Key Points
VCAM-1/VLA-4 triggers reciprocal NF-κB activation in leukemia and stromal cells and mediates cross-talk between leukemia and stromal cells.
VCAM-1/VLA-4 and NF-κB signaling plays a pivotal role in the development of leukemia chemoresistance.
Abstract
Leukemia cells are protected from chemotherapy-induced apoptosis by their interactions with bone marrow mesenchymal stromal cells (BM-MSCs). Yet the underlying mechanisms associated with this protective effect remain unclear. Genome-wide gene expression profiling of BM-MSCs revealed that coculture with leukemia cells upregulated the transcription of genes associated with nuclear factor (NF)-κB signaling. Moreover, primary BM-MSCs from leukemia patients expressed NF-κB target genes at higher levels than their normal BM-MSC counterparts. The blockade of NF-κB activation via chemical agents or the overexpression of the mutant form of inhibitor κB-α (IκBα) in BM-MSCs markedly reduced the stromal-mediated drug resistance in leukemia cells in vitro and in vivo. In particular, our unique in vivo model of human leukemia BM microenvironment illustrated a direct link between NF-κB activation and stromal-associated chemoprotection. Mechanistic in vitro studies revealed that the interaction between vascular cell adhesion molecule 1 (VCAM-1) and very late antigen-4 (VLA-4) played an integral role in the activation of NF-κB in the stromal and tumor cell compartments. Together, these results suggest that reciprocal NF-κB activation in BM-MSCs and leukemia cells is essential for promoting chemoresistance in the transformed cells, and targeting NF-κB or VLA-4/VCAM-1 signaling could be a clinically relevant mechanism to overcome stroma-mediated chemoresistance in BM-resident leukemia cells.
Introduction
Experimental evidence gathered over the last 2 decades has demonstrated that bone marrow mesenchymal stromal cells (BM-MSCs) can prevent spontaneous and chemotherapy-induced apoptosis in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and other types of leukemia.1-4 Undoubtedly, this chemoresistance-enhancing effect has profound clinical significance, because it promotes post-therapy residual disease that retains a greater potential for relapse.
Within the BM microenvironment, BM-MSCs produce cytokines and chemokines and initiate cell adhesion-mediated signals that tightly regulate normal and malignant hematopoietic cell survival and appear to drive the chemoresistance-promoting effect of the BM microenvironment.5-9 Cell-cell adhesion between BM-MSCs and leukemia blasts follows a normal physiological process involving adhesion receptors on the leukemia cell surface (such as integrins β1, β2, and the very late antigen-4 [VLA-4]) interacting with stromal ligands such as vascular cell adhesion molecule 1 (VCAM-1).10-12 This type of adhesive interaction triggers the activation of prosurvival and proliferative pathways in both the blasts and stromal cells that are critical for blast survival.13
Coculture models of ALL cells and BM-MSCs have been used to study the complex and dynamic networks of various growth factors and cytokines in which leukemic blasts and stromal cells cross-talk and reciprocally regulate their cytokine expression.14,15 However, the process by which leukemia-stroma interactions confer chemoresistance to leukemia cells is not fully understood, particularly concerning the requisite changes that occur in BM-MSCs. Such changes are likely, given that leukemia cells promote changes in their BM microenvironment that suppress normal hematopoiesis and enhance leukemia progression.16 Related examples where tumor cells modify their surrounding stroma come from studies in solid tumors reporting that tumor cells can recruit vascular endothelial cells, MSCs, and fibrovascular tumor associated fibroblasts from nearby tissues, as well as from the BM.17-20 Once they are in the tumor microenvironment, these normal cells aid in the promotion of tumor extracellular matrix remodeling, motility, and metastasis.21,22
Recent reports have described nuclear factor (NF)-κB activation in tumor-surrounding stroma on interaction with tumor cells.23-25 Classical activation of NF-κB occurs by factors that stimulate the IκB kinase complex to phosphorylate and degrade IκB, leading to NF-κB nuclear translocation and subsequent target gene expression.26 In this report, we used coculture model systems of human BM-MSCs with human leukemia cells to identify changes induced by their interaction that contribute to the stroma-mediated chemoresistance of leukemia cells. The results presented here demonstrate that the leukemia-stroma interactions induce in these cells reciprocal NF-κB activation along with the ubiquitous upregulation of VCAM-1 in the BM-MSCs, unveiling a possible mechanism that involves integrin engagement and soluble factor-mediated signaling as responsible for this phenomenon.
Methods
Please refer to supplemental Methods (available on the Blood Web site) for detailed descriptions of the methods and reagents used.
Chemicals, reagents, and antibodies
MLN120B (provided by Millennium Pharmaceuticals, Inc.) was dissolved in dimethylsulfoxide and used at a final concentration of 10 µmol/L. CDDO-Me, the C-28 methyl ester derivative of the novel synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), was kindly provided by Dr Edward Sausville (National Cancer Institute, Bethesda, MD) under the Rapid Access to Interventional Development program and by Dr Michael Sporn (Dartmouth Medical College, Hanover, NH) and was used at a concentration of 50 ng/mL. The VLA-4 blocking antibody (CD49d, Cat#555501; BD Biosciences) was used at a concentration of 10 µg/0.5 × 106 cells. Recombinant human interleukin (IL)-1 receptor antagonist (IL1RA; Cat# 200-01RA; PeproTech) was used at concentration of 200 ng/mL. Vincristine (VCR) and Doxo were used at final concentrations of 75 and 50 ng/mL, respectively, unless otherwise indicated. Cytarabidine was used at a final concentration of 1 µmol/L. Allophycocyanin-conjugated anti-human CD90 (Thy-1) and phycoerythrin-conjugated anti-human CD45 antibodies were obtained from BD-Biosciences. 4,6-diamidino-2-phenylindole (DAPI) was obtained from Sigma-Aldrich (St. Louis, MO).
Leukemia cell lines and primary samples
Human OCI-AML3 cells and the pre-B ALL REH cells, NALM6-luciferase-CopGFP, and RS4;11 were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in 5% CO2. Samples of BM or peripheral blood were obtained for in vitro studies from patients with newly diagnosed or recurrent ALL or AML who had a high (>40%) blast count. Informed consent was obtained following institutional guidelines. Clinical data for patient samples are presented in supplemental Table 5. Mononuclear cells were separated by Ficoll-Hypaque (Sigma Chemical) density-gradient centrifugation.
Isolation and culture of primary BM-MSCs
BM-MSCs were isolated from BM of consented AML patients undergoing diagnostic BM aspiration and from healthy donors who were undergoing BM harvest for use in allogeneic BM transplantation. A complete description of the isolation and culture procedures can be found in supplemental Methods.
Isolation and expansion of endothelial colony-forming cells
Human endothelial colony-forming cells (ECFCs) were isolated and expanded as previously described.27 Endothelial growth medium was prepared as suggested by the manufacturer except that fetal bovine serum was replaced with 10% pooled human platelet lysate (pHPL).
Coculture isolation and RNA extraction
Normal BM-MSCs were cocultured with leukemic cell lines or leukemic cells from patient samples and processed as indicated in the supplemental Methods.
mRNA hybridization and gene expression profiling
Total RNA was amplified and hybridized to Illumina HT12 version 4 human whole-genome arrays as described previously.28
In vivo extramedullary bone formation and murine leukemia model
Extramedullary bone formation was performed as previously described.29 Detailed description can be found in supplemental Methods.
Statistical analyses
For the gene analysis study, significance testing for differentially expressed probes (DEPs) was determined by the Wilcoxon rank-sum test applied to individual processed bead values, with false discovery rate significance values (q) established by the method of Benjamini and Hochberg.30 For the other data, the results are expressed as the mean ± standard error of the mean (SEM) for triplicate independent experiments. The Student paired t test was used for statistical comparisons between groups. Differences with P ≤ .05 were considered statistically significant.
Study approval
Animal protocols were approved by the Animal Care and Use Committee of the MD Anderson Cancer Center. Leukemia patient and healthy donor BM samples were obtained following written informed consent in accordance with tissue procurement protocols approved by the Institution Review Board of the MD Anderson Cancer Center. This study was conducted in accordance with the Declaration of Helsinki.
Results
Coculture of human-derived BM-MSCs and leukemia cells induces gene expression in BM-MSCs that is consistent with NF-κB activation
To elucidate molecular mechanisms by which leukemia-stroma interactions within the BM microenvironment could confer chemoresistance to leukemia cells, we used genome-wide gene expression profiling (GEP) to examine human normal BM-MSCs that had been cocultured with the pre-B ALL REH cells and then been separated by fluorescence-activated cell sorter to isolate each individual cell type. As controls, BM-MSCs and REH cells were cultured alone (monocultures) and examined. After 48 hours of coculture, BM-MSCs were sorted and separated from the leukemia cells using the specific markers CD90 and CD45, respectively (supplemental Figures 1A and 2). After sorting, a sample of each purified cell population was checked by flow cytometry, and the cell purity was assessed at >98.5%. Total RNA from 4 cell populations (REH or BM-MSCs, each cultured alone or cocultured and then purified) was generated in 3 independent experiments and taken through standard labeling procedures for hybridization to microarrays for GEP (Gene Expression Omnibus [GEO] accession number GSE55533). Using strict significance criteria (P < .01, false discovery rate q statistic <0.1) for 3 independent experiments, we found 160 DEPs for 154 genes consistently upregulated in cocultured BM-MSCs and 166 DEPs for 159 downregulated genes (supplemental Tables 1 and 2). Based on an analysis of GEP data for REH cells (supplemental Methods), none of the DEPs that were upregulated in BM-MSCs were attributable to contamination of the cocultured and purified BM-MSCs by the REH cells.
Among the upregulated DEPs induced by coculture in BM-MSCs, there was a variety of cytokines and chemokines including IL-8, IL-6, chemokine (C-C motif) ligand 2 (CCL2), and the VLA-4 ligand VCAM-1, as well as other recognized target genes of the NF-κB pathway (supplemental Figure 1B). Gene set enrichment analyses identified 180 gene sets from category C2 of the Molecular Signatures Database that were significantly enriched in cocultured samples (q < 0.1). Table 1 lists the most enriched gene sets, for which more information is available at http://www.broadinstitute.org/gsea/msigdb/search.jsp. The gene set enrichment analysis indicated an activation of NF-κB and NF-κB-related pathways in the cocultured BM-MSCs (supplemental Figure 3; supplemental Tables 3 and 4).
Gene sets differentially regulated in BM-MSCs cocultured with REH leukemia cells compared with BM-MSCs in monoculture
| Name . | Size, n* . | ES . | NES . | False discovery rate q-value . | FWER P value . | Rank at maximum . |
|---|---|---|---|---|---|---|
| HINATA_NFKB_TARGETS_KERATINOCYTE_UP | 44 | 0.74373627 | 2.763994 | 0 | 0 | 910 |
| ZHANG_RESPONSE_TO_IKK_INHIBITOR_AND_TNF_UP | 114 | 0.59999883 | 2.6790879 | 0 | 0 | 864 |
| AMIT_EGF_RESPONSE_60_MCF10A | 33 | 0.7639718 | 2.678291 | 0 | 0 | 490 |
| HINATA_NFKB_TARGETS_FIBROBLAST_UP | 46 | 0.6953736 | 2.640253 | 0 | 0 | 540 |
| KEGG_NOD_LIKE_RECEPTOR_SIGNALING_PATHWAY | 29 | 0.77374154 | 2.575111 | 0 | 0 | 763 |
| KIM_WT1_TARGETS_UP | 159 | 0.5234185 | 2.4591517 | 4.38E-04 | .002 | 2290 |
| MCLACHLAN_DENTAL_CARIES_UP | 92 | 0.57450825 | 2.4543602 | 3.75E-04 | .002 | 306 |
| KEGG_TOLL_LIKE_RECEPTOR_SIGNALING_PATHWAY | 47 | 0.6524064 | 2.453981 | 3.28E-04 | .002 | 1268 |
| ZUCCHI_METASTASIS_DN | 17 | 0.8249239 | 2.4468715 | 4.36E-04 | .003 | 520 |
| GRAHAM_CML_DIVIDING_VS_NORMAL_QUIESCENT_DN | 49 | 0.6465402 | 2.4402559 | 5.20E-04 | .004 | 625 |
| SANA_TNF_SIGNALING_UP | 37 | 0.68556935 | 2.4303193 | 7.04E-04 | .006 | 562 |
| SEKI_INFLAMMATORY_RESPONSE_LPS_UP | 44 | 0.66059566 | 2.4077451 | 9.58E-04 | .009 | 864 |
| DASU_IL6_SIGNALING_UP | 48 | 0.6300051 | 2.3814805 | 0.001368899 | .014 | 969 |
| LIEN_BREAST_CARCINOMA_METAPLASTIC_VS_DUCTAL_UP | 47 | 0.6460621 | 2.3720858 | 0.00127112 | .014 | 952 |
| REACTOME_PEPTIDE_LIGAND_BINDING_RECEPTORS | 36 | 0.666963 | 2.3602338 | 0.001439457 | .017 | 490 |
| MCLACHLAN_DENTAL_CARIES_DN | 105 | 0.53308874 | 2.33769 | 0.002061751 | .026 | 306 |
| KEGG_RIG_I_LIKE_RECEPTOR_SIGNALING_PATHWAY | 28 | 0.6970441 | 2.2951434 | 0.0034468 | .046 | 1268 |
| TENEDINI_MEGAKARYOCYTE_MARKERS | 27 | 0.6971226 | 2.2726986 | 0.004659514 | .065 | 624 |
| KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 78 | 0.55096024 | 2.2703986 | 0.00447959 | .066 | 1258 |
| KEGG_BLADDER_CANCER | 29 | 0.6838998 | 2.2639232 | 0.004569982 | .07 | 1057 |
| Name . | Size, n* . | ES . | NES . | False discovery rate q-value . | FWER P value . | Rank at maximum . |
|---|---|---|---|---|---|---|
| HINATA_NFKB_TARGETS_KERATINOCYTE_UP | 44 | 0.74373627 | 2.763994 | 0 | 0 | 910 |
| ZHANG_RESPONSE_TO_IKK_INHIBITOR_AND_TNF_UP | 114 | 0.59999883 | 2.6790879 | 0 | 0 | 864 |
| AMIT_EGF_RESPONSE_60_MCF10A | 33 | 0.7639718 | 2.678291 | 0 | 0 | 490 |
| HINATA_NFKB_TARGETS_FIBROBLAST_UP | 46 | 0.6953736 | 2.640253 | 0 | 0 | 540 |
| KEGG_NOD_LIKE_RECEPTOR_SIGNALING_PATHWAY | 29 | 0.77374154 | 2.575111 | 0 | 0 | 763 |
| KIM_WT1_TARGETS_UP | 159 | 0.5234185 | 2.4591517 | 4.38E-04 | .002 | 2290 |
| MCLACHLAN_DENTAL_CARIES_UP | 92 | 0.57450825 | 2.4543602 | 3.75E-04 | .002 | 306 |
| KEGG_TOLL_LIKE_RECEPTOR_SIGNALING_PATHWAY | 47 | 0.6524064 | 2.453981 | 3.28E-04 | .002 | 1268 |
| ZUCCHI_METASTASIS_DN | 17 | 0.8249239 | 2.4468715 | 4.36E-04 | .003 | 520 |
| GRAHAM_CML_DIVIDING_VS_NORMAL_QUIESCENT_DN | 49 | 0.6465402 | 2.4402559 | 5.20E-04 | .004 | 625 |
| SANA_TNF_SIGNALING_UP | 37 | 0.68556935 | 2.4303193 | 7.04E-04 | .006 | 562 |
| SEKI_INFLAMMATORY_RESPONSE_LPS_UP | 44 | 0.66059566 | 2.4077451 | 9.58E-04 | .009 | 864 |
| DASU_IL6_SIGNALING_UP | 48 | 0.6300051 | 2.3814805 | 0.001368899 | .014 | 969 |
| LIEN_BREAST_CARCINOMA_METAPLASTIC_VS_DUCTAL_UP | 47 | 0.6460621 | 2.3720858 | 0.00127112 | .014 | 952 |
| REACTOME_PEPTIDE_LIGAND_BINDING_RECEPTORS | 36 | 0.666963 | 2.3602338 | 0.001439457 | .017 | 490 |
| MCLACHLAN_DENTAL_CARIES_DN | 105 | 0.53308874 | 2.33769 | 0.002061751 | .026 | 306 |
| KEGG_RIG_I_LIKE_RECEPTOR_SIGNALING_PATHWAY | 28 | 0.6970441 | 2.2951434 | 0.0034468 | .046 | 1268 |
| TENEDINI_MEGAKARYOCYTE_MARKERS | 27 | 0.6971226 | 2.2726986 | 0.004659514 | .065 | 624 |
| KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 78 | 0.55096024 | 2.2703986 | 0.00447959 | .066 | 1258 |
| KEGG_BLADDER_CANCER | 29 | 0.6838998 | 2.2639232 | 0.004569982 | .07 | 1057 |
ES, enrichment score; FWER, familiar-wise error rate; NES, normalized enrichment score.
Number of genes in each gene set.
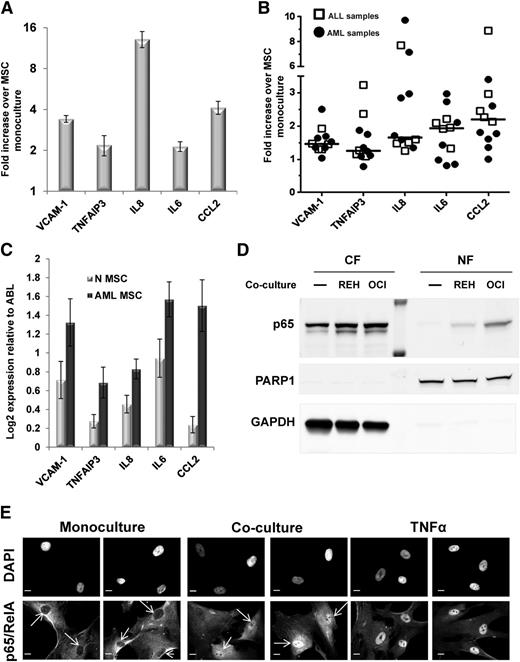
To corroborate the GEP results, we performed a quantitative reverse transcription-polymerase chain reaction (qRT-PCR) validation assay for a group of well-known target genes of NF-κB. Up-regulation of these genes by coculture with REH cells was confirmed, in most cases in the range of two- to fourfold (Figure 1A). To exclude the possibility that the observed upregulation of NF-κB target genes in BM-MSCs was cell line dependent, we cocultured normal BM-MSCs (N-MSCs) with several primary leukemia samples of ALL and AML (supplemental Table 5). After 48 hours, we isolated BM-MSCs from cocultures by fluorescence-activated cell sorter and used qRT-PCR to analyze changes in the expression of selected genes as compared with BM-MSCs cultured alone. Despite some variability between patient samples, the primary leukemia cells significantly up-regulated the expression of the examined NF-κB target genes in N-MSCs, following the same pattern as observed in the REH cells (Figure 1B). In addition, we isolated BM-MSCs from 8 normal donors and from 12 AML patients (supplemental Table 6) and cultured them in the absence of other BM-derived cells for 4 to 8 weeks before RNA extraction. qRT-PCR analysis consistently showed upregulation of NF-κB target genes in AML-derived BM-MSCs (AML-MSCs) compared with N-MSCs (Figure 1C). The GEP and qRT-PCR data suggested that NF-κB activation in BM-MSCs is a common consequence of the leukemia-stroma interaction.
Coculture with leukemia cells induces NF-κB activation in BM-MSCs. (A) A group of array-identified upregulated genes in cocultured BM-MSCs was validated by qRT-PCR. The bars represent qRT-PCR data from 3 independent experiments, and results are expressed as fold difference expression (±SEM) in the cocultured BM-MSCs vs the monocultured BM-MSCs. (B) A total of 5 ALL and 7 AML patient samples were independently cocultured with BM-MSCs and processed as in A. Data from qRT-PCR analysis are expressed as fold difference expression in cocultured BM-MSC vs the monocultured BM-MSCs. Mean expression value for each gene is shown as a bar. (C) qRT-PCR analysis showing expression levels of a selected group of NF-κB target genes in AML-MSCs (n = 12) compared with N-MSCs (n = 8). A total of 12 AML-derived and 8 normal donor-derived primary MSC cultures were analyzed. Bars represent the mean value (±SEM) Log2 expression levels relative to ABL (housekeeping control) expression levels. (D) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of lysates from BM-MSCs cultured alone (−) or cocultured with REH or OCI-AML3 cells for 1 hour. Each well corresponds to 5 µg of total protein. Membranes were probed with rabbit monoclonal anti-p65, mouse monoclonal anti-PARP1 (nuclear fraction loading control), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (cytosolic fraction loading control). (E) Immunofluorescence staining for p65/RelA showing p65 translocation into BM-MSC nuclei on interaction with leukemia cells in coculture conditions. BM-MSCs were cultured alone (monoculture) or cocultured with REH cells for 24 hours and then fixed with 4% paraformaldehyde (PFA). As controls, BM-MSCs cultured alone were treated with vehicle or TNFα (20 ng/mL) for 30 minutes. Nuclei were counterstained with DAPI. Scale bar, 10 µm. Arrows point at absence (monoculture panel) or presence (coculture panel) of p65 in nuclei.
Coculture with leukemia cells induces NF-κB activation in BM-MSCs. (A) A group of array-identified upregulated genes in cocultured BM-MSCs was validated by qRT-PCR. The bars represent qRT-PCR data from 3 independent experiments, and results are expressed as fold difference expression (±SEM) in the cocultured BM-MSCs vs the monocultured BM-MSCs. (B) A total of 5 ALL and 7 AML patient samples were independently cocultured with BM-MSCs and processed as in A. Data from qRT-PCR analysis are expressed as fold difference expression in cocultured BM-MSC vs the monocultured BM-MSCs. Mean expression value for each gene is shown as a bar. (C) qRT-PCR analysis showing expression levels of a selected group of NF-κB target genes in AML-MSCs (n = 12) compared with N-MSCs (n = 8). A total of 12 AML-derived and 8 normal donor-derived primary MSC cultures were analyzed. Bars represent the mean value (±SEM) Log2 expression levels relative to ABL (housekeeping control) expression levels. (D) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of lysates from BM-MSCs cultured alone (−) or cocultured with REH or OCI-AML3 cells for 1 hour. Each well corresponds to 5 µg of total protein. Membranes were probed with rabbit monoclonal anti-p65, mouse monoclonal anti-PARP1 (nuclear fraction loading control), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (cytosolic fraction loading control). (E) Immunofluorescence staining for p65/RelA showing p65 translocation into BM-MSC nuclei on interaction with leukemia cells in coculture conditions. BM-MSCs were cultured alone (monoculture) or cocultured with REH cells for 24 hours and then fixed with 4% paraformaldehyde (PFA). As controls, BM-MSCs cultured alone were treated with vehicle or TNFα (20 ng/mL) for 30 minutes. Nuclei were counterstained with DAPI. Scale bar, 10 µm. Arrows point at absence (monoculture panel) or presence (coculture panel) of p65 in nuclei.
To confirm these data, we sought direct evidence for the activation of the canonical NF-κB pathway in BM-MSCs by interaction with leukemia cells. Such activation causes members of the IκB family of inhibitors, particularly IκBα, to be phosphorylated by the IκB kinase (IKK) complex. This activation results in the ubiquitination and proteasomal degradation of the IκB family proteins and the release and nuclear translocation of the NF-κB heterodimers containing p65 (RelA) or c-Rel.26 Western blot analysis of cytosolic and nuclear fractions from BM-MSCs cocultured with REH cells or OCI-AML3, an AML cell line, showed increased p65 protein level in nuclear extracts from cocultured BM-MSCs compared with BM-MSCs cultured alone (Figure 1D). These results were confirmed by confocal microscopy of BM-MSCs cocultured with REH cells (Figure 1E) and OCI-AML3 cells (supplemental Figure 4).
Chemical inhibition of NF-κB activation blocks stroma-mediated resistance of leukemia cells to chemotherapeutic agents
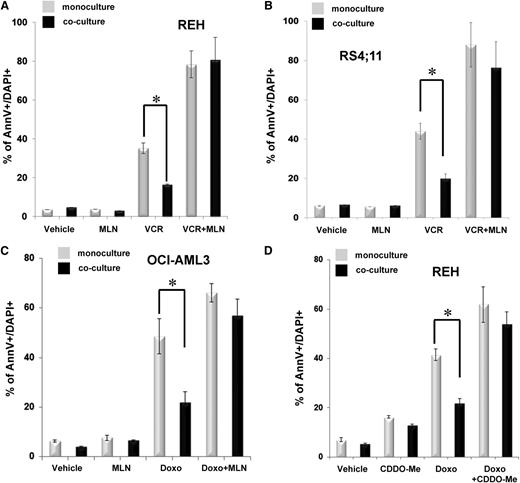
Given that many of the NF-κB target genes (eg, IL-8 and IL-6) that were upregulated in BM-MSCs by coculture have paracrine effects, we hypothesized that the stromal NF-κB activation may contribute to the chemoresistance of leukemia cells. To investigate this hypothesis, we first used the small-molecule IKKβ inhibitor MLN120B31 to block classical NF-κB activation. Treatment of REH cells cultured alone with 10 μmol/L MLN120B did not have an obvious effect on the proliferation or viability of the leukemia cells. However, the toxicity (measured by % of annexin V+DAPI-positive cells) of a sublethal dose (ie, 75 ng/mL) of VCR, a standard chemotherapeutic agent used in ALL therapy, increased from ∼35% when used alone to ∼80% when combined with MLN120B (Figure 2A; supplemental Figure 5A). The coculture with stroma reduced the cytotoxicity of VCR in the leukemia cells (ie, ∼50% less apoptosis than observed in the VCR-treated REH cells cultured alone), which was consistent with a microenvironmental protective effect.4 Moreover, treatment with MLN120B blocked the ability of BM-MSCs to induce chemoresistance to VCR in cocultured REH cells (ie, there was no significant difference between the percentages of apoptotic cells in the monocultured vs the cocultured leukemia cells).
Blockade of NF-κB activation enhances proapoptotic effects of standard chemotherapy. (A,D) REH, (B) RS4;11, and (C) OCI-AML3 cells were cultured alone (monoculture) or cocultured with BM-MSCs as indicated in Methods. Monocultured and cocultured cells were treated for 72 hours with either (A-B) VCR or (C-D) Doxo as monotherapy or in combination with one of the IKKβ inhibitors (A-C) MLN120B (MLN) or (D) CDDO-Me. The percentage of apoptotic cells (annexinV+/DAPI+) was assessed by flow cytometry using annexin V+/DAPI+ staining and counting beads. Data for the absolute number of viable cells are shown in supplemental Figure 5. Results are expressed as the mean of the percentage of annexin V+/DAPI+ (±SEM) of 3 independent experiments. *Statistically significant difference at P ≤ .05. AnnV, annexinV.
Blockade of NF-κB activation enhances proapoptotic effects of standard chemotherapy. (A,D) REH, (B) RS4;11, and (C) OCI-AML3 cells were cultured alone (monoculture) or cocultured with BM-MSCs as indicated in Methods. Monocultured and cocultured cells were treated for 72 hours with either (A-B) VCR or (C-D) Doxo as monotherapy or in combination with one of the IKKβ inhibitors (A-C) MLN120B (MLN) or (D) CDDO-Me. The percentage of apoptotic cells (annexinV+/DAPI+) was assessed by flow cytometry using annexin V+/DAPI+ staining and counting beads. Data for the absolute number of viable cells are shown in supplemental Figure 5. Results are expressed as the mean of the percentage of annexin V+/DAPI+ (±SEM) of 3 independent experiments. *Statistically significant difference at P ≤ .05. AnnV, annexinV.
Similar results were obtained when RS4;11 cells were treated with MLN120B and VCR (Figure 2B; supplemental Figure 5B) and when OCI-AML3 cells were treated with the chemotherapeutic agent doxorubicin (Doxo, 50 ng/mL) in combination with MLN120B (Figure 2C; supplemental Figure 5C). A comparable reduction in BM-MSC-mediated chemoresistance was observed when 50 ng/mL CDDO-Me, another putative NF-κB inhibitor,32,33 was used in combination with 50 ng/mL of Doxo in both REH (Figure 2D; supplemental Figure 5D) and RS4;11 cells (supplemental Figure 5E-F). Inhibition of stroma-mediated chemoresistance was also observed in blasts from 2 of 3 AML patients (patients 13 and 15) treated with the combination of cytarabidine and MLN-120B (supplemental Figure 6; supplemental Table 5). Together, these results indicated that blocking NF-κB activation in both leukemia cells and the stroma cannot only abrogate the stroma-mediated chemoresistance of leukemia cells, but can also increase the apoptotic effects of chemotherapeutic agents, particularly those that induce NF-κB prosurvival pathways.34,35
Overexpression of IκBα “super repressor” in BM-MSCs blocks NF-κB activity and impairs the BM-MSC−mediated chemoresistance in leukemia cells
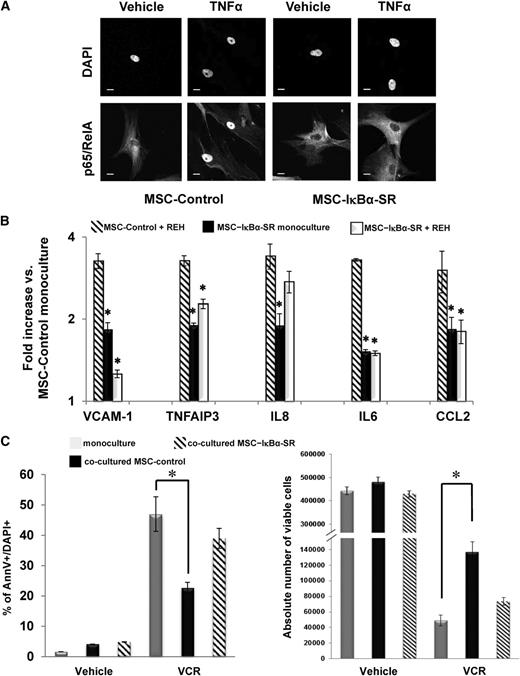
Although the use of chemical inhibitors to block NF-κB activation suggested the involvement of the NF-κB pathway in the cytoprotective response of the leukemia cells to various chemotherapeutic agents, it cannot dissect the relevance of the blocking effects in each particular compartment (ie, BM stromal cells or leukemia cells), and it could certainly produce off-target effects. To investigate whether inhibition of canonical NF-κB activation in BM-MSCs alone is sufficient to reduce stroma-mediated chemoresistance in leukemia cells, we expressed a super repressor form of IκBα (IκBα-SR) in BM-MSCs via a lentiviral vector (supplemental Figure 5A). This mutated form of IκBα cannot be phosphorylated by the IKK complex and consequently inhibits canonical NF-κB activation. The overexpression of IκBα-SR did not markedly affect the proliferation (data not shown) or differentiation potential of the stably transduced BM-MSCs (supplemental Figure 5B). However, it effectively blocked the nuclear localization of p65 after tumor necrosis factor (TNF)α stimulation (Figure 3A), demonstrating the ability of this mutant IκBα to inhibit canonical NF-κB activation. The overexpression of IκBα-SR in the BM-MSCs also blocked or decreased the coculture-induced upregulation of NF-κB target genes (Figure 3B), indicating that NF-κB activation is indeed involved in this process. Most importantly, IκBα-SR overexpression significantly impaired the ability of BM-MSCs to protect the REH cells from apoptosis induced by VCR (75 ng/mL; Figure 3C), illustrating that NF-κB activation in BM-MSC is involved in the chemoresistance of the leukemia cells conferred by their interaction with the stromal cells.
The effects of overexpression of IκBα super repressor (IκBα-SR) in BM-MSCs. (A) Immunofluorescence staining for p65/RelA in BM-MSCs transduced with either empty control (MSC-Control) or IκBα-SR virus (MSC-IκBα-SR). p65/RelA nuclear translocation was determined by confocal microscopy analysis in cells treated with dimethylsulfoxide (vehicle) or TNFα (20 ng/mL) for 30 minutes before fixation with 4% PFA. Approximately 50 fields with 2 to 3 cells per field were analyzed per condition. The nuclei were counterstained with DAPI. Scale bar, 10 µm. (B) MSC-Control or MSC-IκBα-SR cells were cultured alone (monoculture) or cocultured with REH cells for 48 hours as indicated. Total RNA was extracted, and qRT-PCR was carried out to detect the expression of a selected group of NF-κB target genes. Results of 3 independent experiments are expressed as the mean fold difference expression (±SEM) in different culture conditions over the expression levels in the monocultured MSC-Control. (C) REH cells were cultured alone or cocultured with either MSC-Control or MSC-IκBα-SR and then treated with VCR for 72 hours. As described for Fig. 2, (left) the percentage of apoptotic cells and (right) absolute number of viable cells were assessed by flow cytometry using annexin V+/DAPI+ staining and counting beads. Results are expressed as the mean of the percentage of annexin V+/DAPI+ (±SEM) and the mean of the absolute number of viable cells (±SEM) of 2 independent experiments. *P ≤ .05.
The effects of overexpression of IκBα super repressor (IκBα-SR) in BM-MSCs. (A) Immunofluorescence staining for p65/RelA in BM-MSCs transduced with either empty control (MSC-Control) or IκBα-SR virus (MSC-IκBα-SR). p65/RelA nuclear translocation was determined by confocal microscopy analysis in cells treated with dimethylsulfoxide (vehicle) or TNFα (20 ng/mL) for 30 minutes before fixation with 4% PFA. Approximately 50 fields with 2 to 3 cells per field were analyzed per condition. The nuclei were counterstained with DAPI. Scale bar, 10 µm. (B) MSC-Control or MSC-IκBα-SR cells were cultured alone (monoculture) or cocultured with REH cells for 48 hours as indicated. Total RNA was extracted, and qRT-PCR was carried out to detect the expression of a selected group of NF-κB target genes. Results of 3 independent experiments are expressed as the mean fold difference expression (±SEM) in different culture conditions over the expression levels in the monocultured MSC-Control. (C) REH cells were cultured alone or cocultured with either MSC-Control or MSC-IκBα-SR and then treated with VCR for 72 hours. As described for Fig. 2, (left) the percentage of apoptotic cells and (right) absolute number of viable cells were assessed by flow cytometry using annexin V+/DAPI+ staining and counting beads. Results are expressed as the mean of the percentage of annexin V+/DAPI+ (±SEM) and the mean of the absolute number of viable cells (±SEM) of 2 independent experiments. *P ≤ .05.
Blockade of NF-κB activation in BM stroma in the humanized extramedullary BM model reduces leukemia burden following chemotherapy in vivo
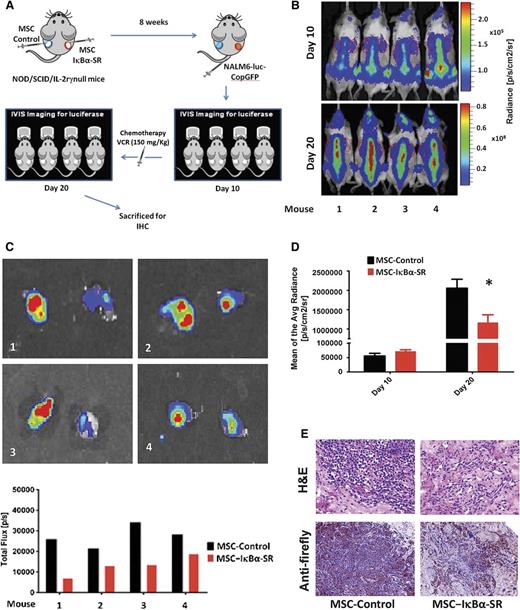
To assess the contribution of NF-κB activation to the stroma-mediated chemoresistance of leukemia cells in vivo, we used an approach recently developed in our laboratory to generate ectopic human-derived extramedullary bone and BM in immunodeficient mice.29 Briefly, primary human BM-MSC are mixed with primary human endothelial precursors (ECFCs) and Matrigel and injected subcutaneously in mice to generate the humanized extramedullary BM niches hosting human hematopoietic and/or xenotransplanted leukemic cells. We used BM-MSCs that had been stably transduced with either empty vector or IκBα-SR, injecting them with ECFCs and Matrigel subcutaneously into contralateral flanks of NOD/SCID/IL-2rγ-null mice (Figure 4A).
Blockade of NF-κB activation in BM-MSCs reduces in vivo leukemia burden. (A) A mixture of Matrigel, ECFCs, and either MSC-Control or MSC-IκBα-SR were injected subcutaneously into contralateral flanks of NOD/SCID/IL-2rγ−null mice (a representative example of 2 independent experiments with 4 mice, n = 8, is shown). NALM6-luciferase-CopGFP leukemia cells were intravenously transplanted into the mice, and tumor burden was monitored throughout the experiment by bioluminescence imaging with the IVIS system. Ten days after leukemia cell engraftment, mice were administered VCR for another 10 days. (B) Animals were imaged and analyzed for luciferase signal (upper) right before chemotherapy treatment started (day 10) and (lower) on the last day of treatment (day 20). (C) (Upper) Both extramedullary bones from each mouse were surgically removed at the end of the experiment, and (lower) the intensity of signal irradiating from them was quantified and plotted. (D) Average radiance measurement of luciferase signal corresponding to extramedullary bone areas derived from MSC-Control or MSC-IκBα-SR before and after 10 days of treatment with VCR, indicating a decrease in leukemia burden in extramedullary bones derived from MSC-IκBα-SR. Average radiance is expressed as photons per second per centimeter squared per steradian (p/s/cm2/sr). *Statistically significant difference at P ≤ .05. (E) Immunohistochemical analysis of artificial BM sections stained with hematoxylin and eosin or anti-luciferase antibody.
Blockade of NF-κB activation in BM-MSCs reduces in vivo leukemia burden. (A) A mixture of Matrigel, ECFCs, and either MSC-Control or MSC-IκBα-SR were injected subcutaneously into contralateral flanks of NOD/SCID/IL-2rγ−null mice (a representative example of 2 independent experiments with 4 mice, n = 8, is shown). NALM6-luciferase-CopGFP leukemia cells were intravenously transplanted into the mice, and tumor burden was monitored throughout the experiment by bioluminescence imaging with the IVIS system. Ten days after leukemia cell engraftment, mice were administered VCR for another 10 days. (B) Animals were imaged and analyzed for luciferase signal (upper) right before chemotherapy treatment started (day 10) and (lower) on the last day of treatment (day 20). (C) (Upper) Both extramedullary bones from each mouse were surgically removed at the end of the experiment, and (lower) the intensity of signal irradiating from them was quantified and plotted. (D) Average radiance measurement of luciferase signal corresponding to extramedullary bone areas derived from MSC-Control or MSC-IκBα-SR before and after 10 days of treatment with VCR, indicating a decrease in leukemia burden in extramedullary bones derived from MSC-IκBα-SR. Average radiance is expressed as photons per second per centimeter squared per steradian (p/s/cm2/sr). *Statistically significant difference at P ≤ .05. (E) Immunohistochemical analysis of artificial BM sections stained with hematoxylin and eosin or anti-luciferase antibody.
Within 8 weeks after implantation, we observed the development of well-defined, vascularized bone-like tissues in both flanks of all mice. In vivo staining with OsteoSense 750, a fluorescent agent that targets hydroxylapatite, confirmed high osteoblast activity originating from the BM-MSC-ECFC-Matrigel-derived tissues in the flanks of the mice (supplemental Figure 8). After the formation of the humanized extramedullary bone, we intravenously injected the pre-B-ALL cell line NALM6-luciferase-CopGFP into each mouse and monitored tumor burden by bioluminescence imaging with the In Vivo Imaging System (IVIS). After 10 days of NALM6-luciferase-CopGFP cell engraftment, luciferase activity was observed not only in the calvaria and vertebral column but also in the flanks of mice where the BM-MSC-ECFC-Matrigel mixtures had been implanted (Figure 4B), suggesting that the leukemia cells engrafted not only into murine BM reservoirs but also into both control- and IκBα-SR-transduced BM-MSC-derived extramedullary bones.
Immediately after confirmation of leukemia cell engraftment, the mice were administered VCR (150 µg/kg) every 3 days for a total of 10 days. Whereas there were no obvious differences between the luciferase signals in both flanks before treatment (Figure 4B,D), at the end of the treatment (ie, 20 days after the transplantation), there was a statistically significant reduction in leukemia cell burden in extramedullary bones derived from the IκBα-SR-transduced BM-MSCs (Figure 4B, lower; Figure 4C-D). These observations were confirmed by hematoxylin and eosin staining and by immunohistochemical staining with anti-luciferase antibody (Figure 4E). These results indicated that the NF-κB activity in BM-MSCs is an integral part of the stroma-mediated chemoresistance imparted by the BM microenvironment to the leukemic cells.
Transcriptional activation of NF-κB target genes in BM-MSC is linked to interaction between VCAM-1 and VLA-4
Both cDNA array and qRT-PCR data indicated that the leukemia-stroma interaction induced the transcriptional up-regulation of NF-κB target genes in BM-MSCs. Notably, one of the consistently up-regulated genes was VCAM-1. The VCAM-1 protein is an integrin receptor cell adhesion molecule that acts as the primary ligand for VLA-4, which is an interaction that participates in leukocyte-endothelial cell signal transduction. A previous study demonstrated that the interaction between endothelial cells and monocytes via the VCAM-1-VLA-4 engagement caused the activation of NF-κB in both cell types. The activating mechanism also apparently involved IL-1β and the IL-1 receptor (IL-1R).36
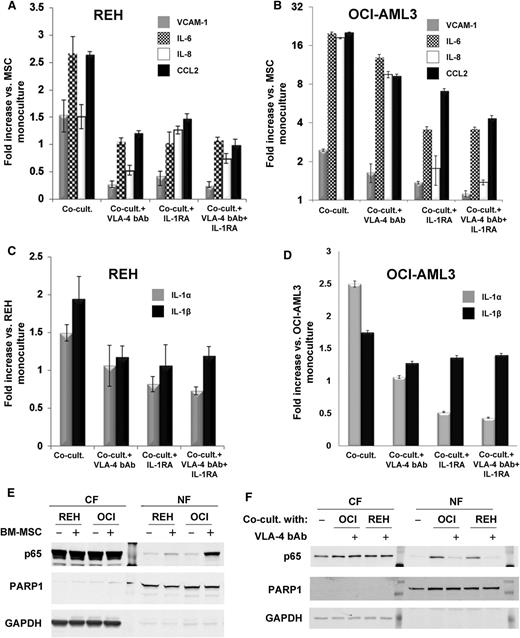
To test whether VCAM-1-VLA-4 signaling could play a role in leukemia cell-mediated NF-κB activation in cells constituting the microenvironment, we used VLA-4 blocking antibody (VLA-4 bAb). Preincubation of REH or OCI-AML3 cells with the VLA-4 bAb prior to coculture significantly reduced expression of selected NF-κB target genes in BM-MSCs (Figure 5A-B, respectively). Furthermore, and in agreement with previous data,36 IL-1 blockade using IL1RA had an even more pronounced inhibitory effect. Accordingly, the inhibition of the VLA-4 and VCAM-1 interaction also impaired the autocrine IL-1α mRNA expression in cocultured REH (Figure 5C) and OCI-AML3 (Figure 5D), albeit the effect on IL-1β was modest. This result suggested that BM-MSC coculture could induce the reciprocal activation of NF-κB and IL-1α production in the leukemic cells. Indeed, western blot analyses revealed that coculture with BM-MSCs induced p65 translocation to the nuclear fraction of both REH and OCI-AML3 cells compared with the same cells cultured alone (Figure 5E), although to a lesser extent to that observed for BM-MSCs (ie, compared with Figure 1D). Moreover, and in agreement with the qRT-PCR data (Figure 5A-B), blockade of VCAM-1-VLA-4 signaling by preincubating REH and OCI-AML3 cells with VLA-4 bAb significantly reduced p65 nuclear translocation in cocultured BM-MSCs (Figure 5F).
Blockade of VLA-4/VCAM-1 signaling impairs NF-κB activation and transcription of NF-κB downstream target genes. Normal BM-MSCs were cocultured for 24 hours with (A) REH or (B) OCI-AML3 cells that were either untreated or preincubated with VLA-4 bAb for 1 hour. When indicated, 200 ng/mL of IL1RA was added to the coculture medium. BM-MSCs were cultured alone (monocultured) as controls. After separating the cells (as indicated in Methods), total RNA from BM-MSCs and leukemic cells was extracted. (A-B) Expression of selected NF-κB target genes in cocultured BM-MSCs was determined by qRT-PCR. Bars represent qRT-PCR data from triplicate samples, and results are expressed as fold difference expression (±SEM) in each coculture condition vs the monocultured BM-MSCs. (C-D) Total RNA from (C) REH and (D) OCI-AML3 ells (cocultured with MSCs in experiments shown in A and B, respectively) was extracted, and IL-1α and IL-1 β mRNA expression levels were quantified by qRT-PCR. Bars represent qRT-PCR data from triplicate samples, and results are expressed as fold difference expression (±SEM) in each coculture condition vs the monocultured OCI-AML3 cells. (E) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of lysates from REH and OCI-AML3 cultured alone (−) or cocultured with BM-MSC (+) for 1 hour. (F) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of BM-MSC lysates cultured alone (−) or cocultured with OCI-AML3 or REH cells for 1 hour. Leukemic cells were preincubated with VLA-4 bAb when indicated (+) before coculture. Each well corresponds to 5 µg of total protein. Membranes were probed with rabbit monoclonal anti-p65, mouse monoclonal anti-PARP1 (nuclear fraction loading control), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (cytosolic fraction loading control).
Blockade of VLA-4/VCAM-1 signaling impairs NF-κB activation and transcription of NF-κB downstream target genes. Normal BM-MSCs were cocultured for 24 hours with (A) REH or (B) OCI-AML3 cells that were either untreated or preincubated with VLA-4 bAb for 1 hour. When indicated, 200 ng/mL of IL1RA was added to the coculture medium. BM-MSCs were cultured alone (monocultured) as controls. After separating the cells (as indicated in Methods), total RNA from BM-MSCs and leukemic cells was extracted. (A-B) Expression of selected NF-κB target genes in cocultured BM-MSCs was determined by qRT-PCR. Bars represent qRT-PCR data from triplicate samples, and results are expressed as fold difference expression (±SEM) in each coculture condition vs the monocultured BM-MSCs. (C-D) Total RNA from (C) REH and (D) OCI-AML3 ells (cocultured with MSCs in experiments shown in A and B, respectively) was extracted, and IL-1α and IL-1 β mRNA expression levels were quantified by qRT-PCR. Bars represent qRT-PCR data from triplicate samples, and results are expressed as fold difference expression (±SEM) in each coculture condition vs the monocultured OCI-AML3 cells. (E) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of lysates from REH and OCI-AML3 cultured alone (−) or cocultured with BM-MSC (+) for 1 hour. (F) Western blot analysis of cytosolic (CF) and nuclear (NF) fractions of BM-MSC lysates cultured alone (−) or cocultured with OCI-AML3 or REH cells for 1 hour. Leukemic cells were preincubated with VLA-4 bAb when indicated (+) before coculture. Each well corresponds to 5 µg of total protein. Membranes were probed with rabbit monoclonal anti-p65, mouse monoclonal anti-PARP1 (nuclear fraction loading control), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (cytosolic fraction loading control).
Discussion
Many studies have illustrated the ability of BM stromal cells, in particular BM-MSCs, to protect leukemia cells from spontaneous and chemotherapy-induced apoptosis. Moreover, increasing evidence indicates that cross-talk between leukemia cells and BM stromal cells results in reciprocal modulation of each other’s functions13,37-39 to create a leukemia-promoting “soil” that is able to nurture the malignant “seed” cells.23 In this regard, most of the previous studies have focused on characterizing changes in leukemia cells that were caused by interaction with stromal cells. In this study, we focused instead on changes in BM-MSCs caused by leukemia cells, through which the leukemia cells essentially modify their own microenvironment.
The whole-genome gene expression profiling we carried out in this study identified consistent transcriptional changes suggesting NF-κB activation in the BM-MSCs, which was apparently caused by leukemia cells (Figure 1A-C; supplemental Figures 1B and 3). Our subsequent experiments confirmed that the coculture with leukemic cells activated NF-κB in BM-MSCs, at least in part through the canonical pathway, as demonstrated by the nuclear translocation of p65/RelA (Figure 1D-E; supplemental Figure 4). These data are consistent with our observation that phosphorylated p65 (Ser276) is localized in the nuclei of CD90-positive BM stroma cells in BM biopsies of patients with ALL (supplemental Figure 9) and that AML-derived BM-MSCs display higher transcriptional activity of NF-κB target genes even after several passages, suggesting that these leukemia-induced changes can persist for prolonged time even in the absence of leukemia cells (Figure 1C).
We further showed that the blockade of NF-κB activation in BM-MSCs, either by chemical inhibition or by the overexpression of a super-repressor form of IκBα, could notably impair their ability to confer chemoresistance to the leukemia cells (Figures 3–5). This mechanism could, at least in part, contribute to the increased efficacy of the proteasome inhibitor bortezomib, which is known to block NF-κB signaling, in combination with standard chemotherapy in patients with advanced ALL.40 Bortezomib is used in the treatment of multiple myeloma41 and, in combination with other agents, has been the focus of several clinical trials for the treatment of AML.42-44
In recent years, studies conducted in mice have shown NF-κB activation in tumor-surrounding stroma on interaction with tumor cells. In a mouse model of squamous skin carcinoma, carcinoma cells were shown to educate normal fibroblasts into tumor-promoting stromal cells through the activation of NF-κB.24 In a recent report and using a murine model of chronic myeloid leukemia (CML), Schmidt et al showed that CML cells can induce the expression of placental growth factor by BM-MSCs in an NF-κB-dependent manner.23 Unfortunately, little is known about the role of activated NF-κB in human BM-derived MSCs. Our results extend these data by showing for the first time in a bona-fide human BM model of acute leukemia a direct link between NF-κB activation and stromal-associated chemoprotection. Our in vivo model allowed us to illustrate that the blockade of NF-κB activation with IκBα-SR in BM-MSCs increased the induction of apoptosis in leukemia cells by chemotherapy (Figure 4). Furthermore, in vitro chemical inhibition of NF-κB in both stromal and leukemia cells increased the toxicity of standard chemotherapeutic agents, suggesting that the inhibition of NF-κB in combination with traditional chemotherapy, especially those known to activate NF-κB-mediated prosurvival pathways, could improve the chances of eradicating circulating and residual BM leukemia cells.
A role for adhesion-mediated signals in the activation of NF-κB was shown in monocytes whereby binding of monocyte-associated VLA-4 to VCAM-1 expressed on the surface of vascular endothelial cells induces NF-κB activation in both cell types.36 Our data demonstrate the up-regulation of VCAM-1 in cocultured BM-MSCs, and our subsequent observations indicated that direct contact between leukemia cells and BM-MSCs is needed to achieve maximal NF-κB activation in BM-MSCs (supplemental Figure 10). Specifically, the expression of VLA-4 on the leukemic cells and VCAM-1 on BM-MSCs has been implicated in the maintenance of residual disease in AML and in resistance of stroma-adherent AML cells to chemotherapy.7 Interestingly, experiments conducted using human recombinant VCAM-1-coated dishes indicated that this adhesion molecule is sufficient to stimulate NF-κB activation in leukemic cells and such activation can be blocked with MLN-120B (supplemental Figure 11A). Despite the NF-κB activation, immobilized VCAM-1 failed to confer resistance to chemotherapy treatment (supplemental Figure 11B), suggesting the need for stromal cells capable of responding to activated leukemic cells. In agreement with these data, only conditioned medium from cocultured BM-MSCs and not from monocultured BM-MSCs was capable of protecting leukemic cells from chemotherapy-induced apoptosis (supplemental Figure 11C-D). These data indicate that direct contact between leukemic cells and BM-MSCs (likely through VCAM-1/VLA4 interaction) is needed for a reciprocal activation of NF-κB in both cellular compartments and to trigger the stroma-mediated chemoresistance. In addition, our results using VLA-4 blocking antibody indicated that the interaction between VLA-4 and VCAM-1 is, at least in part, responsible for the activation of NF-κB in the BM-MSCs (Figure 5 A-B,F). Notably, a recent report showed dramatic improvement of survival of mice transplanted with primary human ALL cells, on combination of Natalizumab, an antifunctional VLA-4 antibody, with chemotherapy.45 Our data further indicated that coculture with BM-MSCs can also induce NF-κB activation in leukemia cells (Figure 5E) and that blockade of the VLA-4-VCAM-1 interaction during coculture can impair levels of IL-1α, and to a less extent IL-1β, mRNA expression in REH and OCI-AML3 leukemia cells (Figure 5C-D). In addition, disruption of the VLA-4-VCAM-1 interaction during coculture impaired the nuclear translocation of NF-κB in BM-MSC (Figure 5F). We also found that interference of IL-1α and IL-1β signaling using IL1RA resulted in enhanced blockade of NF-κB activation in BM-MSCs (Figure 5A-B) and on the transcriptional up-regulation of IL-1α and IL-1β mRNA in REH and OCI-AML3 cells (Figure 5C-D). These data are in agreement with a recent publication from Weinberg’s group showing that carcinoma cell-derived IL-1 signals through the IL-1R on tumor-associated MSCs, inducing the expression of prostaglandin E2 and production of cytokines including IL-6 and IL-8.46 This integrin-mediated signaling involving the VLA-4 and VCAM-1 interaction could be one of the mechanisms cooperating to establish communication between the leukemia and stromal cells. We envision that a combination of cell adhesion-mediated signals (VLA-4/VCAM-1) and soluble factor-mediated signals (IL-1) is needed for this “stroma education” phenomenon to occur (Figure 6). These findings suggest future avenues exploring targeted inhibition of selective pathways with the goal to abrogate microenvironment-mediated chemoresistance in leukemias.
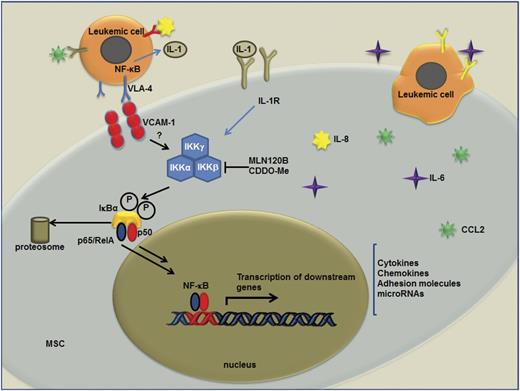
Schematic representation of leukemic cell−BM-MSC cross-talk. Interactions of leukemic cells with BM-MSCs promote transcriptional changes in the BM stroma that ultimately impact leukemic cells proliferation and survival. Activation of NF-κB and transcription of NF-κB downstream target genes (IL-8, IL-6, CCL2, and VCAM-1, etc.) are triggered in BM-MSCs by these interactions. Communication between these 2 cell types can be achieved by integrins and their receptors (ie, VCAM-1/VLA-4) or by soluble factors that are directly secreted to the extracellular milieu (such as IL-1). VCAM-1/VLA-4 interaction between leukemia cells and BM-MSC may provide a possible mechanism for the activation of NF-κB in both BM-MSCs and leukemia cells.
Schematic representation of leukemic cell−BM-MSC cross-talk. Interactions of leukemic cells with BM-MSCs promote transcriptional changes in the BM stroma that ultimately impact leukemic cells proliferation and survival. Activation of NF-κB and transcription of NF-κB downstream target genes (IL-8, IL-6, CCL2, and VCAM-1, etc.) are triggered in BM-MSCs by these interactions. Communication between these 2 cell types can be achieved by integrins and their receptors (ie, VCAM-1/VLA-4) or by soluble factors that are directly secreted to the extracellular milieu (such as IL-1). VCAM-1/VLA-4 interaction between leukemia cells and BM-MSC may provide a possible mechanism for the activation of NF-κB in both BM-MSCs and leukemia cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Lizee for providing recombinant IL-1α; L. Pham, I. Samudio, S. Duvuri, and T. McQueen for technical support; and E. Rozengurt and N. Hail Jr. for advice and helpful comments on the manuscript.
This work was supported by the National Institutes of Health/National Cancer Institute grants P01 CA55164, MD Anderson's National Cancer Institute Cancer Center Support Grant P30 CA016672, Leukemia SPORE P50 CA100632, and Lymphoma SPORE P50 CA136411, and the Paul and Mary Haas Chair in Genetics (M.A.) and National Institutes of Health/National Cancer Institute grant R01 CA155056, Leukemia and Lymphoma Society grant CDP-01, and Leukemia Spore DRP 5 P50 CA100632-08 (M.K.).
Authorship
Contribution: R.J., M.K., and M.A. were involved in the conception and design of the study; R.J., R.E.D., M.K., and M.A. developed the methodology; R.J., Y.C., V.L.B., P.R., and M.H.N. acquired the data; R.J., Y.C., Z.W., W.M., M.Z., E.L.S., Y.W., R.E.D., M.K., and M.A. provided the analysis and interpretation of the data; R.J., R.E.D., M.K., and M.A. wrote, reviewed, and revised the manuscript; W.D.S., E.J.S., M.H.N., C.E.B.-R., D.S., K.S., P.Y.M. and S.K. provided administrative, technical, and material support; and M.K. and M.A. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Konopleva, Section of Molecular Hematology and Therapy, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0448, Houston, TX 77030; e-mail: mkonople@mdanderson.org; and Michael Andreeff, Section of Molecular Hematology and Therapy, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0448, Houston, TX 77030; e-mail: mandreef@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal