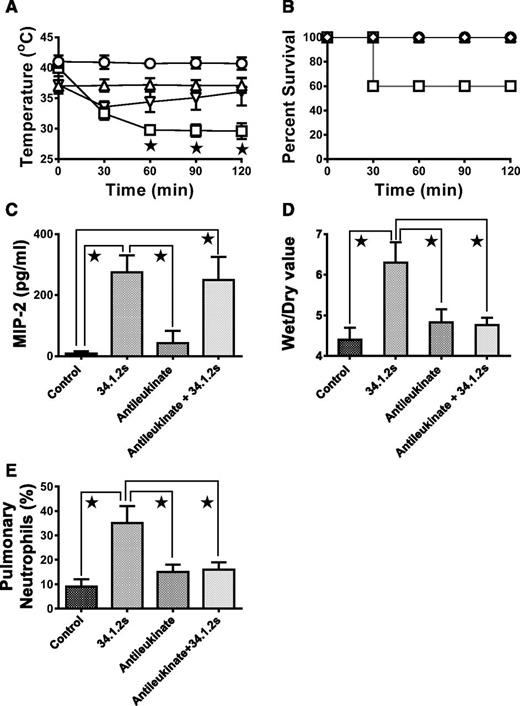
We next determined whether in vivo MIP-2 chemokine production by peripheral blood monocytes was required for antibody-mediated TRALI by blocking its CXCR with antileukinate peptide. Compared with control mice, mice treated in vivo with antileukinate showed that the treatment rescued the 34.1.2s-reduced rectal temperatures (Figure 3A) and prevented mortality (Figure 3B). Treatment with the CXCR13 antagonist did not affect MIP-2 production (Figure 3C) but significantly reduced the ability of 34.1.2s to induce lung damage (Figure 3D). Pulmonary neutrophil accumulation was also abrogated by antileukinate treatment when compared with control mice (Figure 3E).
Intraperitoneal administration of antileukinate abrogates TRALI in SCID mice. (A) Rectal temperatures and (B) survival time courses of SCID mice either not injected (control, ○) or injected with 50 µg 34.1.2s (□), 1 mg antileukinate (△), or 1 mg antileukinate + 50 µg 34.1.2s (▿). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± SD of the indicated parameters (y-axes) in A and C-E and as means in panel B (n = 6 to 11 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA. All other comparisons were not significant except for control vs antileukinate and antileukinate + 34.1.2s (E, P < .05).
Intraperitoneal administration of antileukinate abrogates TRALI in SCID mice. (A) Rectal temperatures and (B) survival time courses of SCID mice either not injected (control, ○) or injected with 50 µg 34.1.2s (□), 1 mg antileukinate (△), or 1 mg antileukinate + 50 µg 34.1.2s (▿). Two hours after injection of the indicated proteins, the mice were euthanized, (C) serum MIP-2 levels were measured, (D) wet:dry lung weight measurements were made, and (E) the percentage of pulmonary neutrophils was measured. Data are expressed as means ± SD of the indicated parameters (y-axes) in A and C-E and as means in panel B (n = 6 to 11 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA. All other comparisons were not significant except for control vs antileukinate and antileukinate + 34.1.2s (E, P < .05).
Repletion of monocytes into GdCl3-treated SCID mice restores 34.1.2s-mediated TRALI
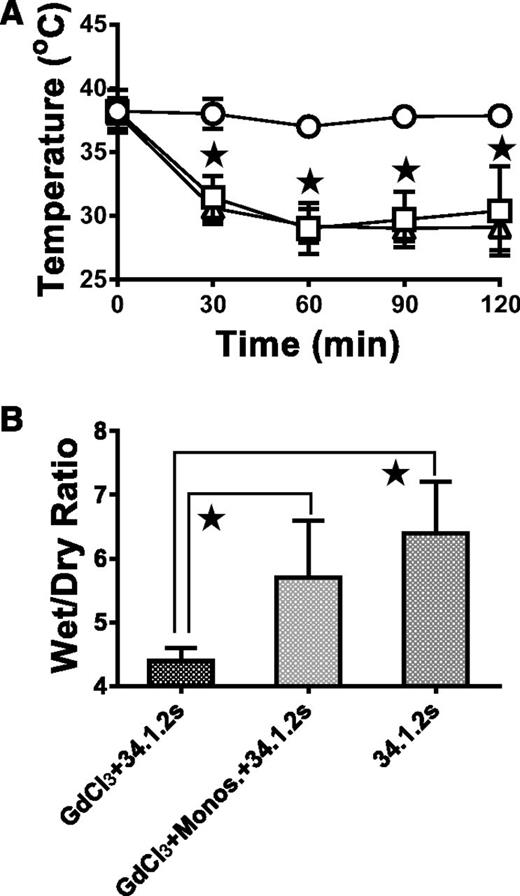
After we depleted peripheral monocytes with GdCl3, the next thing we set out to establish was whether repletion of monocytes prior to 34.1.2s injection would restore antibody-mediated TRALI. Compared with mice treated with GdCl3 24 hours before 34.1.2s injection, mice treated with GdCl3 and repleted with 3.5 × 105 F4/80-purified monocytes prior to 34.1.2s injection had significantly lower rectal temperatures (Figure 4A) and significantly higher wet:dry lung weight ratios (Figure 4B).
Monocyte repletion after GdCl3 treatment restores TRALI in SCID mice. (A) Time course of rectal temperatures in naive SCID mice (○) or in SCID mice injected with 50 µg 34.1.2s (□) or in GdCl3-treated SCID mice (△) repleted with 3.5 × 105 purified monocytes. (B) Wet:dry lung weight ratios of lungs from GdCl3-treated SCID mice 2 hours after injection with either 50 μg of 34.1.2s (first column) or 2 hours after repletion with 3.5 × 105 purified monocytes and injection with 34.1.2s (second column) compared with those of SCID mice 2 hours after injection with only 34.1.2s (third column). Data in both panels are expressed as means ± SD of the indicated parameters (y-axes) (n = 5 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA.
Monocyte repletion after GdCl3 treatment restores TRALI in SCID mice. (A) Time course of rectal temperatures in naive SCID mice (○) or in SCID mice injected with 50 µg 34.1.2s (□) or in GdCl3-treated SCID mice (△) repleted with 3.5 × 105 purified monocytes. (B) Wet:dry lung weight ratios of lungs from GdCl3-treated SCID mice 2 hours after injection with either 50 μg of 34.1.2s (first column) or 2 hours after repletion with 3.5 × 105 purified monocytes and injection with 34.1.2s (second column) compared with those of SCID mice 2 hours after injection with only 34.1.2s (third column). Data in both panels are expressed as means ± SD of the indicated parameters (y-axes) (n = 5 mice in each group). The ★ indicates significance (P < .05) between mouse groups and was determined by using one-way ANOVA.
Discussion
It was postulated that the mechanism of lung damage in murine antibody–mediated TRALI was dependent upon neutrophils and their FcγRs.20 Additionally, it was demonstrated that not only neutrophils but also platelets could be sequestered in the lungs of mice, and depletion of either neutrophils or platelets was protective.21,23,24 Moreover, it was suggested that activated platelets induced the development of neutrophil extracellular traps in both human and murine TRALI.23,24,27 We have shown, in addition to the role neutrophils play in TRALI, that the presence of recipient CD8+ and CD4+ T cells is capable of significantly reducing the severity of TRALI reactions and that prophylactic treatment with intravenous immunoglobulin or treatment with intravenous immunoglobulin following the onset of symptoms was able to abrogate and reduce TRALI symptoms at the level of neutrophil ROS production.22,28 More recently, however, evidence suggested that the induction of this disease involves macrophages via C5a stimulation rather than neutrophils and their FcRs.25 It was also shown by Strait et al25 that depletion of neutrophils and platelets in BALB/c mice was unable to attenuate 34.1.2s-mediated TRALI. Therefore, we set out to determine what role the components of the 34.1.2s IgG molecule play in the instigation of TRALI and to ascertain whether monocytes or neutrophils are the predominant cell type involved in TRALI induction. Our results show that the intact 34.1.2s IgG molecule was required for full TRALI induction; however, it appears that the antibody binding to its cognate antigen was responsible for a MIP-2 chemokine response. Looney et al20 previously hypothesized that neutrophils are sequestered in the lung via Fc interactions with MHC class I antibodies bound to endothelial cells. Our results, however, suggest that the source of the MIP-2 was peripheral blood monocytes and that both were essential for the initiation of TRALI. Depletion of peripheral monocytes significantly reduced MIP-2 levels and pulmonary neutrophil sequestration, which suggests that the source of MIP-2 production and neutrophil activation is from circulating monocytes as opposed to other cell types such as pulmonary endothelium. The results suggest that TRALI-inducing anti-MHC antibodies first encounter circulating monocytes after infusion leading to their production of MIP-2; these events alone were partially responsible for subsequent accumulation of pulmonary neutrophils, and these events occur relatively early. Previous work by our group has also shown that the production of MIP-2 occurs within 30 minutes of 34.1.2s infusion into SCID mice.22
Neither the F(ab′)2 nor the Fc fragments of 34.1.2s on their own could induce TRALI symptoms (Figure 1). This was also true if both F(ab′)2 and Fc fragments were transfused together (Figure 2C-D). The only observable response against the fragments was that, like intact antibody, F(ab′)2 fragments induced production of MIP-2 (Figure 2C). MIP-2 is a 6-kD CXCL chemokine and is the murine equivalent of human interleukin-8.32 It is primarily produced by monocytes and/or macrophages subsequent to their stimulation with, for example, bacterial endotoxins.31,33 MIP-2 is extremely chemotactic for neutrophils and has been shown to induce neutrophil degranulation but does not enhance the production of ROS.32,34 Our data support this latter contention with intact 34.1.2s; its binding to its cognate antigen on monocytes caused a strong production of MIP-2 that correlated with significant pulmonary neutrophil accumulation (supplemental Figure 3). Of interest, the 34.1.2s F(ab′)2 fragments also caused significant MIP-2 stimulation, but pulmonary neutrophil accumulation was significantly less than that produced by intact antibody, indicating that the Fc domain of the antibody may also be responsible for some of the pulmonary neutrophil chemotaxis (Figure 2). Furthermore, even though the F(ab′)2 portion was still capable of stimulating MIP-2 production, the added infusion of Fc fragments into SCID mice failed to induce TRALI. Nonetheless, early MIP-2 production was essential for TRALI induction because the CXCL receptor antagonist antileukinate totally blocked TRALI induction (Figure 3C). Taken together, the data suggest that an early event of anti-MHC antibody-mediated TRALI induction is the antibody’s ability to bind to its cognate antigen that is responsible for early but significant chemokine production and at least partially responsible for pulmonary neutrophil accumulation. All the subsequent events leading to full TRALI induction were dependent on the Fc domain of the intact antibody.
Previous work has implicated macrophages in the induction of anti-MHC–mediated TRALI,25 and depletion of alveolar macrophages has previously been shown to arrest different forms of lung injury.35-42 GdCl3, a rare earth metal lanthanide, has previously been shown to be taken up exclusively by phagocytic mononuclear cells, and once inside the phagocytic cell, the metal induces apoptosis.36 Intravenous administration of GdCl3 in a rat model has been shown to significantly attenuate pulmonary water content, neutrophil infiltration, and pulmonary apoptosis in an LPS-induced acute lung injury.41 In addition, depletion of pulmonary macrophages by intratracheal GdCl3 administration significantly reduced levels of pulmonary intravascular macrophages and inhibited lung inflammation.42 Our results, however, show that only peripheral blood monocytes were significantly depleted with intravenous and intraperitoneal administration of GdCl3, leaving alveolar macrophages intact (supplemental Figure 1). Nonetheless, GdCl3 administration caused a significant reduction in the serum levels of MIP-2 after administration of 34.1.2s, suggesting that peripheral monocytes were the primary cell type required for the early MIP-2 chemokine response and the instigation of TRALI. Additionally, the depletion of peripheral monocytes completely prevented hypothermia and pulmonary edema induced in 34.1.2s-treated control SCID mice, resulting in complete survival (Figure 1A-B). This correlated with reduced levels of pulmonary neutrophils induced by 34.1.2s, suggesting that peripheral monocytes and not alveolar macrophages are the critical cell type in initiating antibody-induced TRALI. Additionally, the repletion of F4/80 sorted monocytes into GdCl3-treated mice restored the ability of 34.1.2s to reduce rectal temperatures (Figure 4A) and significantly increase pulmonary edema (Figure 4B), and this was comparable to values seen in mice treated with 34.1.2s alone. These findings further support the hypothesis that peripheral monocytes are crucial in the initiation of antibody-induced TRALI. Although the exact site of the initiation of TRALI is currently unidentified, the mechanism of TRALI induction may be related to the antibody-bound monocytes migrating from the peripheral circulation recruiting neutrophils to the lung and subsequently causing lung damage in the pulmonary vascular beds.
Our work with 34.1.2s may not explain how TRALI occurs with the infusion of granulocyte-specific antibodies. Monocytes do not contain HNA antigens, and there are no equivalent HNA-1 or -2 models available; however, recent studies have suggested that anti-HNA-3 antibodies cause fatal TRALI more frequently than other anti-neutrophil antibodies.8,43 Additionally, a recent study has shown that human anti-HNA-3a induces TRALI in a murine model.44 This study, however, focuses on TRALI associated with anti-MHC antibodies and how these antibodies may interact with recipient cells. Whether the results presented here are related to anti-HNA antibodies is unknown at present.
In summary, we propose a 2-step mechanism that is responsible for anti-MHC– mediated TRALI. The first step in this model is the interaction of peripheral monocytes with 34.1.2s via its cognate MHC class I antigens, resulting in the release of the neutrophil chemoattractant MIP-2. The second step is the ability of the antibody-bound monocytes to somehow influence the full activation of and damage to the pulmonary endothelium. These results suggest that in combination with previously published reports,15,21 both peripheral monocytes and the CXCL chemokine MIP-2 play critical roles in the initiation and propagation of antibody-mediated TRALI, and that modulating these early events could be considered a potential therapeutic target to minimize or abrogate the TRALI.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christopher Spring for assisting in the purification of monocytes.
This work was supported by a Health Canada/Canadian Blood Services Priority Research Grant. C.G.J.M. is the recipient of a postdoctoral fellowship from Canadian Blood Services.
Authorship
Contribution: C.G.J.M. designed research, performed all experiments, collected, analyzed and interpreted data, performed statistical analysis, and wrote the first draft of the manuscript; M.K., T.K.S., and Y.M. performed experiments and collected, analyzed, and interpreted data; J.F. designed research, analyzed and interpreted data, and edited the manuscript; and J.W.S. provided financial resources, designed research, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St. Michael’s Hospital, 30 Bond Street, Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.ca.