Abstract
Monoclonal antibody therapy has revolutionized cancer treatment by significantly improving patient survival both in solid tumors and hematologic malignancies. Recent technological advances have increased the effectiveness of immunotherapy leading to its broader application in diverse treatment settings. Immunoconjugates (ICs) consist of a cytotoxic effector covalently linked to a monoclonal antibody that enables the targeted delivery of its therapeutic payload to tumors based on cell-surface receptor recognition. ICs are classified into 3 groups based on their effector type: immunotoxins (protein toxin), radioimmunoconjugates (radionuclide), and antibody drug conjugates (small-molecule drug). Optimization of each individual component of an IC (antibody, linker, and effector) is essential for therapeutic efficacy. Clinical trials have been conducted to investigate the effectiveness of ICs in hematologic malignancies both as monotherapy and in multiagent regimens in relapsed/refractory disease as well as frontline settings. These studies have yielded encouraging results particularly in lymphoma. ICs comprise an exciting group of therapeutics that promise to play an increasingly important role in the management of hematologic malignancies.
Introduction
A formidable challenge in curing cancer is the difficulty in administering a sufficiently high dose of tumoricidal agents to eradicate systemic disease while minimizing adverse effects on normal tissues. Tumor-targeted delivery can effectively increase the amount of cytotoxic agent that can be safely given and thereby improve patient survival. Development of a therapeutic with the ability to home to a malignant cell based on surface receptors was realized with the advent of monoclonal antibody therapy.1 Although it required over 20 years from the description of hybridoma technology by Kohler and Millstein to the 1997 US Food and Drug Administration (FDA) approval of rituximab for B-cell non-Hodgkin lymphoma (NHL), unconjugated antibodies have proven to be an essential component of many contemporary treatment regimens for hematologic malignancies.2,3
The ascendance of immunotherapy has not been without obstacles. Initial enthusiasm for antibodies as “magic bullets” was quickly tempered by the realization that immunoglobulins of murine origin were highly immunogenic and neutralized by the same tumor immune surveillance system that these agents sought to enhance.4 Efforts to humanize murine-derived antibodies and create fully human antibodies have largely overcome this impediment.5,6 Unconjugated antibodies such as rituximab exert antitumor effects through complement- or antibody-dependent cell–mediated cytotoxicity facilitated by Fc binding and by activation of apoptotic pathways by cognate antigen binding. Most antibodies exhibit only modest efficacy as single agents and have generally been used in combination with chemotherapy. Attempts to augment antibody activity have included modifications of the immunoglobulin scaffold to enhance immune activation or trigger direct cell death.7-9
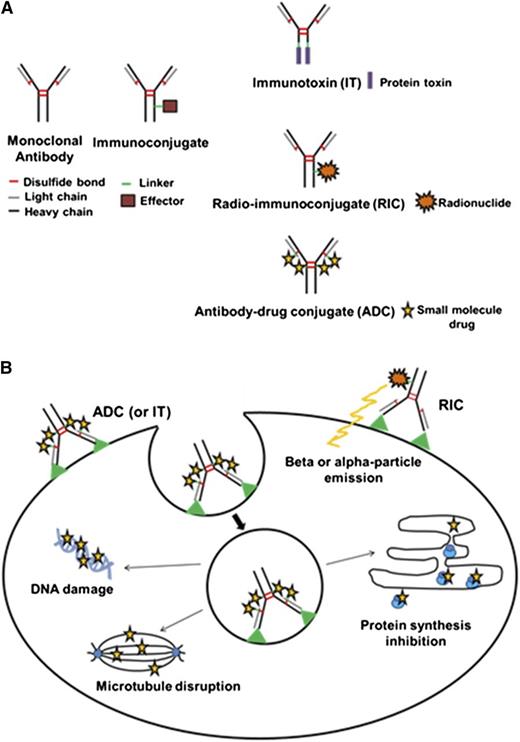
Immunoconjugates (ICs) harness the targeting function of antibodies to specifically deliver a lethal payload to cancer cells.10-12 ICs rely upon a covalently attached effector moiety for therapeutic activity. The effector type classifies ICs into 3 general groups: immunotoxins (ITs), radioimmunoconjugates (RICs), and antibody drug conjugates (ADCs) (Figure 1A). Antibody targeting focuses higher concentrations of the covalently linked toxin, radionuclide, or small-molecule drug to the tumor while reducing exposure to normal tissues, effectively expanding the therapeutic window. In this review, we emphasize the progress in using RICs and ADCs for the treatment of hematologic malignancies. An accompanying article in this series will focus specifically on ITs.
IC structure and mechanism of action. (A) IC types. Schematic diagrams of both a monoclonal antibody and an IC are depicted. An IC consists of a monoclonal antibody, linker, and effector molecule. The 3 general categories of ICs linked to different effector molecules are shown. An IT contains a protein toxin while an RIC possesses a radionuclide. An ADC carries a small-drug molecule. (B) Mechanism of IC activity. The mechanisms of action for the various ICs are illustrated. All ICs recognize and bind to a cognate tumor antigen or receptor. For ITs and ADCs, internalization via receptor-mediated endocytosis is required for entry into the target cell. Subsequent release of the effector moiety from the IC occurs via the conditional cleavage of the linker or protease degradation of the antibody within the endosomal/lysosomal compartment. The released effector toxin or drug diffuses into the cytoplasm and inhibits tumor growth by disruption of microtubules (ADC), damage to DNA (ADC), or inhibition of protein synthesis (IT). For RICs, internalization is not required for cell penetration and damage by the emitted α- or β-particles from the effector radionuclide.
IC structure and mechanism of action. (A) IC types. Schematic diagrams of both a monoclonal antibody and an IC are depicted. An IC consists of a monoclonal antibody, linker, and effector molecule. The 3 general categories of ICs linked to different effector molecules are shown. An IT contains a protein toxin while an RIC possesses a radionuclide. An ADC carries a small-drug molecule. (B) Mechanism of IC activity. The mechanisms of action for the various ICs are illustrated. All ICs recognize and bind to a cognate tumor antigen or receptor. For ITs and ADCs, internalization via receptor-mediated endocytosis is required for entry into the target cell. Subsequent release of the effector moiety from the IC occurs via the conditional cleavage of the linker or protease degradation of the antibody within the endosomal/lysosomal compartment. The released effector toxin or drug diffuses into the cytoplasm and inhibits tumor growth by disruption of microtubules (ADC), damage to DNA (ADC), or inhibition of protein synthesis (IT). For RICs, internalization is not required for cell penetration and damage by the emitted α- or β-particles from the effector radionuclide.
Features of an IC
An IC consists of: (1) the targeting antibody, (2) the effector molecule, and (3) the linker joining the effector to the antibody. Each part plays an essential role in defining the therapeutic activity of the IC.10,11
Several factors are critically important in the selection of an antibody and its cognate cancer antigen or receptor. Ideally, the antigen is preferentially expressed at a high level by neoplastic cells, located on the cell surface with minimal shedding into the surrounding environment and internalizes either constitutively or upon antibody binding (Figure 1B). The latter is critical for ADCs and ITs which carry effectors that inhibit intracellular targets but less so for RICs which emit β- or α-particles that are not restricted by membrane barriers. Endocytic uptake is in fact detrimental for RICs containing iodine-131 (131I) due to lysosomal degradation and release of free 131I or 131I-tyrosine into the blood.13
An ideal antibody penetrates quickly and homogeneously into tumor tissue and is rapidly cleared from systemic circulation after maximal binding of available receptors. The antibody need not possess intrinsic antitumor activity because this is conferred by the effector molecule although affinity maturation can improve antibody binding efficiency and potentiate IC activity.14 Targets investigated for hematologic malignancies include the internalizing receptors CD19, CD22, CD30, CD33, and CD79b as well as the more surface stable receptors CD20 and CD45.
ICs are differentiated by their effector type: protein toxin (IT), radionuclide (RIC), or small-molecule drug (ADC). Judicious selection, modification, and conjugation of effector molecules can enhance IC efficacy. A potent effector is essential because cellular delivery is limited by the number of surface-bound ICs. Most effector molecules are too toxic to use without conjugation and are delivered by ICs in a prodrug form. Synthetic derivatives of natural compounds with enhanced toxicity such as maytansinoids or auristatins have commonly been used.15,16 For RICs, ionizing radiation affects not only the bound cell but neighboring cells as well (“crossfire effect”), therefore the use of α-emitting radionuclides with higher energy and shorter path lengths than the more commonly used β-emitters is being investigated.17,18 Protein engineering can remove immunogenic sequences from ITs that generate neutralizing antibodies. Modification of a drug to a membrane-impermeable form can reduce toxicity stemming from nonspecific uptake of unconjugated effector or premature diffusion out of the target cell after release.19 The number of effector molecules conjugated and their position within the antibody can affect aggregation, antigen binding, and clearance from the circulation as well as potency and tolerability.20
Advances in linker technology have greatly accelerated the development of potent ICs.16,19,21 An ideal linker prevents premature effector release in the circulation yet permits its liberation in the tumor. Unstable linkers lead to nonspecific distribution or rapid clearance accompanied by either intolerable toxicity or reduced potency. ITs and ADCs are typically internalized by receptor-mediated endocytosis and trafficked to the lysosome. Cleavable linkers conditionally release the cytotoxic agent in the presence of a reducing environment (disulfide bond), acid (hydrazone linkage), or lysosomal enzymes (peptide bond) in the endocytic compartment. In contrast, noncleavable linkers (thioether or hindered disulfide bonds) rely upon degradation of the antibody to its constituent amino acids in the lysosome for cytotoxin release. Modification of amino acid residues to control conjugation sites or recombinant DNA technology to generate fusion proteins can overcome difficulties associated with the production of heterogeneous species by traditional chemical conjugation approaches.22 The latter is an inherent advantage of third-generation recombinant ITs and permits large-scale purification from Escherichia coli bulk cultures contributing to reduced complexity of manufacturing and lower production cost compared with chemically conjugated ADCs.23
RICs
Radioimmunotherapy (RIT) has proven effectiveness in hematologic malignancies. The most extensive clinical experience has been with RICs containing the β-particle–emitting isotopes 131I or 90yttrium (90Y) which possess advantageous characteristics including favorable emission profiles, availability, and stable antibody attachment (Table 1). Initial studies in the early 1990s used 131I-labeled monoclonal anti-CD20 antibodies for the treatment of NHL.24,25 The long path length of emitted β-particles produces an advantageous “crossfire effect” on nearby cancer cells not expressing target antigen, though this phenomenon can also produce toxicities in neighboring normal tissues. In contrast, α-particle–emitting radionuclides possess shorter path lengths, exhibit less oxygen dependency for cell killing, and confer a higher linear energy transfer resulting in greater cytotoxicity. However, the limited availability, more difficult radiolabeling chemistry, and short half-lives of most α-emitters have limited their clinical utility to date. Only a few α-emitters, like 213bismuth (213Bi), 211astatine (211At), and 225actinium (225Ac), are practical for clinical use.
RICs
| Antibody . | Target . | Isotope . | Indication . | Stage of development . |
|---|---|---|---|---|
| Anti-Tac antibody (90Y-HAT) | CD25 | 90Y | T-cell NHL, HL | Phase 1 NCT00001575 |
| BB4 antibody | CD138 | 131I | MM | Phase 1 NCT01296204 |
| BC8 antibody-streptavidin conjugate | CD45 | 131I, 90Y | AML, ALL, MDS | Phase 1 NCT00988715 |
| Daclizumab (CHX-A daclizumab) | CD25 | 90Y | HL | Phase 1/2 NCT01468311 |
| Epratuzumab | CD22 | 90Y | B-cell NHL, WM | Phase 1/2 NCT01101581, NCT00004107 |
| Ibritumomab tiuxetan | CD20 | 90Y | B-cell NHL | Approved 2002 |
| Lintuzumab | CD33 | 225Ac | AML | Phase 1/2 NCT01756677 |
| Tositumomab | CD20 | 131I | B-cell NHL | Approved 2003; to be discontinued February 2014 |
| Antibody . | Target . | Isotope . | Indication . | Stage of development . |
|---|---|---|---|---|
| Anti-Tac antibody (90Y-HAT) | CD25 | 90Y | T-cell NHL, HL | Phase 1 NCT00001575 |
| BB4 antibody | CD138 | 131I | MM | Phase 1 NCT01296204 |
| BC8 antibody-streptavidin conjugate | CD45 | 131I, 90Y | AML, ALL, MDS | Phase 1 NCT00988715 |
| Daclizumab (CHX-A daclizumab) | CD25 | 90Y | HL | Phase 1/2 NCT01468311 |
| Epratuzumab | CD22 | 90Y | B-cell NHL, WM | Phase 1/2 NCT01101581, NCT00004107 |
| Ibritumomab tiuxetan | CD20 | 90Y | B-cell NHL | Approved 2002 |
| Lintuzumab | CD33 | 225Ac | AML | Phase 1/2 NCT01756677 |
| Tositumomab | CD20 | 131I | B-cell NHL | Approved 2003; to be discontinued February 2014 |
HAT, humanized anti-Tac; WM, Waldenstrom macroglobulinemia.
To date, RIT has demonstrated the most efficacy in NHL.26 The only RICs currently approved by the FDA are 131I-tositumomab and 90Y-ibritumomab tiuxetan, which both target CD20, a lineage-specific tetrapass phosphoprotein expressed on normal and malignant B lymphocytes. 90Y-ibritumomab is approved for treatment of relapsed/refractory low-grade B-cell NHL or follicular lymphoma (FL) or previously untreated FL after partial response (PR) or complete response (CR) to initial chemotherapy. 131I-tositumomab is approved for similar indications as well as for transformed and rituximab-resistant or refractory NHL. Targeting CD20 with RICs labeled with either 131I- or 90Y-radioisotopes achieves high overall response rate (ORR) and CR rates (50%-80% and 20%-40%, respectively) in extensively pretreated and refractory patients with low-grade or transformed NHL.27,28 Toxicity is generally minor, with delayed myelosuppression occurring 4 to 8 weeks later being the dose-limiting toxicity. Delayed myelodysplasia (MDS) and secondary acute myelogenous leukemia (AML) are uncommon but potentially serious late sequelae of RIT. CD22 has also been examined as a target for RIT of NHL. Fractionated doses of 90Y-epratuzumab were administered to patients with relapsed/refractory NHL as a single agent with an ORR of 62% (48% CR) and a median progression-free survival (PFS) of 9.5 months.29 Dual-targeted RIC and unlabeled antibody has been explored.30 Combining 90Y-epratuzumab with the anti-CD20 antibody veltuzumab was well-tolerated and yielded an ORR of 53% in relapsed/refractory aggressive NHL.31
Incorporating RIT into frontline therapy has also been investigated. A phase 2 study administering a single therapeutic dose of 131I-tositumomab as initial therapy for advanced FL yielded a remarkable 95% ORR (75% CR) and a median PFS of 6.1 years.32 A phase 2 study of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy followed by 131I-tositumomab (SWOG 9911) showed excellent results with an ORR of 91% (69% CR) in patients with previously untreated FL with 60% of patients remaining progression-free for >10 years.33 A subsequent phase 3 trial (SWOG S0016) randomized newly diagnosed advanced-stage FL patients to CHOP plus rituximab (CHOP-R) for 6 cycles vs CHOP for 6 cycles followed by consolidation with 131I-tositumomab (CHOP-RIT).34 There was a trend toward a better 5-year PFS favoring the RIT group (76% CHOP-R vs 80% CHOP-RIT) but it did not reach statistical significance, nor was there an improvement in overall survival (OS) (97% CHOP-R and 93% CHOP-RIT after a median follow-up of 4.9 years). Phase 2 studies have also examined the utility of 90Y-ibritumomab as either a single agent or following chemotherapy in the frontline treatment of FL.35-37 Frontline monotherapy produced an ORR of 87% (56% CR) with a PFS of 26 months after follow-up of 30.6 months.38 Results of a phase 3 trial using 90Y-ibritumomab as consolidation after first remission in advanced-stage FL showed an 8-year PFS of 41% for patients receiving RIT consolidation compared with 22% for patients in the control arm not receiving RIT (P < .001). The time-to-next treatment was prolonged by 5.1 years in patients receiving RIT, although the OS rates were similar.39 There was a higher annualized incidence rate of MDS/AML in the 90Y-ibritumomab–treated group (0.50% vs 0.07%; P = .042).
RIT has been studied in the setting of hematopoietic stem cell transplantation (HSCT) in hopes of improving durable responses. Early studies using myeloablative doses of 131I–anti-CD20 RICs (approximately fivefold higher doses of 131I than conventional RIT) followed by autologous HSCT showed objective remissions in 85% to 95% of patients with multiply relapsed/refractory B-cell NHL and demonstrated durable 10- to 20-year remissions in 40% to 50% of patients.24,40,41 This approach has subsequently been validated using 90Y-ibritumomab with equally promising results.42-44 However, a recent phase 3 trial adding conventional, low doses of 131I-tositumomab to the BEAM regimen (BiCNU [carmustine], etoposide, cytarabine [Ara-C], melphalan) in the setting of autologous HSCT for relapsed/refractory diffuse large B cell lymphoma (DLBCL) did not improve outcomes compared with the control arm (BEAM-rituximab).45 Conversely, a randomized phase 2 trial of 90Y-ibritumomab added to BEAM showed a significantly improved OS for patients receiving BEAM-RIT compared with control patients receiving BEAM alone (92% vs 61%, P = .05).46 A confirmatory phase 3 trial is currently under way (NCT00463463).
The utility of RIT for other hematologic malignancies is being actively explored. RICs targeting CD33, CD45, or CD66 for AML have been examined.47-54 Early-phase clinical trials studying 131I- or 213Bi-conjugated to the humanized anti-CD33 antibody lintuzumab (HuM195) showed tolerability and moderate efficacy in AML patients.47,53,54 To circumvent the short 46-minute half-life of 213Bi, 225Ac has been used in subsequent phase 1/2 trials of RIT with lintuzumab for AML. A series of phase 1/2 studies combining 131I-BC8 anti-CD45 antibody with allogeneic HSCT for AML, acute lymphoblastic leukemia (ALL), and MDS has demonstrated the feasibility, safety, and efficacy of this approach.48,49 A phase 1 dosimetry study showed the feasibility of targeting CD138 in multiple myeloma (MM)55 and an 131I-CD5 antibody has been investigated for cutaneous T-cell lymphoma.56
Multistep pretargeted RIT (PRIT) is a strategy to improve tumor-to-organ ratios of absorbed radioactivity compared with conventional 1-step RIT by separating the slow distribution phase of the antibody from administration of the radionuclide. Nonradiolabeled antibody is administered and allowed to bind at tumor sites then followed by the infusion of a radioisotope which has a high affinity for a conjugated adaptor molecule on the antibody. Radiation exposure to normal organs is limited as the small radioisotope can quickly penetrate the tumor while the unbound radiolabeled ligand is rapidly cleared from the circulation through renal excretion. Addition of a “clearing agent” before the second step can further improve specificity by complexation of excess unbound antibody in the bloodstream, which is subsequently removed by hepatic receptors recognizing the complexes. Several preclinical studies have validated the advantages of this approach utilizing the affinity of streptavidin or avidin for biotin.57-62 Other attractive PRIT strategies use bispecific (antitumor × antiligand) antibodies,63,64 “dock and lock” methods that exploit binding between the regulatory subunits of cAMP-dependent protein kinase and the anchoring domains of A-kinase anchor proteins,65,66 complementary hybridization of phosphorodiamidate morpholino oligomers (MORFs),67 or cyclooctene-modified antibodies with radiolabeled tetrazine ligands.68 Early trials investigating PRIT have yielded encouraging results in hematologic malignancies.69,70 Four of 7 patients with advanced NHL who had failed multiple prior therapies including HSCT and were treated with CD20-streptavidin conjugate and 90Y-DOTA-biotin PRIT had objective responses (3 CR and 1 PR).70 A phase 1 trial of PRIT in AML patients using anti-CD45 antibody (BC8) streptavidin conjugate and 90Y-DOTA biotin prior to total body irradiation and allogeneic HSCT is ongoing (NCT00988715).
Regrettably, despite encouraging clinical results, RIT has not been widely embraced as a treatment modality. The recent decision to discontinue manufacture and distribution of 131I-tositumomab in February 2014 was based on the anticipated decline in its use as a result of the recent emergence of multiple other alternatives for relapsed/refractory NHL, including bendamustine, ibrutinib, idelalisib, and ABT-199. Logistical issues involving the transfer of care from the treating oncologist/hematologist to the nuclear medicine physician, economic concerns about insufficient reimbursement and expense, and an exaggerated emphasis on delayed effects such as marrow damage and secondary malignancies have contributed to the limited use of RIT.71 Importantly, the inability to administer RIT locally at community practice sites with the resultant need for referral to distant centers has been a major economic disincentive. Although the development of strategies to further improve RIT efficacy and extend its use to other hematologic malignancies is continuing, reducing the logistic hurdles to RIT administration will be essential for more widespread adoption of the next generation of RICs.
ADCs
ADCs are inarguably the most active current area of IC development. Although the voluntary withdrawal in 2010 of the first approved ADC for the treatment of a hematologic malignancy (gemtuzumab ozogamicin [GO]) transiently diminished the enthusiasm for ADCs, the approval of brentuximab vedotin a year later, as well as ado-trastuzumab emtansine for metastatic breast cancer in early 2013, has buoyed the ADC field. Multiple ADCs are in clinical development (Table 2). Targets include CD19, CD22, CD33, and CD79b. Several recent clinical trials have demonstrated the therapeutic promise of ADCs for a variety of malignancies.11
ADCs
| Antibody . | Target . | Drug . | Indication . | Stage of development . |
|---|---|---|---|---|
| BV | CD30 | Monomethyl auristatin E | HL, ALCL | Approved 2011 |
| BT062 | CD138 | DM4 (Maytansinoid) | MM | Phase 2 NCT01001442, NCT01638936 |
| Polatuzumab vedotin (DCDS4501A) | CD79b | Monomethyl auristatin E | DLBCL, FL | Phase 2 NCT01691898 |
| GO | CD33 | Calicheamicin | AML | Approved 2000; withdrawn June 2010 |
| INO (CMC-544) | CD22 | Calicheamicin | B-cell NHL, B-cell ALL | Phase 3 NCT01564784, NCT01232556 |
| IMGN529 | CD37 | DM1 (Maytansinoid) | B-cell NHL, B-cell CLL | Phase 1 NCT01534715 |
| Milatuzumab-doxorubicin (hLL1-Dox; IMMU-110) | CD74 | Doxorubicin | MM, CLL, NHL | Phase 1/2 NCT01101594 |
| PV (DCDT2980S) | CD22 | Monomethyl auristatin E | DLBCL, FL | Phase 2 NCT01691898 |
| SAR-3419 | CD19 | DM4 (Maytansinoid) | DLBCL, B-cell ALL | Phase 2 NCT01472887, NCT01440179 |
| SGN-CD19A | CD19 | Monomethyl auristatin F | B-cell NHL, B-cell ALL | Phase 1 NCT01786135, NCT01786096 |
| SGN-CD33A | CD33 | Pyrrolobenzodiazepine dimer | AML | Phase 1 NCT01902329 |
| Antibody . | Target . | Drug . | Indication . | Stage of development . |
|---|---|---|---|---|
| BV | CD30 | Monomethyl auristatin E | HL, ALCL | Approved 2011 |
| BT062 | CD138 | DM4 (Maytansinoid) | MM | Phase 2 NCT01001442, NCT01638936 |
| Polatuzumab vedotin (DCDS4501A) | CD79b | Monomethyl auristatin E | DLBCL, FL | Phase 2 NCT01691898 |
| GO | CD33 | Calicheamicin | AML | Approved 2000; withdrawn June 2010 |
| INO (CMC-544) | CD22 | Calicheamicin | B-cell NHL, B-cell ALL | Phase 3 NCT01564784, NCT01232556 |
| IMGN529 | CD37 | DM1 (Maytansinoid) | B-cell NHL, B-cell CLL | Phase 1 NCT01534715 |
| Milatuzumab-doxorubicin (hLL1-Dox; IMMU-110) | CD74 | Doxorubicin | MM, CLL, NHL | Phase 1/2 NCT01101594 |
| PV (DCDT2980S) | CD22 | Monomethyl auristatin E | DLBCL, FL | Phase 2 NCT01691898 |
| SAR-3419 | CD19 | DM4 (Maytansinoid) | DLBCL, B-cell ALL | Phase 2 NCT01472887, NCT01440179 |
| SGN-CD19A | CD19 | Monomethyl auristatin F | B-cell NHL, B-cell ALL | Phase 1 NCT01786135, NCT01786096 |
| SGN-CD33A | CD33 | Pyrrolobenzodiazepine dimer | AML | Phase 1 NCT01902329 |
ALCL, anaplastic large cell lymphoma; CLL, chronic lymphocytic leukemia.
GO retains the dubious distinction of being both the first ADC approved under an accelerated approval program in May 2000 and the first withdrawn 10 years later. It is composed of a humanized anti-CD33 antibody linked to calicheamicin via an acid-labile hydrazone linker. It was approved on the basis of multicenter phase 2 trials demonstrating its efficacy and safety in 141 AML patients in first relapse with an ORR of 30% (16% CR).72 A confirmatory phase 3 trial in 2004 was initiated to determine whether addition of GO to induction and postconsolidation therapy improved OS in newly diagnosed younger AML patients. The trial was halted after no clinical benefit was demonstrated and more deaths were observed due to liver toxicity in the GO plus chemotherapy arm than in the arm with chemotherapy alone.73 Although GO was withdrawn in 2010, subsequent studies have strongly suggested a benefit in a defined AML patient population raising hope that this ADC may be resurrected for use in the future.74
Brentuximab vedotin (BV; SGN-35) was approved in 2011 for treatment of relapsed/refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL). It is composed of a chimeric anti-CD30 antibody linked to the microtubule inhibitor monomethyl auristatin E (MMAE) via a protease-cleavable linker. The development of BV was recently reviewed.75 The parental unconjugated anti-CD30 antibody (SGN-30) exhibited modest efficacy in phase 2 studies with clinical responses observed in 7 of 41 sALCL patients and 0 of 38 HL patients.76 In contrast, the pivotal phase 2 studies administering BV demonstrated impressive clinical activity, including an ORR of 80% (57% CR) in patients with relapsed/refractory sALCL77 and an ORR of 75% (34% CR) in relapsed/refractory HL.78 Common adverse events (≥10%) reported in both studies included peripheral sensory neuropathy, nausea, fatigue, neutropenia, pyrexia, diarrhea, emesis, pruritis, myalgia, and alopecia. The most common grade ≥3 toxicities included neutropenia (20%-21%), peripheral sensory neuropathy (8%-12%), and thrombocytopenia (14% in sALCL). Addition of BV to frontline chemotherapy regimens is the subject of ongoing clinical trials in HL and sALCL.79,80 Phase 1 study results of 26 previously untreated sALCL patients receiving BV at the standard 1.8 mg/kg dose combined with standard dose CHP (cyclophosphamide, doxorubicin, and prednisone) yielded an ORR of 100% (88% CR).79 Interim phase 1 study results combining BV with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or AVD (doxorubicin, vinblastine, dacarbazine) in newly diagnosed advanced stage HL patients showed tolerability up to 1.2 mg/kg of BV.80 Pulmonary adverse events were observed in 7 of 25 patients on the combination ABVD arm leading to omission of bleomycin from subsequent cycles of therapy, though 5 of the 7 were able to safely continue treatment with BV plus AVD. All 10 patients who had completed therapy achieved CR. Phase 3 studies investigating frontline use of ABVD vs BV combined with AVD in advanced classical HL (NCT01712490) or combined with CHP vs CHOP in CD30-positive mature T-cell lymphomas (NCT01777152) are ongoing. Additional studies have suggested utility in other settings including relapse after allogeneic HSCT.81 CD30 expression identifies a unique subset of DLBCL82 and BV is being explored both as monotherapy in relapsed/refractory DLBCL (NCT01421667) and as frontline therapy with R-CHOP (NCT01925612).
Another promising ADC in clinical development is inotuzumab ozogamicin (INO; CMC-544). INO consists of a humanized IgG4 anti-CD22 monoclonal antibody attached to calicheamicin via an acid-labile linker and showed favorable antitumor activity in mouse xenograft models of B-cell NHL and ALL.83,84 A phase 2 study demonstrated encouraging results in both adults and children with relapsed/refractory ALL who were treated with single-agent INO at a dose of 1.8 mg/kg every 3 weeks with an ORR of 57%, with 28 of 49 patients achieving either CR (18%) or marrow CR (39%).85 The most common nonhematologic adverse events reported were drug-related fever (59%), elevated aminotransferase (57%), elevated bilirubin (29%), drug-related hypotension (27%), and nausea (49%). A phase 3 trial investigating single-agent INO compared with the investigator’s choice of chemotherapy (FLAG [fludarabine and cytarabine], high-dose cytarabine, or cytarabine and mitoxantrone) in relapsed/refractory adult ALL is ongoing (NCT01564784). Phase 2 study results in relapsed/refractory pediatric ALL patients receiving INO as a single agent at 1.8 mg/kg or as split weekly doses reported 3 of 5 responses (1 CR in bone marrow and normal peripheral counts and 2 with morphologic remissions in the bone marrow but with platelets <100 000).86 Although toxicities included fever, sepsis, and liver function abnormalities, the ADC was generally well tolerated. A phase 3 trial of pediatric ALL patients is planned.
Results of INO in NHL have been mixed. A phase 1 study of single-agent INO enrolling 79 patients with relapsed/refractory B-cell NHL yielded an ORR of 68% in FL and 15% in DLBCL at a dose of 1.8 mg/m2 given every 3 to 4 weeks.87 A phase 1/2 study of INO combined with rituximab showed impressive ORRs of 87%, 74%, and 20% for relapsed FL, relapsed DLBCL, and refractory aggressive NHL, respectively, with a 2-year PFS of 68% for FL and 42% for DLBCL.88 Toxicities were manageable and included thrombocytopenia, neutropenia, hyperbilirubinemia, and transaminitis. However, a phase 3 study (NCT01232556) of monthly 1.8 mg/kg INO with rituximab vs investigator’s choice chemotherapy (bendamustine or gemcitabine) with rituximab in relapsed/refractory aggressive CD22+ B-cell NHL was halted in May 2013 after an independent data monitoring committee concluded that the ADC experimental arm would not meet the primary objective of improving OS compared with the chemotherapy arm. Another phase 3 study comparing INO with rituximab vs R-CVP or R-FND (fludarabine, mitoxantrone, dexamethasone) in FL had previously been discontinued due to slow accrual (NCT00562965).
Several other ADCs are undergoing phase 1/2 studies. Two of these use the same protease-cleavable linker to MMAE as BV but replace the anti-CD30 antibody with antibodies targeting either the internalizing receptor CD22 (pinatuzumab vedotin [PV]; DCDT2980S) or CD79b (polatuzumab vedotin; DCDS4501A), a component of the B-cell receptor. A phase 2 study randomizing patients with relapsed/refractory FL or DLBCL to either DCDT2980S or DCDS4501A in combination with rituximab is ongoing (NCT01691898). Results from the prior phase 1 studies suggested possible greater efficacy of the anti-CD79b ADC than the anti-CD22 ADC with an ORR of 55% vs 30% as a single agent and 78% vs 33% when combined with rituximab.89,90 Toxicities observed included neutropenia and peripheral neuropathy which were not unexpected given the previous clinical experience with BV. Two other ADCs use maytansinoids as effectors and target either CD19 (SAR-3419) in DLBCL or ALL or CD138 (BT-062) in MM.91,92 In a phase 1 study enrolling relapsed/refractory B-cell NHL patients, single-agent SAR3419 was found to have a maximum tolerated dose of 160 mg/m2 with 6 of 35 patients (17%) achieving an objective response.93 The notable dose-limiting toxicity in this trial was reversible bilateral corneal epitheliopathy, which has also been observed with other ADCs incorporating DM4. ADCs in phase 1 testing include an anti-CD74 antibody conjugated to doxorubicin,94 an anti-CD37 antibody conjugated to maytansinoid,95 an anti-CD19 antibody conjugated to monomethyl auristatin F,96 and an anti-CD33 antibody conjugated to pyrrolobenzodiazepine.97 Antibodies fused to a cell-signaling molecule (immunocytokines) comprise another increasingly recognized group of ICs with activity in hematologic malignancies.98 A tetrameric interferon-α construct attached to veltuzumab showed promising activity in a lymphoma mouse xenograft model.99
Drug and linker affect the efficacy and toxicity profile of ADCs. Microtubule inhibitors such as maytansine and dolastatin derivatives and DNA damaging agents including calicheamicin and pyrrolobenzodiazepine comprise the majority of small-molecule drug effectors currently incorporated into ADCs (Table 3). Maytansinoids and auristatins, like the vinca alkaloids and taxanes, cause neuropathy by virtue of a common mechanism of tubulin disruption. However, the relative membrane permeability of the released drug can impact severity. Hydrophobic effectors such as DM4 produce less neuropathy than hydrophilic effectors like DM1 and MMAE which diffuse across the cell membrane to affect bystander cells. Membrane permeability can be modulated by linker attachment. Intracellular processing of specific linker-drug combinations result in charged metabolites preventing drug escape and uptake by neighboring cells. However, local bystander effects can prove beneficial in tumors heterogeneously expressing the targeted cell-surface antigen. Free MMAE released by intracellular processing of BV by rare CD30-expressing Reed-Sternberg cells is believed to enhance the tumoricidal efficacy in HL.100 Bystander effects may also prove beneficial when intact ADCs have difficulty penetrating deep into bulky tumors. Selection of ADCs targeting the same antigen may depend upon the side effect profile conferred by the linker drug. In choosing between CD22-targeted ADCs PV and INO, for example, preexisting severe neuropathy would exclude PV whereas prior HSCT may conversely prohibit INO use. Emerging trial data clarifying the advantages and disadvantages of individual ADCs should provide further guidance to the clinician.
Small-molecule drug effectors
| Effector drug . | Ozogamicin . | MMAE . | Maytansinoid DM1 (mertansine) . | Maytansinoid DM4 . |
|---|---|---|---|---|
| Origin | Semi-synthetic derivative of γ-calicheamicin (Micromonospora echinospora calichensis–Actinomycete soil bacterium) | Synthetic derivative of dolastatin 10 (Dolabella auricularia–Sea hare) | Synthetic derivative of maytansine (Maytenus serrata–Ethiopian shrub) | Synthetic derivative of maytansine (M serrata–Ethiopian shrub); DM1 with 2 additional methyl groups |
| Class of molecule | Enediyne-containing antibiotic | Linear cytotoxic pentapeptide | Ansamycin macrolide antibiotic | Ansamycin macrolide antibiotic |
| Mechanism of action | Intercalates in the minor groove of DNA causing double-stranded breaks | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest |
| Example ADCs (target antigen) | GO (CD33) | BV (CD30) | Ado-trastuzumab emtansine (Her2/neu) | SAR3419 (CD19) |
| INO (CD22) | PV (CD22) | IMGN529 (CD37) | BT062 (CD138) | |
| Polatuzumab vedotin (CD79b) | ||||
| Major toxicities including phase 1 study DLTs | Thrombocytopenia (DLT); neutropenia (DLT); hepatotoxicity | Thrombocytopenia (DLT); neutropenia (DLT); hyperglycemia (DLT); peripheral neuropathy; pulmonary toxicity | Thrombocytopenia (DLT); hepatotoxicity; interstitial lung disease; peripheral neuropathy | Ocular/corneal toxicity (DLT); peripheral neuropathy (DLT); neutropenia; thrombocytopenia |
| Effector drug . | Ozogamicin . | MMAE . | Maytansinoid DM1 (mertansine) . | Maytansinoid DM4 . |
|---|---|---|---|---|
| Origin | Semi-synthetic derivative of γ-calicheamicin (Micromonospora echinospora calichensis–Actinomycete soil bacterium) | Synthetic derivative of dolastatin 10 (Dolabella auricularia–Sea hare) | Synthetic derivative of maytansine (Maytenus serrata–Ethiopian shrub) | Synthetic derivative of maytansine (M serrata–Ethiopian shrub); DM1 with 2 additional methyl groups |
| Class of molecule | Enediyne-containing antibiotic | Linear cytotoxic pentapeptide | Ansamycin macrolide antibiotic | Ansamycin macrolide antibiotic |
| Mechanism of action | Intercalates in the minor groove of DNA causing double-stranded breaks | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest | Binds tubulin and inhibits normal microtubule polymerization causing mitotic arrest |
| Example ADCs (target antigen) | GO (CD33) | BV (CD30) | Ado-trastuzumab emtansine (Her2/neu) | SAR3419 (CD19) |
| INO (CD22) | PV (CD22) | IMGN529 (CD37) | BT062 (CD138) | |
| Polatuzumab vedotin (CD79b) | ||||
| Major toxicities including phase 1 study DLTs | Thrombocytopenia (DLT); neutropenia (DLT); hepatotoxicity | Thrombocytopenia (DLT); neutropenia (DLT); hyperglycemia (DLT); peripheral neuropathy; pulmonary toxicity | Thrombocytopenia (DLT); hepatotoxicity; interstitial lung disease; peripheral neuropathy | Ocular/corneal toxicity (DLT); peripheral neuropathy (DLT); neutropenia; thrombocytopenia |
DLT, dose-limiting toxicity.
Conclusions
ICs represent an exciting class of biologics that have increasingly established a place in the treatment of hematologic malignancies. RIT has proven to be an effective although underutilized modality in the treatment of NHL and is being studied for other neoplasms. Several ADCs are in clinical development for a variety of indications and may soon be incorporated into frontline treatment regimens. Continued research to improve components of ICs including linker optimization and development of more potent and specific effector molecules may further expand their use in a variety of hematologic cancers.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute grants: K08CA163603 (M.C.P.-W.), P50 CA083636, (M.C.P.-W.), R01EB002991 (O.W.P.), R01 CA076287 (O.W.P.), R01 CA109663 (O.W.P.), R01 CA154897 (O.W.P.), R01 CA136639 (O.W.P.), and P01 CA044991 (O.W.P.); Washington State Life Sciences Discovery Fund #2496490 (O.W.P. and M.C.P.-W.); the Wayne D. Kuni & Joan E. Kuni Foundation, and the Kuni family through the 3725 Fund of the Oregon Community Foundation (M.C.P.-W.).
Authorship
Contribution: M.C.P.-W. and O.W.P. wrote the manuscript.
Conflict-of-interest disclosure: O.W.P. and M.C.P.-W. have received research funding from Roche/Genentech. O.W.P. was a consultant for Roche/Genentech.
Correspondence: Oliver W. Press, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109; e-mail: press@uw.edu.