Abstract
We and others have shown that the B cell Translocation Gene 1 (BTG1) locus is affected by genomic deletions in 9% of pediatric acute lymphoblastic leukemia (ALL) patients. The fact that multiple subclones carrying distinct deletions can be present in individual patients suggests that interfering with normal BTG1 function provides a selective growth advantage to leukemic cells. However, it remains unclear how loss of BTG1 promotes clonal outgrowth.
Although BTG1 possesses no catalytic activity, it functions as a transcriptional co-regulator that acts by recruiting Protein Arginine Methyl Transferase 1 (PRMT1) to transcription factor complexes. By in vitro methylation assays with purified proteins we showed that ATF4 is directly methylated by PRMT1 on a single arginine residue. In addition we found that the PRMT1 interacting domain in BTG1, while dispensable for the BTG1-ATF4 interaction, is essential for the BTG1 mediated suppression of ATF4 function. In search for additional evidence for the functional interaction between BTG1 and ATF4 we performed global expression analysis on murine cells expressing the B cell marker B220. This revealed a significant deregulation of ATF4 target genes in BTG1 knockout cells when compared to wildtype cells.
Together, our data indicate that BTG1 suppresses activation of ATF4 in response to cellular stress. Loss of BTG1 function, as it occurs during leukemia development, enhances ATF4 activity, thereby promoting cell survival under cellular stress conditions such as nutrient deprivation or ER stress. Leukemic cells carrying BTG1 deletions may thus benefit from this increased resistance to cellular stress, not only during leukemia development but also during treatment. Hence, targeting the ATF4 stress response pathway may prevent relapse of therapy-resistant leukemic clones.
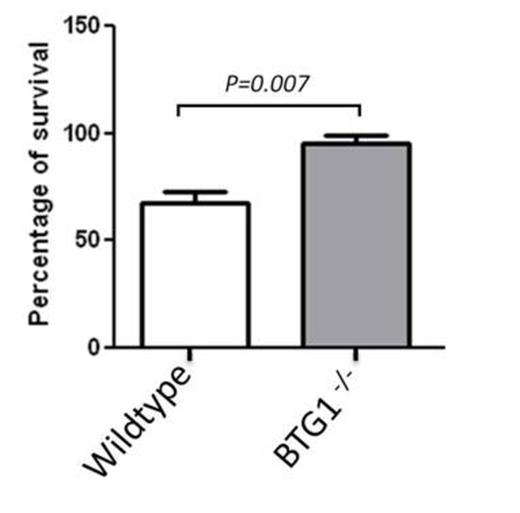
Cells were treated with 2 IU/L Asparaginase for 24 hours. After treatment, cell viability was measured using an MTT assay. The average of 4 independent experiments is plotted with error bars representing the standard error of the mean.
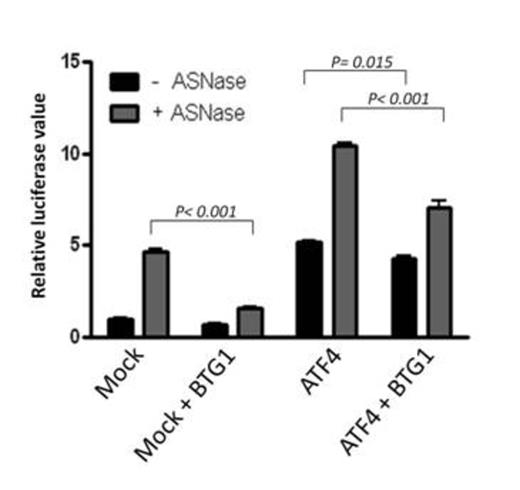
A luciferase reporter gene controlled by the ATF4 responsive ASNS promoter region was transfected into HEK293 cells. Asparaginase treatment induces endogenous ATF4 expression, which results in an increase in luciferase signal (Mock transfected cells). Co-expression of BTG1 represses both endogenous ATF4 activity as well as ectopically expressed ATF4 activity as detected by a decrease in luciferase signal. The average of 2 independent experiments is plotted with error bars representing the standard deviation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal