Key Points
JAK2V617F homozygosity drives a phenotypic switch between myeloproliferative neoplasms.
JAK2V617F homozygosity is insufficient to sustain clonal expansion.
Abstract
Genomic regions of acquired uniparental disomy (UPD) are common in malignancy and frequently harbor mutated oncogenes. Homozygosity for such gain-of-function mutations is thought to modulate tumor phenotype, but direct evidence has been elusive. Polycythemia vera (PV) and essential thrombocythemia (ET), 2 subtypes of myeloproliferative neoplasms, are associated with an identical acquired JAK2V617F mutation but the mechanisms responsible for distinct clinical phenotypes remain unclear. We provide direct genetic evidence and demonstrate that homozygosity for human JAK2V617F in knock-in mice results in a striking phenotypic switch from an ET-like to PV-like phenotype. The resultant erythrocytosis is driven by increased numbers of early erythroid progenitors and enhanced erythroblast proliferation, whereas reduced platelet numbers are associated with impaired platelet survival. JAK2V617F-homozygous mice developed a severe hematopoietic stem cell defect, suggesting that additional lesions are needed to sustain clonal expansion. Together, our results indicate that UPD for 9p plays a causal role in the PV phenotype in patients as a consequence of JAK2V617F homozygosity. The generation of a JAK2V617F allelic series of mice with a dose-dependent effect on hematopoiesis provides a powerful model for studying the consequences of mutant JAK2 homozygosity.
Introduction
Genome-wide single nucleotide polymorphism analyses have revealed the presence of multiple regions of acquired loss of heterozygosity in many tumor types.1 Some loss-of-heterozygosity regions contain oncogenic gain-of-function mutations and are copy number neutral uniparental disomy (UPD) as a result of mitotic recombination (eg, JAK2,2-6 FLT3,7,8 MPL9 ). Homozygosity for such oncogenes may result in distinct tumorigenic consequences, but direct evidence has been elusive.
Myeloproliferative neoplasms (MPNs) arise in the hematopoietic stem cell (HSC) compartment and include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis.10 An acquired JAK2V617F mutation is found in >95% of PV patients and in approximately 60% of those with ET or primary myelofibrosis,3-6 but the mechanisms responsible for different clinical phenotypes remain unclear.
JAK2V617F homozygosity as a consequence of UPD was found in granulocytes from approximately 30% of PV patients but was rare in patients with ET.3-6 Because PV is distinguished mainly by the presence of marked erythrocytosis, and JAK2 mediates erythropoietin (EPO) signaling, acquired homozygosity may contribute to the phenotypic differences. Theories that assert a simple causal role of JAK2V617F homozygosity in driving the PV phenotypes are complicated by a number of observations. Some PV patients have small or undetectable homozygous clones and approximately 50% of ET patients harbor small homozygous clones,11 indicating that homozygosity is neither necessary nor sufficient for a PV phenotype. Moreover, heterozygous mutant erythroblasts from patients with PV and ET have distinct transcriptional profiles that precede acquisition of homozygosity and reflect differential activation of phosphorylated signal transducer and activator of transcription (STAT)1, which is known to modulate erythropoiesis and megakaryopoiesis.12
To study JAK2V617F at physiological levels in blood cells, several groups have developed knock-in models that demonstrate that a heterozygous JAK2V617F mutation is sufficient to produce myeloproliferation with varying degrees of erythrocytosis and thrombocytosis.13-17 One group generated mice that express homozygous mutant mouse Jak2 in all hematopoietic cells; these mice developed higher platelet counts but hemoglobin (Hb) levels were unchanged or reduced relative to heterozygous mutant mice.14
In this article, we demonstrate that acquisition of JAK2V617F homozygosity results in a switch from an ET-like to PV-like phenotype with the development of a profound and transplantable erythrocytosis accompanied by reduced platelet survival. Our results also indicate that additional genetic lesions are required to produce a clonal advantage at the level of the HSC.
Methods
Generation of mice with homozygous JAK2V617F expression
To generate mice with homozygous JAK2V617F, we adopted the strategy outlined in supplemental Figure 1A, which is further described in the supplemental Methods on the Blood Web site.
Blood and tissue histological analysis
Peripheral blood was collected into EDTA-coated tubes and automated blood cell counts were performed using a Woodley ABC blood counter (Woodley, United Kingdom [UK]). For histological analysis, specimens were collected from 6- to 8-week-old mice into 4% buffered formaldehyde (CellPath PLC, UK). Mice were kept in specific pathogen-free conditions and all procedures were performed according to the UK Home Office regulations. For details on individual staining, see the supplemental Methods.
BM transplantation
When donor mice were 129SvEvBrd/C57Bl/6 (CD45.2+) background, recipients were 129SvEvBrd/C57Bl/6 (CD45.1+CD45.2+) F1 mice generated by crossing C57Bl/6 (CD45.1) mice with 129SvEvBrd mice (CD45.2). Where donor mice had been crossed onto a C57Bl/6 (CD45.2) background, C57Bl/6 (CD45.1) mice were used as recipients. All recipients were lethally irradiated with a split dose of 1100 cGY, separated by >4 hours. Donor cells were from either JAK2R/R, JAK2R/+, or JAK2+/+ 6- to 8-week-old littermates. For noncompetitive transplantation, 2 × 106 bone marrow (BM) cells were injected into recipient mice. Peripheral blood was obtained for full blood and donor chimerism analysis by flow cytometry each month for up to 4 months posttransplantation. For competitive transplantation, donor BM cells from JAK2R/R, JAK2R/+, or JAK2+/+ littermates were mixed at a 1:1 ratio with wild-type (WT) competitor BM cells. A total of 1 × 106 BM cells containing both donor and competitor cells were injected into recipient animals. For purified HSC transplantations, 50 E-SLAM HSCs of each genotype were sorted into a single tube and distributed across 5 recipient mice (ie, 10 HSCs per mouse). For transplantation analysis details, see the supplemental Methods.
Statistics
The statistical significance was determined by a 2-tailed, unpaired Student t test unless indicated in the figure legends.
Results
JAK2V617F homozygosity drives a switch from an ET-like to a PV-like phenotype
We previously described a knock-in mouse in which human JAK2V617F was conditionally expressed under control of the endogenous Jak2 locus.17 Because the floxed allele is null and JAK2 is essential for fetal development, it cannot be bred to homozygosity; therefore, we adopted a strategy using StellaCre mice18 as outlined in supplemental Figure 1A. The resultant JAK2R/+StellaCre/+ offspring have a recombined allele in their germline and were born at a normal Mendelian ratio. The interbreeding of JAK2R/+ mice allowed the generation of cohorts that were WT (JAK2+/+), heterozygous (Het, JAK2R/+), or homozygous (Hom, JAK2R/R) for JAK2V617F as determined by genomic polymerase chain reaction (supplemental Figure 2A). Levels of JAK2V617F expression were comparable (supplemental Figure 2B), and homozygous JAK2V617F was associated with increased STAT5 phosphorylation in the presence or absence of EPO (supplemental Figure 2C), although it remains unclear whether this is due to an increased amount of STAT5 or its increased phosphorylation.
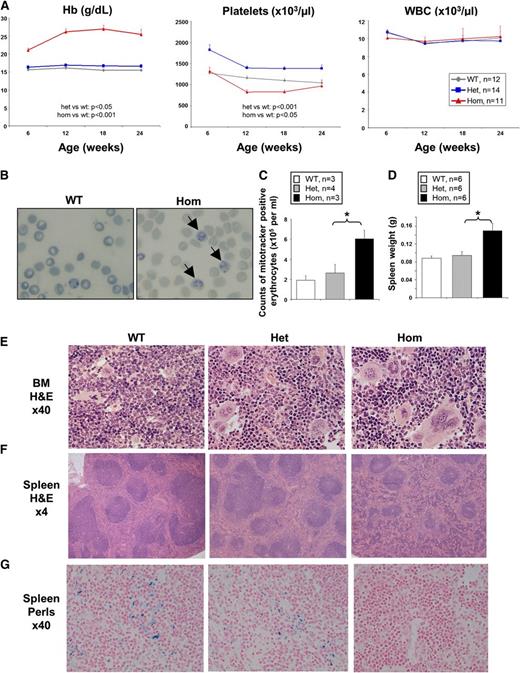
Heterozygous JAK2V617F mice developed a phenotype resembling human ET with raised platelet counts and a small but significant increase in Hb, similar to the phenotype previously described using Mx1-Cre.17 By contrast, homozygous JAK2V617F mice were readily distinguished from littermates by their plethoric feet (supplemental Figure 1B) and exhibited a striking erythrocytosis (average hematocrit 80%-90%, Hb 220-250g/L across multiple cohorts) together with platelet counts that were similar or lower than WT but significantly lower than those of heterozygous mutant mice (Figure 1A; supplemental Figure 3A-B). Overall, homozygous JAK2V617F mice did not show an increase in average numbers of leukocytes, granulocytes, or thrombocytes across all cohorts (Figure 1A and data not shown), but about 10% of the homozygous mice displayed increased leukocytes and thrombocytes over time (supplemental Figure 3C). Homozygous JAK2V617F mice also showed an increased number of reticulocytes (Figure 1B-C; supplemental Figure 4). All homozygous JAK2V617F mice developed splenomegaly by 2 months (Figure 1D; supplemental Figure 5A). BM cellularity was not significantly altered in homozygous JAK2V617F mice compared with heterozygous and WT littermates (supplemental Figure 5B). Histological analysis of BM sections of JAK2R/R mice revealed marked erythrocytic and megakaryocytic hyperplasia with pleomorphic megakaryocytes including abnormally large forms, nuclear hyperlobation, and clustering (Figure 1E). Marked erythroid hyperplasia was also present in the spleen, resulting in red pulp expansion and loss of normal architecture, with occasional megakaryocyte clusters (Figure 1F). By contrast, the BM and spleen of JAK2R/+ mice lacked significant erythroid hyperplasia and showed less megakaryocytic hyperplasia and atypia (Figure 1E-F). Reticulin staining was not increased in BM or spleen from JAK2R/+ or JAK2R/R mice (supplemental Figure 5C) in young animals. However, in older animals, spleens were further enlarged (supplemental Figure 6A) and increased reticulin staining was observed in the BM and spleen of approximately 10% of mice analyzed (supplemental Figure 6B). JAK2R/R spleens also had a reduction of stainable iron compared with JAK2R/+ or WT littermates (Figure 1G), although neither reduced mean corpuscular volume nor microcytoses were observed (data not shown).
Homozygous (Hom) JAK2V617F expression induces severe erythrocytosis in JAK2R/R knock-in mice. (A) JAK2R/R mice show strikingly increased Hb, lower platelet levels (relative to JAK2R/+), but normal white blood cell counts. Peripheral blood was collected from 6- to 8-week-old WT, JAK2R/+, and JAK2R/R mice. Significant differences are indicated and were determined using a linear mixed-effect model to accommodate the longitudinal and grouped data structure. (B) New methylene blue staining of peripheral blood showing increased numbers of reticulocytes (as indicated by arrows) in JAK2R/R mice. (C) Increased number of reticulocytes in JAK2R/R mice compared with WT and JAK2R/+ as shown by increased number of CD71+Mitotracker+ erythrocytes. (D) Spleen size was significantly increased in 8-week-old JAK2R/R mice. Asterisks indicate significant differences as determined by Student t test (*P < .05). Data are shown as mean ± standard error of the mean (SEM). BM (E) and spleen (F) sections stained with hematoxylin and eosin (H&E), examined using ×40 and ×4 objective lenses, respectively. (G) Spleen sections stained with Perls’ stain for iron. Het, heterozygosity.
Homozygous (Hom) JAK2V617F expression induces severe erythrocytosis in JAK2R/R knock-in mice. (A) JAK2R/R mice show strikingly increased Hb, lower platelet levels (relative to JAK2R/+), but normal white blood cell counts. Peripheral blood was collected from 6- to 8-week-old WT, JAK2R/+, and JAK2R/R mice. Significant differences are indicated and were determined using a linear mixed-effect model to accommodate the longitudinal and grouped data structure. (B) New methylene blue staining of peripheral blood showing increased numbers of reticulocytes (as indicated by arrows) in JAK2R/R mice. (C) Increased number of reticulocytes in JAK2R/R mice compared with WT and JAK2R/+ as shown by increased number of CD71+Mitotracker+ erythrocytes. (D) Spleen size was significantly increased in 8-week-old JAK2R/R mice. Asterisks indicate significant differences as determined by Student t test (*P < .05). Data are shown as mean ± standard error of the mean (SEM). BM (E) and spleen (F) sections stained with hematoxylin and eosin (H&E), examined using ×40 and ×4 objective lenses, respectively. (G) Spleen sections stained with Perls’ stain for iron. Het, heterozygosity.
These data provide direct genetic evidence that JAK2V617F homozygosity causes marked erythrocytosis together with platelet counts lower than those seen in heterozygous JAK2V617F mice.
The PV-like phenotype is cell-intrinsic and associated with reduced survival
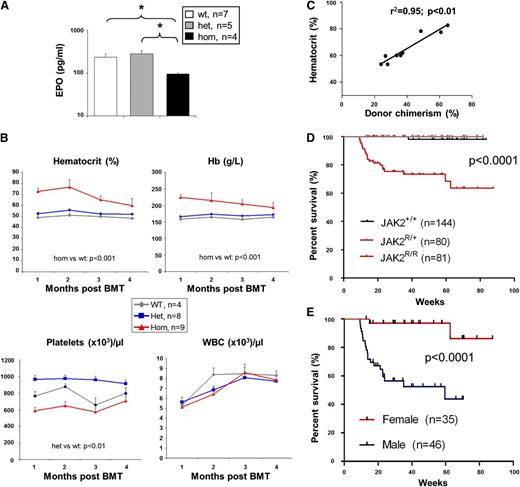
The marked erythrocytosis in JAK2R/R mice might be cell-intrinsic or secondary to increased EPO levels. To distinguish between these possibilities, plasma EPO levels were quantified and found to be significantly reduced in JAK2R/R mice compared with JAK2R/+ or WT littermates (Figure 2A). This observation indicates that the erythrocytosis is cell-intrinsic and is reminiscent of EPO suppression observed in patients with PV.
The PV-like phenotype in JAK2R/R mice is cell-intrinsic and the severity is dependent on homozygous JAK2V617F allele burden. (A) Plasma EPO level was significantly reduced in JAK2R/R mice compared with JAK2R/+ and WT littermates. (B) The PV-like phenotype in JAK2R/R mice is transplantable. BM cells (2 × 106 per recipient) were transplanted into irradiated 129SvEvBrd/C57Bl/6 F1 recipients. Donors were StellaCre−/− background. Time course of blood counts are shown for 1 to 4 months following transplantation. Significant differences are indicated and were determined using a linear mixed-effect model. (C) The severity of the PV-like phenotype corresponds to JAK2R/R donor cell chimerism (ie, homozygous-JAK2V617F allele burden). The graph shows a positive correlation between hematocrit and JAK2R/R donor chimerism at 2 months posttransplantation. The data were analyzed by Spearman rank correlation coefficient test; r2 and P values are indicated. (D) Kaplan-Meier analysis shows that JAK2R/R mice have significantly reduced survival compared with both WT and JAK2R/+ mice. (E) Kaplan-Meier analysis show that male JAK2R/R mice have significantly reduced survival compared with female JAK2R/R mice; P values are indicated. BMT, BM transplantation; WBC, white blood cell.
The PV-like phenotype in JAK2R/R mice is cell-intrinsic and the severity is dependent on homozygous JAK2V617F allele burden. (A) Plasma EPO level was significantly reduced in JAK2R/R mice compared with JAK2R/+ and WT littermates. (B) The PV-like phenotype in JAK2R/R mice is transplantable. BM cells (2 × 106 per recipient) were transplanted into irradiated 129SvEvBrd/C57Bl/6 F1 recipients. Donors were StellaCre−/− background. Time course of blood counts are shown for 1 to 4 months following transplantation. Significant differences are indicated and were determined using a linear mixed-effect model. (C) The severity of the PV-like phenotype corresponds to JAK2R/R donor cell chimerism (ie, homozygous-JAK2V617F allele burden). The graph shows a positive correlation between hematocrit and JAK2R/R donor chimerism at 2 months posttransplantation. The data were analyzed by Spearman rank correlation coefficient test; r2 and P values are indicated. (D) Kaplan-Meier analysis shows that JAK2R/R mice have significantly reduced survival compared with both WT and JAK2R/+ mice. (E) Kaplan-Meier analysis show that male JAK2R/R mice have significantly reduced survival compared with female JAK2R/R mice; P values are indicated. BMT, BM transplantation; WBC, white blood cell.
To determine whether the PV-like phenotype was transplantable, BM from JAK2R/R, JAK2R/+, or WT mice was transplanted into irradiated recipients. Compared with WT BM, recipients of JAK2R/+ BM displayed raised platelet counts, but no significant increases in Hb, hematocrit, or white cell counts were observed (Figure 2B). The increases in Hb and hematocrit were not as striking as the changes observed in our previous inducible heterozygous knock-in model and likely reflect the lower chimerism observed in recipient animals. However, recipients of homozygous BM developed marked erythrocytosis with platelet levels significantly lower than recipients of JAK2R/+ BM (Figure 2B). Moreover, there was a tight correlation between the levels of donor chimerism and the hematocrit of each recipient that received JAK2R/R BM (Figure 2C; supplemental Figure 7), reminiscent of the correlation between hematocrit and JAK2V617F allele burden in patients with PV.19,20
To assess the influence of JAK2V617F homozygosity on survival, JAK2R/R mice were monitored together with JAK2R/+ and WT littermates (n = 81, 80, and 144, respectively). JAK2R/R mice had significantly increased mortality compared with JAK2R/+ and WT mice (Figure 2D), which was almost exclusively restricted to males (Figure 2E), although gender did not significantly impact hematological parameters (data not shown). Immediate postmortem examination was performed in 2 mice and revealed engorged thoracic blood vessels and intrathoracic hemorrhage (supplemental Figure 8), suggesting a lethal vascular event in these 2 mice.
Together, these results demonstrate that the PV-like phenotype seen in JAK2R/R mice results in reduced survival, is transplantable, and is associated with suppressed EPO levels, indicating that the effects of JAK2V617F homozygosity are cell-intrinsic.
JAK2V617F homozygosity results in increased megakaryocyte numbers and reduced platelet survival
The observation that JAK2R/+ mice develop thrombocytosis that is abrogated in JAK2R/R mice (Figure 1A) is reminiscent of data obtained from studies of human PV in which a lower platelet count is associated with a higher proportion of JAK2V617F-homozygous erythroid colonies and/or higher JAK2V617F allele burdens in granulocyte DNA.21-23 These observations suggest that the level of JAK2V617F-homozygous precursors may be a significant determinant of platelet count in human PV. We therefore investigated the mechanism responsible for the reduction in platelet counts in JAK2R/R mice.
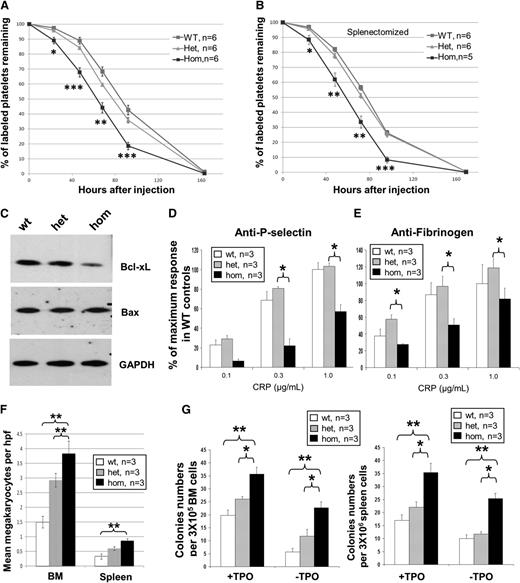
We found that platelets from JAK2R/R mice had significantly reduced survival compared with either JAK2R/+ or JAK2+/+ mice (Figure 3A). In this experiment, survival reflects the length of time that platelets circulate as measured by the percentage of remaining NHS-biotin labeled platelets, which could either be due to changes in platelet lifespan or clearance. Because it is possible that the reduction in labeled platelets in JAK2R/R mice was due to splenomegaly, the same experiment was performed in splenectomized mice. Splenectomy was associated with increased mean platelet levels and increased variability of platelet counts within each genotype (supplemental Figure 9A). However, the studies after splenectomy showed persistent reduction in the percentage of labeled platelets in JAK2R/R compared with JAK2R/+ or JAK2+/+ mice (Figure 3B), demonstrating that the reduced platelet survival was not merely a consequence of splenomegaly. These data suggest that platelet survival is intrinsic and at least partly contributes to the observed reduction in platelet counts in homozygous mice; however, we cannot exclude additional differences in proplatelet formation or maturation. Compared with WT littermate controls, purified platelets from homozygous mice showed a reduction in Bcl-xL levels (Figure 3C) and a significant reduction in platelet P-selectin and fibrinogen in response to C-reactive protein (CRP) (Figure 3D-E) but not thrombin (supplemental Figure 9B). Of note, an increased response to CRP was observed in heterozygous JAK2V617F mice induced by Mx1Cre.24 The reduced response to CRP in JAK2R/R mice might reflect a suppressive effect of JAK2V617F homozygosity in platelet function. Alternatively, homozygosity for mutant JAK2 might result in platelet hyperreactivity and increased clearance, with the reduced response to CRP representing the activity of remaining, less reactive platelets. The latter explanation would be consistent with the reduced platelet lifespan or clearance in vivo. In either case, the reduced platelet function might be relevant to the thoracic hemorrhage noted postmortem (supplemental Figure 8).
Homozygous JAK2V617F expression is associated with increased megakaryopoiesis but reduced platelet survival. (A) Platelets from JAK2R/R mice have reduced survival compared with WT and JAK2R/+ mice as measured by labeling platelets on day 0 and monitoring the labeled cells remaining over a 7-day time course. (B) Splenectomized JAK2R/R mice also show reduced platelet survival. Asterisks indicate significant differences between JAK2R/R and JAK2R/+ mice at each time point using Student t test (*P < .05; **P < .01; ***P < .001). Data are shown as mean ± SEM. (C) Western blot analysis shows reduced Bcl-xL but not Bax in homozygous platelets. Homozygous platelets show reduced level of P-selectin (D) and fibrinogen binding (E) in response to CRP. The histogram represent % of maximum response in WT controls. (F) JAK2R/R mice have increased numbers of megakaryocytes in BM and spleen compared with JAK2R/+ and WT mice. Megakaryocytes were visualized by histology and counted throughout 10 high-power fields (hpf, ×40 objective) per section. (G) Megakaryocyte colony-forming unit assays showed an increase in megakaryocyte progenitors from 8-week-old JAK2R/R mice in BM (left panel) and spleen (right panel) with and without thrombopoietin (TPO). Asterisks indicate significant differences by Student t test (*P < .05; **P < .01). Data are shown as mean ± SEM.
Homozygous JAK2V617F expression is associated with increased megakaryopoiesis but reduced platelet survival. (A) Platelets from JAK2R/R mice have reduced survival compared with WT and JAK2R/+ mice as measured by labeling platelets on day 0 and monitoring the labeled cells remaining over a 7-day time course. (B) Splenectomized JAK2R/R mice also show reduced platelet survival. Asterisks indicate significant differences between JAK2R/R and JAK2R/+ mice at each time point using Student t test (*P < .05; **P < .01; ***P < .001). Data are shown as mean ± SEM. (C) Western blot analysis shows reduced Bcl-xL but not Bax in homozygous platelets. Homozygous platelets show reduced level of P-selectin (D) and fibrinogen binding (E) in response to CRP. The histogram represent % of maximum response in WT controls. (F) JAK2R/R mice have increased numbers of megakaryocytes in BM and spleen compared with JAK2R/+ and WT mice. Megakaryocytes were visualized by histology and counted throughout 10 high-power fields (hpf, ×40 objective) per section. (G) Megakaryocyte colony-forming unit assays showed an increase in megakaryocyte progenitors from 8-week-old JAK2R/R mice in BM (left panel) and spleen (right panel) with and without thrombopoietin (TPO). Asterisks indicate significant differences by Student t test (*P < .05; **P < .01). Data are shown as mean ± SEM.
To determine the effect of JAK2V617F homozygosity on platelet production, we assessed megakaryocyte numbers in histological sections. In both BM and spleen, megakaryocyte numbers per high-power field (×40) were increased in JAK2R/+ compared with JAK2+/+ and were further increased in JAK2R/R littermates (Figure 3F). We also observed increased numbers of megakaryocyte progenitors in JAK2R/R mice compared with WT littermate controls, but these changes were not associated with differences in apoptosis (supplemental Figure 10A-C). Numbers of megakaryocyte colony-forming units in BM and spleen were also increased in JAK2R/R mice, an effect that was most marked in the absence of thrombopoietin (Figure 3G). Previously we reported an increase in megakaryocyte colony-forming units in young heterozygous mice and no difference in older mice.17 It is important to note that these previous assays were performed at different ages and in different backgrounds (ie, JAK2V617F induced by Mx1-cre), which may explain the differences observed.
In summary, these results demonstrate that the number of megakaryocytes and their precursors were further increased by JAK2V617F homozygosity, indicating that reduced platelets in JAK2R/R mice (relative to JAK2R/+ littermates) did not reflect diminished megakaryopoiesis. Instead, JAK2V617F homozygosity was associated with splenomegaly-independent reduced platelet survival.
JAK2V617F homozygosity increases erythroblast proliferation, but not survival, and does not alter erythrocyte half-life
The erythrocytosis observed in JAK2R/R mice might reflect increased red blood cell survival. Mice were therefore injected with NHS-biotin and erythrocyte survival analyzed at various time points by flow cytometry. No significant difference was observed in erythrocyte lifespan of JAK2R/R mice compared with JAK2R/+ or WT littermates (supplemental Figure 11).
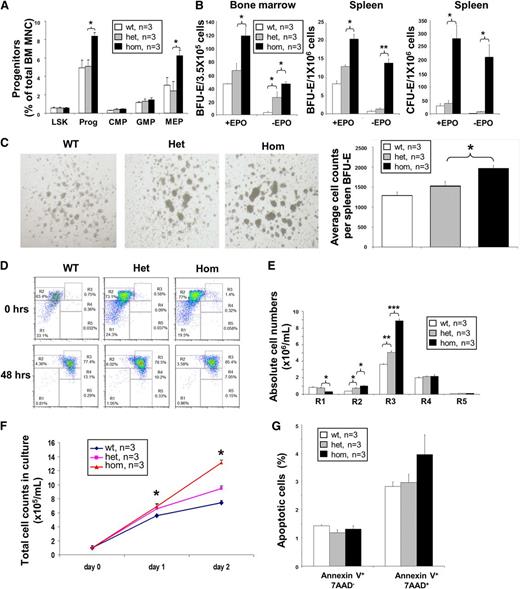
To investigate erythroblast generation, multipotent hematopoietic progenitors were analyzed. While frequencies of lin−Sca-1+c-Kit+ (LSK), common myeloid progenitors, and granulocyte/monocyte progenitors were not significantly altered (Figure 4A), the number of myeloid progenitors and megakaryocyte/erythrocyte progenitors (MEP) were significantly increased in JAK2R/R mice compared with both JAK2R/+ and JAK2+/+ littermates (Figure 4A; supplemental Figure 12).
Homozygous JAK2V617F expression increases the number of erythroid progenitors and fetal liver erythroblast cell proliferation, but does not reduce apoptosis. (A) JAK2R/R mice show increased total progenitors, mostly from a significant increase in MEP numbers. Frequencies of stem and progenitor cells in WT, JAK2R/+, and JAK2R/R mice as determined by flow cytometry. (B) JAK2R/R mice show increased erythroid colony formation. BM BFU-E (left panel), spleen BFU-E (middle panel), and spleen CFU-E colony assays (right panel), performed with or without EPO. (C) JAK2R/R mice produce larger spleen BFU-E colonies (left panel), which contain more erythroblasts per colony (right panel). Cell counts per BFU-E were derived by recording colony numbers and total cell number per dish at day 12. (D) Representative flow cytometry plots show Ter119 and CD71 expression before and after 48 hours of in vitro culture of lineage-negative E14.5 fetal liver cells. (E) Increased production of erythroblasts by JAK2R/R lineage-negative fetal liver cells in culture. Cells were analyzed by flow cytometry after 48 hours in culture. The histograms show the number of cells at individual erythroid differentiation stages (R1-R5). (F) Line graph shows the total cell number in culture. Equal numbers of lineage-negative fetal liver cells were plated on day 0 and cell number was counted on days 1 and 2. (G) JAK2V617F expression does not affect fetal liver erythroblast apoptosis. Erythroblasts were analyzed 48 hours after culture of the lineage-negative fetal liver cells by flow cytometry using Annexin-V antibody and 7-AAD on CD71+Ter119+. The histograms show percentages of apoptotic cells in the CD71+Ter119+ erythroblast population. Asterisks indicate significant differences by Student t test (*P < .05; **P < .01; ***P < .001). Data are shown as mean ± SEM.
Homozygous JAK2V617F expression increases the number of erythroid progenitors and fetal liver erythroblast cell proliferation, but does not reduce apoptosis. (A) JAK2R/R mice show increased total progenitors, mostly from a significant increase in MEP numbers. Frequencies of stem and progenitor cells in WT, JAK2R/+, and JAK2R/R mice as determined by flow cytometry. (B) JAK2R/R mice show increased erythroid colony formation. BM BFU-E (left panel), spleen BFU-E (middle panel), and spleen CFU-E colony assays (right panel), performed with or without EPO. (C) JAK2R/R mice produce larger spleen BFU-E colonies (left panel), which contain more erythroblasts per colony (right panel). Cell counts per BFU-E were derived by recording colony numbers and total cell number per dish at day 12. (D) Representative flow cytometry plots show Ter119 and CD71 expression before and after 48 hours of in vitro culture of lineage-negative E14.5 fetal liver cells. (E) Increased production of erythroblasts by JAK2R/R lineage-negative fetal liver cells in culture. Cells were analyzed by flow cytometry after 48 hours in culture. The histograms show the number of cells at individual erythroid differentiation stages (R1-R5). (F) Line graph shows the total cell number in culture. Equal numbers of lineage-negative fetal liver cells were plated on day 0 and cell number was counted on days 1 and 2. (G) JAK2V617F expression does not affect fetal liver erythroblast apoptosis. Erythroblasts were analyzed 48 hours after culture of the lineage-negative fetal liver cells by flow cytometry using Annexin-V antibody and 7-AAD on CD71+Ter119+. The histograms show percentages of apoptotic cells in the CD71+Ter119+ erythroblast population. Asterisks indicate significant differences by Student t test (*P < .05; **P < .01; ***P < .001). Data are shown as mean ± SEM.
To study erythroblast generation at a clonal level, erythroid colonies were analyzed. BM from JAK2R/R mice gave rise to increased numbers of burst forming unit-erythroid (BFU-E) colonies with or without EPO, compared with both JAK2R/+ and WT littermates (Figure 4B), but no change in granulocyte/monocyte colony number (supplemental Figure 13). Increased numbers of splenic BFU-E and colony-forming unit erythroid (CFU-E) colonies were also observed (Figure 4B). BFU-E colonies from JAK2R/R mice were larger than those from JAK2R/+ or WT mice, an observation confirmed by quantification of the mean number of cells per colony (Figure 4C). Our results therefore demonstrate that JAK2V617F homozygosity causes increased production of MEP, BFU-E, and CFU-E cells together with an increase in the number of erythroblasts generated by individual BFU-E cells.
We then used fetal liver as a tractable model of terminal erythroid differentiation25 in which cells undergoing terminal erythroid differentiation give rise to a relatively pure population of erythroblasts after 48 hours in culture (Figure 4D). Cultures of lineage-negative cells from JAK2R/R fetal liver generated more CD71+Ter119+ erythroblasts than littermates (Figure 4E-F). This observation did not reflect reduced levels of apoptosis (Figure 4G; supplemental Figure 14A), but instead was associated with a higher percentage of erythroblasts in S-phase of the cell cycle, consistent with increased proliferation (supplemental Figure 15).
The effect of JAK2V617F homozygosity on adult erythropoiesis was then assessed. Compared with JAK2R/+ or JAK2+/+ littermates, the percentage of CD71+Ter119+ erythroblasts in JAK2R/R mice was markedly increased in BM and spleen (Figure 5A), with no significant effect on either myeloid or lymphoid lineages (supplemental Figure 16). The increase in erythroblasts was not accompanied by significantly reduced apoptosis in either BM or spleen (Figure 5B; supplemental Figure 14B-C). Compared with cells from JAK2+/+ and JAK2R/+ littermates, those from JAK2R/R mice generated more S-phase erythroblasts (Figure 5C). Together, our results indicate that JAK2V617F homozygosity not only increases the generation of erythroid progenitors, but also increases the proliferation of differentiating CD71+Ter119+ erythroblasts without affecting their survival.
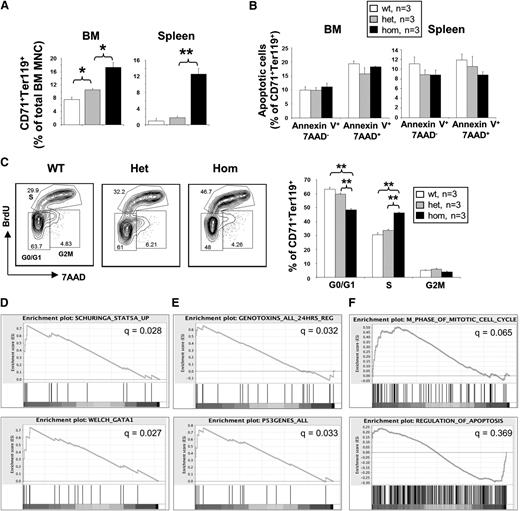
Homozygous JAK2V617F expression increases the number of adult erythroblasts with increased proliferation but does not reduce apoptosis; these changes are associated with expression of genes related to cell cycle and/or mitosis. (A) JAK2R/R mice show a marked increase in CD71+Ter119+ erythroblasts. Flow cytometry was performed on BM and spleen mononuclear cells (MNCs) using Ter119 and CD71 antibodies. (B) JAK2V617F expression does not affect erythroblast apoptosis. Flow cytometry was performed using Annexin-V and 7-AAD on BM and spleen erythroblasts. Histograms show percentages of apoptotic cells in CD71+Ter119+ erythroblast populations from both BM and spleen. (C) Homozygous JAK2V617F expression increased erythroblast proliferation. Lineage-negative BM cells were cultured to assess erythroid differentiation in vitro. A 5-bromo-2′-deoxyuridine (BrdU) cell proliferation assay was performed after 4 days in culture on the gated erythroblast population as shown in the plots with the G0/G1, S, and G2/M phases indicated. Asterisks indicate significant differences by Student t test (**P < .01). Data are shown as mean ± SEM. (D) Gene set enrichment analysis (GSEA) showing enrichment for STAT5 and GATA1 target genes in JAK2R/R samples relative to non-JAK2R/R samples (pooled datasets from 3 biological replicates each of WT and JAK2V617FR/+ littermates). (E) GSEA showing enrichment of gene sets associated with DNA-damaging agents and the p53 response in JAK2R/R samples. (F) GSEA showing enrichment in the JAK2R/R samples for gene sets related to regulation of the cell cycle, but no enrichment for genes associated with apoptosis.
Homozygous JAK2V617F expression increases the number of adult erythroblasts with increased proliferation but does not reduce apoptosis; these changes are associated with expression of genes related to cell cycle and/or mitosis. (A) JAK2R/R mice show a marked increase in CD71+Ter119+ erythroblasts. Flow cytometry was performed on BM and spleen mononuclear cells (MNCs) using Ter119 and CD71 antibodies. (B) JAK2V617F expression does not affect erythroblast apoptosis. Flow cytometry was performed using Annexin-V and 7-AAD on BM and spleen erythroblasts. Histograms show percentages of apoptotic cells in CD71+Ter119+ erythroblast populations from both BM and spleen. (C) Homozygous JAK2V617F expression increased erythroblast proliferation. Lineage-negative BM cells were cultured to assess erythroid differentiation in vitro. A 5-bromo-2′-deoxyuridine (BrdU) cell proliferation assay was performed after 4 days in culture on the gated erythroblast population as shown in the plots with the G0/G1, S, and G2/M phases indicated. Asterisks indicate significant differences by Student t test (**P < .01). Data are shown as mean ± SEM. (D) Gene set enrichment analysis (GSEA) showing enrichment for STAT5 and GATA1 target genes in JAK2R/R samples relative to non-JAK2R/R samples (pooled datasets from 3 biological replicates each of WT and JAK2V617FR/+ littermates). (E) GSEA showing enrichment of gene sets associated with DNA-damaging agents and the p53 response in JAK2R/R samples. (F) GSEA showing enrichment in the JAK2R/R samples for gene sets related to regulation of the cell cycle, but no enrichment for genes associated with apoptosis.
To investigate the molecular mechanisms involved in the phenotypic switch to PV-like phenotypes in the JAK2R/R mice, expression microarray analysis was performed using BM Ter119+CD71+ erythroblasts (experiment name: Bone Marrow Erythroblast Arrays, ArrayExpress accession: E-MTAB-2427). Erythroblasts were sorted by gating the central portion of the Ter119+CD71+ population to reduce possible differences between populations, although subtle erythroid maturation differences could still exist. Gene Set Enrichment Analysis identified pathways upregulated in the JAK2R/R compared with the other (JAK2R/+ or WT) samples. Using the MSigDB Chemical and Genetic Perturbations database, 36 and 219 gene sets were enriched in the JAK2R/R and non-JAK2R/R samples, respectively (supplemental Table 1). Gene sets enriched in the JAK2R/R samples included STAT5 and GATA1 target genes (Figure 5D), consistent with the known roles of these transcription factors downstream of JAK2 and in driving erythropoiesis. We also observed enrichment of gene sets associated with DNA-damaging agents and the p53 response in JAK2R/R samples (Figure 5E), keeping with previous evidence that JAK2V617F is associated with DNA damage.17,26 These analyses demonstrate that JAK2V617F homozygosity is associated with upregulation of key erythroid regulatory genes and a more marked DNA damage response. Using gene sets in the MSigDB Gene Ontology database, 45 and 12 gene sets were enriched in the JAK2R/R and non-JAK2R/R samples, respectively (supplemental Table 2). Strikingly, 10 of the gene sets showing enrichment in the JAK2R/R samples were related to cell cycle and/or mitosis (q values 0.057-0.092), whereas there was no enrichment in either sample group for 9 gene sets related to apoptosis (q values 0.329-0.798) (Figure 5F). These data support our findings that JAK2V617F homozygosity is predominantly associated with increased erythroblast proliferation, rather than with reduced apoptosis.
JAK2V617F homozygosity reduces HSC frequency and impairs HSC function
We previously reported that mice heterozygous for human JAK2V617F have normal numbers of LSK cells 6 to 8 weeks after Cre induction together with a functional HSC defect that was subtle in primary recipients and more marked in secondary competitive transplants.17 We therefore investigated HSC number and function in JAK2R/R mice compared with JAK2R/+ and JAK2+/+ littermates. The numbers of highly purified E-SLAM (EPCR+CD45+CD150+CD48−)27 or LSKCD34−Flt3− HSCs in JAK2R/R mice were substantially reduced compared with both JAK2R/+ and WT littermates (Figure 6A; supplemental Figures 17-20). In the BM, but not the spleen, of old homozygous animals, all stem/progenitor cell fractions showed reduced frequency except for common myeloid progenitors and MEPs (supplemental Figure 21), further demonstrating the progressive loss of stem and progenitor cells.
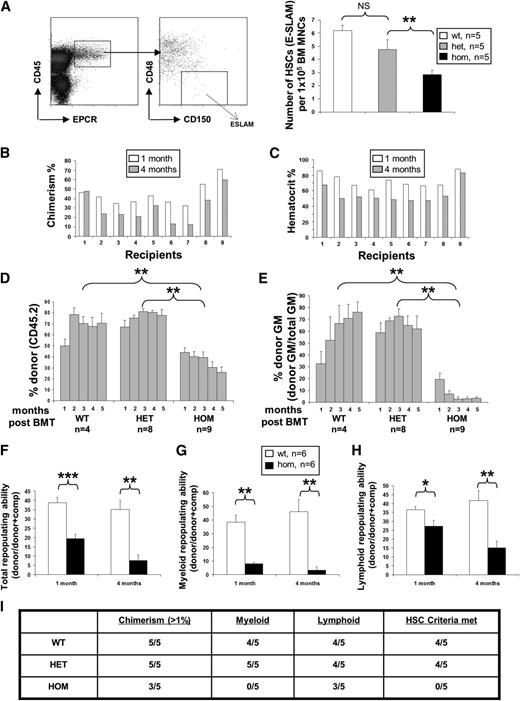
Homozygous JAK2V617F expression compromises HSC self-renewal. (A) Representative flow cytometry plot for analysis of the E-SLAM HSC population (EPCR+CD45+CD150+CD48−). The histogram shows significantly reduced frequency of E-SLAM HSCs in BM from JAK2R/R mice at 6 to 8 weeks of age compared with WT and JAK2R/+. (B) JAK2R/R chimerism (homozygous JAK2V617F allele burden) was reduced in noncompetitive transplant recipients at 4 months compared with 1 month; (C) the reduction of the allele burden was associated with a decline in hematocrit levels in 9 individual recipients of JAK2R/R BM cells. (D) Histograms show the percentage of total donor chimerism (% of CD45.2+ cells) in noncompetitive transplant recipients over time. (E) Histograms show the percentage of donor chimerism in myeloid cells (% of CD45.2+ cells in total Mac-1+ and/or Ly6G+ cells) in noncompetitive transplant recipients over time. (F) Histograms show reduced total repopulating capacity of BM cells from 6- to 8-week-old donor mice (backcrossed 10 generations onto C57/Bl6 background) in competitive transplant recipients. Histograms are presented as a ratio of donor/(donor+comp). (G,H) Histograms show the repopulating capacity of donor cells in the myeloid and lymphoid lineages in competitive transplant recipients (ratio of donor/(donor+comp) in total Mac-1+ and/or Ly6G+ cells). Asterisks indicate significant differences by Student t test (*P < .05; **P < .01). Data are shown as mean ± SEM. (I) HSCs from JAK2R/R mice display a repopulating defect. Ten E-SLAM cells from 8- to 10-week-old JAK2+/+, JAK2R/+, and JAK2R/R mice on a C57Bl/6 background were injected per recipient (C57Bl/6 CD45.1), with total of 5 recipients for each genotype. The table shows data from 6 months posttransplantation in which donor cells were assessed for overall chimerism as well as contributions to lymphoid and myeloid elements of the blood system. Animals were considered to have received an HSC if they had >1% contribution to each lineage at 24 weeks posttransplantation. JAK2R/R HSC recipients showed lower overall repopulation levels and an absence (<0.5%) of granulocytes and macrophages.
Homozygous JAK2V617F expression compromises HSC self-renewal. (A) Representative flow cytometry plot for analysis of the E-SLAM HSC population (EPCR+CD45+CD150+CD48−). The histogram shows significantly reduced frequency of E-SLAM HSCs in BM from JAK2R/R mice at 6 to 8 weeks of age compared with WT and JAK2R/+. (B) JAK2R/R chimerism (homozygous JAK2V617F allele burden) was reduced in noncompetitive transplant recipients at 4 months compared with 1 month; (C) the reduction of the allele burden was associated with a decline in hematocrit levels in 9 individual recipients of JAK2R/R BM cells. (D) Histograms show the percentage of total donor chimerism (% of CD45.2+ cells) in noncompetitive transplant recipients over time. (E) Histograms show the percentage of donor chimerism in myeloid cells (% of CD45.2+ cells in total Mac-1+ and/or Ly6G+ cells) in noncompetitive transplant recipients over time. (F) Histograms show reduced total repopulating capacity of BM cells from 6- to 8-week-old donor mice (backcrossed 10 generations onto C57/Bl6 background) in competitive transplant recipients. Histograms are presented as a ratio of donor/(donor+comp). (G,H) Histograms show the repopulating capacity of donor cells in the myeloid and lymphoid lineages in competitive transplant recipients (ratio of donor/(donor+comp) in total Mac-1+ and/or Ly6G+ cells). Asterisks indicate significant differences by Student t test (*P < .05; **P < .01). Data are shown as mean ± SEM. (I) HSCs from JAK2R/R mice display a repopulating defect. Ten E-SLAM cells from 8- to 10-week-old JAK2+/+, JAK2R/+, and JAK2R/R mice on a C57Bl/6 background were injected per recipient (C57Bl/6 CD45.1), with total of 5 recipients for each genotype. The table shows data from 6 months posttransplantation in which donor cells were assessed for overall chimerism as well as contributions to lymphoid and myeloid elements of the blood system. Animals were considered to have received an HSC if they had >1% contribution to each lineage at 24 weeks posttransplantation. JAK2R/R HSC recipients showed lower overall repopulation levels and an absence (<0.5%) of granulocytes and macrophages.
Consistent with an HSC defect, most recipients of noncompetitive JAK2R/R BM transplants showed a progressive decline in levels of chimerism by 4 months following transplantation (Figure 6B) accompanied by a decline in hematocrit (Figure 6C). By contrast, recipients of JAK2+/+ and JAK2R/+ maintained consistent levels of chimerism (Figure 6D). Myeloid engraftment was particularly poor in recipients of JAK2R/R BM, with a donor contribution less than 5% in most recipients by 4 months following transplantation (Figure 6E). Such low levels of myeloid engraftment are associated with a failure to reconstitute secondary recipients and the existence of a marked defect in HSC self-renewal.28
Competitive BM transplantation was then performed to investigate HSC function in 6- to 8-week-old mice, using equal numbers of WT competitor and test cells from JAK2R/R, JAK2R/+, or JAK2+/+ littermates, respectively. Compared with JAK2R/+ or JAK2+/+ BM cells, those from JAK2R/R mice showed significantly less repopulation 1 and 4 months after transplantation (Figure 6F; supplemental Figure 23A). Previously, we documented a subtle but significant decrease in competitive reconstituting ability of JAK2V617F heterozygous BM using larger cohorts of mice.17 Here, contribution from the heterozygous JAK2V617F cells is reduced (supplemental Figure 23A-C), but this change is not significant, likely because of lower numbers of transplanted animals. Repopulation of both myeloid and lymphoid lineages was reduced by JAK2V617F expression in a dose-dependent manner (Figure 6G-H; supplemental Figure 23B-C), accompanied by a reduction in the relative number of stem and progenitor cells (supplemental Figure 22). The low myeloid chimerism, reduced donor stem and progenitor cells, and the hematocrit normalization in recipient mice with decreasing chimerism over time all predict a further HSC disadvantage in secondary animals.27,28 We also found no difference in the ability of purified E-SLAM HSCs to home to the BM 36 hours posttransplantation (supplemental Figure 23D). The reduced HSC function could still result from fewer transplanted HSCs in the original test BM suspension, and we therefore performed purified HSC transplantation experiments using 10 E-SLAM HSCs to test the functional capacity of individual HSCs. Compared with their WT littermate controls, JAK2V617F homozygous HSCs (but not JAK2V617F heterozygous HSCs) from young mice were less able to repopulate recipient animals (Figure 6I), although as demonstrated previously, HSCs from older JAK2V617F heterozygous mice also have a disadvantage.29 Taken together, these data demonstrate that JAK2V617F homozygosity reduces HSC number and function, and that JAK2R/R HSC are unable to sustain a PV-like phenotype over time in transplants.
Discussion
There has been a growing recognition that acquired UPD is a common occurrence in many malignancies.1 Such regions of UPD contain gain-of-function mutations in genes including MPL,9,30 NRAS,8 FLT3,7,8 and JAK2.3-6 However, there is surprisingly little direct evidence that homozygosity for individual gain-of-function mutations confers clinically relevant consequences. In this article, we present direct genetic evidence that JAK2V617F homozygosity plays a causal role in the development of a PV-like phenotype. Whereas heterozygous mutant mice develop a condition resembling mild JAK2V617F-positive ET, homozygous mutant mice develop marked erythrocytosis. The increases in spleen size, myeloid cells, and platelets are relatively mild compared with some other heterozygous knock-in models (reviewed in van Etten et al31 and Li et al13 ), but are consistent with the modest increase in platelets observed in a proportion of patients and suggest the need for collaborating mutations to drive the complete ET disease phenotype. Our results indicate that UPD on chromosome 9p plays a causal role in the PV phenotype in patients as a consequence of JAK2V617F homozygosity, although they do not exclude a cooperative role for other loci within the region of UPD. The generation of an allelic series with a dose-dependent effect on hematopoiesis provides a powerful system for studying the cellular and molecular consequences of homozygosity for the JAK2 mutation.
Several lines of circumstantial evidence agree with our results and indicate that the strength of mutant-JAK2 signaling influences clinical phenotype. (1) Peripheral blood JAK2V617F allele burden is substantially higher in most patients with PV compared with those with ET.22,32 (2) Large numbers of homozygous mutant BFU-E are found in many patients with PV and are present at much lower levels in patients with ET.11,33 (3) JAK2 exon 12 mutations are associated exclusively with PV and not with ET, and activate signaling pathways more strongly than the V617F mutation.34,35 (4) Higher levels of mutant JAK2 expression results in PV-like phenotypes, whereas lower levels were associated with ET-like phenotypes in a transgenic mouse model.36 Our demonstration that JAK2V617F homozygosity results in a phenotypic switch, from ET-like to PV-like, is also consistent with the fact that transformation to PV occurs in a small minority of ET patients,37,38 an event that is likely to be underreported because transformation would be masked in those ET patients who are started on cytoreductive therapy.
Human PV is a heterogeneous disease and disease evolution is similarly variable. For example, only 40% to 50% of patients have splenomegaly at presentation and more than 60% have normal white cell and platelet counts.39 Our results demonstrate that, compared with heterozygous human JAK2V617F, homozygosity gives rise to erythrocytosis and is associated with the development in some mice with features seen in some (but not all) patients with PV (eg, leukocytosis, transformation, decreased platelet count). Indeed, each knock-in mouse model that has been generated has its own unique aspects (reviewed in van Etten et al31 and Li et al13 ), including extremely high platelet counts,16 no increase in platelets,15 or high rates of myelofibrosis.14 The reason for the different phenotypes could relate to technical issues associated with the different targeting strategies or to inherent differences in mutant human and mouse proteins.
Recent observations reconcile our data with evidence that, in some patients, acquisition of JAK2V617F homozygosity is insufficient for the development of PV. It is now recognized that JAK2V617F homozygosity is recurrently acquired and that small homozygous mutant subclones are present in patients with both ET and PV, with the 2 diseases being distinguished by the expansion of a dominant subclone in PV patients, most likely as a result of additional advantageous alterations.11 Moreover, homozygous mutant BFU-E are undetectable in a subset of PV patients despite use of low-EPO conditions,11,22 an observation likely to reflect the existence of acquired genetic or epigenetic lesions that can phenocopy the effects of JAK2V617F homozygosity.
JAK2 is essential for normal definitive erythropoiesis and plays a central role in signaling from the EPO receptor (EPOR).40 EPO signaling in erythroblasts acts primarily to regulate apoptosis,40,41 but also to modulate cell-cycle pathways.42 Our data demonstrate that JAK2V617F homozygosity enhances erythropoiesis at multiple levels. The generation of early erythroid progenitors (MEP and BFU-E) is increased and more erythroblasts are produced per BFU-E. In addition, our functional studies and array data indicate that JAK2V617F homozygosity increases proliferation of Ter119+CD71+ erythroblasts with no discernable effect on apoptosis, although this does not exclude an effect at other stages of erythroid differentiation.43 Together, our results indicate that the consequences of JAK2V617F homozygosity are not identical to those resulting from enhanced EPOR signaling. This observation is likely to reflect that EPOR is only 1 of multiple cytokine receptors that signal through JAK244 and/or that JAK2V617F has functions that are qualitatively distinct from WT JAK2.
JAK2V617F homozygosity also results in reduced numbers of platelets, consistent with the lower platelet levels seen in PV compared with ET.32 Moreover, JAK2V617F homozygosity was associated with reduced platelet survival that did not merely reflect hypersplenism and instead is likely to reflect increased platelet apoptosis and/or clearance. Our data therefore raise the possibility that increased JAK2V617F signaling may enhance (or reduce) platelet reactivity, a concept that may be relevant to thrombotic (or hemorrhagic) complications in patients with PV. Aberrant platelet activation has been reported to varying degrees in MPN patients,45 although interindividual differences in the clonal burden of JAK2-mutant platelets make these data difficult to interpret, and no consistent differences have been reported between ET and PV patients.
Several other JAK2V617F knock-in mouse models have been reported, all expressing mutant mouse Jak2,14-16 but the consequences of homozygosity have only been reported in 1 study.14 These homozygous mutant mice developed Hb levels that were lower or unchanged and platelet counts that were higher than heterozygous mutant controls.14 The explanation for the different homozygous mutant phenotype compared with our knock-in mouse is currently unclear, but could relate to the different targeting strategies or to intrinsic differences in the signaling properties of mutant human compared with mutant mouse proteins.13
We previously reported that JAK2V617F heterozygous mice have reduced HSC function that was clearly evident in secondary transplants.17 In knock-in models using mouse JAK2V617F,14,15 original reports described either an increased HSC function or no change, but did not use purified HSC fractions or secondary transplantation to determine HSC function. When secondary transplantation studies were subsequently undertaken in therapy-related studies, JAK2 mutant HSCs showed no advantage in vehicle treated controls.46,47 Here we show that homozygous mutant mice exhibit a profound HSC defect, consistent with several features of human MPNs: the JAK2V617F mutation is present in only a minority of hematopoietic stem and progenitor cells48-50 ; xenograft experiments show that JAK2V617F-positive CD34+ cells fail to outcompete normal cells51,52 ; ET and PV patients harbor multiple small homozygous clones, but only PV patients possess an expanded dominant homozygous clone, suggesting the acquisition of additional lesions.11 Similar observations have been noted in chronic myelogenous leukemia in which BCR-ABL–positive cells are present at low levels in the HSC and progenitor compartments53 and that HSCs exhibit reduced self-renewal.54 Interestingly, in this latter model,55 it was recently shown that although HSC numbers were reduced in the BM, their individual repopulating activity was increased and the spleens of these mice contained an increased frequency of transplantable HSCs.
Together, our data indicate that JAK2V617F homozygosity is neither sufficient for clonal expansion nor the complete phenotype observed in MPNs (eg, leukocytosis), but instead may provide a barrier reminiscent of oncogene-induced senescence.56 The underlying molecular mechanisms are unclear, but studies of single HSCs indicate that JAK2V617F impairs HSC self-renewal while skewing their progeny toward differentiation and proliferation.29 Clonal expansion likely requires additional lesions10 and it will be critical to elucidate the mechanisms by which cooperating events drive clonal expansion of JAK2V617F mutant cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to the authors thank Wanfeng Zhao for histology; Anna Petrunkina-Harrison, Reiner Schulte, Veronika Romashova, and Susan Kennelly for flow cytometry assistance; Peter Campbell and Elli Papaemmanuil for microarray analysis; and Cavan Bennett for helpful information.
Work in the A.R.G. laboratory is supported by the Leukemia and Lymphoma Research, Cancer Research UK, the Kay Kendall Leukaemia Fund, the National Institute for Health Research Cambridge Biomedical Research Centre, the Cambridge Experimental Cancer Medicine Centre, and the Leukemia & Lymphoma Society of America. This work was supported by the Kay Kendall Leukaemia Fund (A.L.G., J.N.), the Canadian Institutes of Health Research, and Lady Tata Memorial Trust (D.G.K.).
Authorship
Contribution: J.L. designed and performed experiments and analyzed the data with input from A.L.G. on cell survival, western blots, EPO assay, microarray, megakaryocyte colony, and histology; D.G.K. performed hematopoietic stem cell and progenitor assays, BMT, and homing assays; H.M. performed platelet flow cytometry analysis and western blots; A.A. performed 5-bromo-2′-deoxyuridine proliferation assay; K.S. performed splenectomy; E.C., J.F., J.N., and X.Z. performed fetal liver erythroid flow cytometry, BMT, histological analysis, and polymerase chain reaction analysis, respectively; R.S., T.L.H., D.C.P., and Y.S. provided assistance in generation and phenotypic analysis of the mice cohorts; C.G. collaborated on platelet analysis; P.L. provided StellaCre and collaborated in the generation of the knock-in model; J.L. and A.R.G. wrote the paper with input from A.L.G. and D.G.K.; and A.R.G. directed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony R. Green, Cambridge Institute for Medical Research, Hills Rd, Cambridge, CB2 0XY, UK; e-mail: arg1000@cam.ac.uk.
References
Author notes
J.L. and D.G.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal