Abstract
Hematopoietic stem cells (HSCs) harboring PIGA mutations acquire a survival advantage under immune pressure compared to normal HSCs in patients with acquired aplastic anemia (AA). Cytotoxic T cells (CTLs) specific to glycosylphosphatidylinositol-anchored proteins (GPI-APs) are reportedly involved in this survival advantage, because PIGA mutant HSCs cannot present GPI-AP-derived peptides via class I HLAs. However, there is no convincing evidence that CTLs specific to GPI-AP-derived peptides are involved in the “escape” hematopoiesis by PIGA mutant HSCs. We recently demonstrated that 31.4-99.4% HLA-A allele-lacking leukocytes (HLA-LLs) were detectable in approximately 13% of AA patients as a result of escape hematopoiesis by HSCs with uniparental disomy in the short arm of chromosome 6, and that some patients possessed both GPI-AP-deficient (GPI-AP-) leukocytes and HLA-LLs (Katagiri, et al. Blood 2011). We hypothesized that if GPI-AP-derived peptides serve as a target for CTLs that elicit the development of AA, HLA-LLs may always be detectable only in the GPI-AP+ leukocyte population, because PIGA mutant HSCs do not require the lack of HLA class Is for the escape from the attack by GPI-AP peptide-specific CTLs.
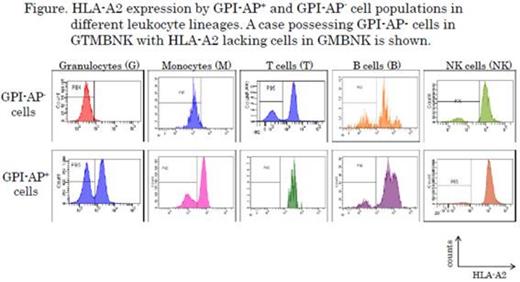
To examine this hypothesis, the GPI-AP expression was analyzed in various leukocyte lineages in 32 (nine at diagnosis and 23 previously treated) AA patients possessing HLA-LLs by a flow cytometry (FCM) analysis with liquid fluorescent aerolysin.
A total of 0.01%-50% GPI-AP- granulocytes (GPI-AP- Gs) were detected in 22 (69%) of the 32 HLA-LLs (+) patients. Of the 22 patients possessing both HLA-LLs and increased GPI-AP- Gs, HLA-LLs were detectable in GPI-AP+ cells alone in 19 patients (86%). However, in the remaining three patients, HLA-LLs were shown in both GPI-AP+ and GPI-AP- populations. To determine which mutation occurs first in HSCs with a PIGA mutation and 6pUPD, the lineage diversity of GPI-AP- HLA-LLs was determined in the three patients. In two of the three patients, the lineage diversity of GPI-AP- cells (G/monocytes (M)/T cells (T)/B cells (B)/NK cells (NK)) and G/M/T) was greater than that of the HLA-A-lacking cells (G/M/B/NK and G/M) suggesting that PIGA mutations occurred earlier in the maturation of HSCs than did 6pUPD. The lineage diversity was the same in the GPI-AP- cells and HLA-LLs in one patient
The presence of HLA-LLs in the GPI-AP- leukocyte population and lower lineage diversity in HLA-LLs than GPI-AP- leukocytes suggest that CTLs specific to GPI-APs are not involved in the escape of PIGA mutant HSCs in AA, and that other mechanisms, such as a lower sensitivity to myelosuppressive cytokines than wild-type HSCs, may contribute to the survival advantage of PIGA mutant HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal