Abstract
The presence of HLA-A allele-lacking leukocytes (HLA-LLs) due to uniparental disomy in the short arm of chromosome 6 is compelling evidence for the immune pathophysiology of acquired aplastic anemia (AA). Our previous study revealed that 31.4-99.4% HLA-A allele-lacking granulocytes (HLA-LGs) were detectable in approximately 13% of AA patients with various disease durations. However, many issues still need to be determined regarding HLA-LLs, including the prevalence in AA patients at the time of diagnosis, the clone size of HLA-LGs, the lineage diversity of HLA-LLs and their relationship to the response to immunosuppressive therapy (IST).
To clarify these issues, we examined the peripheral blood leukocytes obtained from 175 AA patients (71 at diagnosis and 104 after treatments; 73 with severe and 102 with non-severe AA) for the presence of HLA-LLs using flow cytometry (FCM) with monoclonal antibodies (mAbs) specific to A24, A2, A26, A31 and A11. For patients whose HLA-A alleles had not been determined, the cryopreserved peripheral blood mononuclear cells (PBMCs) were used to obtain thawed monocytes (M), which were subjected to FCM with anti-CD33 mAbs after HLA-A allele data became available. The results of the M were compared to those of granulocytes (G) obtained later. The other lineage makers used for FCM included CD11b for G, CD19 for B cells (B) and CD3 for T cells (T).
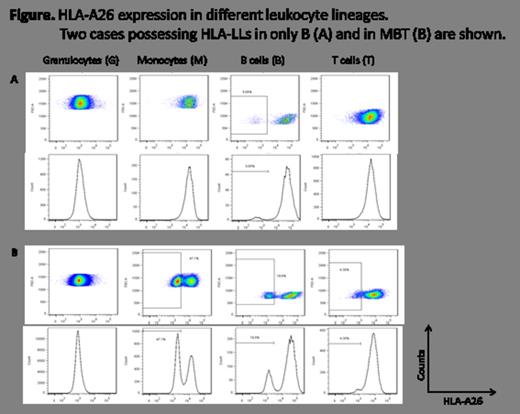
A total of 120 (68.6%) patients were assessable for the presence of HLA-LLs owing to their HLA-A heterozygosity. Nine (18.4%, six with severe and three with non-severe AA) of the 49 patients at diagnosis and 23 (32.4%) of the 71 patients after treatments were positive for HLA-LLs with median G or M percentages of 8.5% (0.8%-88.8%) and 57.5% (0.44-99.4%), respectively. The lineage combinations in HLA-LLs of the 32 patients were GMBT in seven, GMB in 11, GM in 10, MBT in two and B in two. These different combination patterns persisted for 1-40 months in the 23 patients whose peripheral blood samples were available for the sequential analyses. Of the nine HLA-LLs (+) patients at diagnosis, four patient's blood samples could be examined again when the patients achieved remission after IST. The percentage of HLA-LLs did not change, except in one patient who showed a decrease in HLA-LGs from 11.7% to 1.0%. A total of 16 (four with and 12 without HLA-LLs at the diagnosis) patients were treated with antithymocyte globulin plus cyclosporine, and four (100%) of the HLA-LLs (+) and six (50.0%) of the HLA-LLs (-) patients responded.
Various percentages of HLA- LLs were detectable in approximately 18% of AA patients at the time of diagnosis, regardless of the disease severity. The fact that the percentages of HLA-LLs did not change after successful IST suggests that the cytotoxic T-cell attack against HSCs triggers the development of AA, but that the main mechanism responsible for AA is cytokine-mediated BM suppression. The presence of HLA-LLs with limited lineage diversity and HLA-LBs alone further supports this hypothesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal