Key Points
More than 60% of primary AML blasts constitutively produce high levels of NOX-derived reactive oxygen species (ROS), which drives AML proliferation.
High ROS AMLs show depleted antioxidant defenses but evade the oxidative stress response through suppression of p38MAPK signaling.
Abstract
Excessive production of reactive oxygen species (ROS) is frequently observed in cancer and is known to strongly influence hematopoietic cell function. Here we report that extracellular ROS production is strongly elevated (mean >10-fold) in >60% of acute myeloid leukemia (AML) patients and that this increase is attributable to constitutive activation of nicotinamide adenine dinucleotide phosphate oxidases (NOX). In contrast, overproduction of mitochondrial ROS was rarely observed. Elevated ROS was found to be associated with lowered glutathione levels and depletion of antioxidant defense proteins. We also show for the first time that the levels of ROS generated were able to strongly promote the proliferation of AML cell lines, primary AML blasts, and, to a lesser extent, normal CD34+ cells, and that the response to ROS is limited by the activation of the oxidative stress pathway mediated though p38MAPK. Consistent with this, we observed that p38MAPK responses were attenuated in patients expressing high levels of ROS. These data show that overproduction of NOX-derived ROS can promote the proliferation of AML blasts and that they also develop mechanisms to suppress the stress signaling that would normally limit this response. Together these adaptations would be predicted to confer a competitive advantage to the leukemic clone.
Introduction
Reactive oxygen species (ROS) are a heterogeneous group of molecules and free radicals that are increasingly implicated in a variety of normal cellular processes1 and are frequently elevated in cancer cells.2 ROS are generated from multiple sources,3 including the mitochondrial electron transport chain4 or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) proteins.5 Both of these sources initiate ROS production with the univalent reduction of oxygen to produce superoxide radicals.6 Superoxide is the precursor to several biologically important molecules including hydrogen peroxide (H2O2), a membrane-permeable, mildly pro-oxidant molecule that can act as a second messenger.7 Indeed, NOX-derived H2O2 is known to play important roles in hematopoiesis8 and hematopoietic growth factor signaling.9,10 Excessive ROS production or a defect in antioxidant defense can lead to a state known as oxidative stress, characterized by oxidative damage to DNA, membrane lipids, and proteins, as well as augmented ROS signaling.11 To prevent the establishment of oxidative stress, cells possess an extensive network of antioxidant defenses. Important among these are the glutathione/glutathione peroxidase12 and peroxiredoxin systems,13 which destroy H2O2 in cycles of sacrificial oxidation and subsequent regeneration by the NADPH-dependent thioredoxin system.14 In the event of oxidative stress, normal cells respond by activation of stress-activated protein kinases (SAPKs), particularly p38MAPK, which is phosphorylated in response to ROS-induced stress15 and mediates cell cycle arrest.16
In the context of hematopoietic malignancy, there is evidence of elevated ROS and/or accompanying oxidative stress in acute lymphoblastic leukemia (ALL),17 myelodysplastic syndrome (MDS),18 chronic myeloid leukemia (CML),19,20 and in some models of acute myeloid leukemia (AML)21,22 ; however, little is known about the extent of ROS overproduction in primary AML blasts nor have the sources and consequences of ROS production in AML blasts been systematically investigated. Here we report that primary AML blasts constitutively produce highly elevated NOX-derived ROS compared with normal blasts and that these high levels of ROS are associated with defective p38MAPK stress signaling. We also show that extracellular ROS promotes the proliferation of AML cell lines and primary AML blasts, and that inhibition of p38MAPK signaling augments ROS-driven proliferation, suggesting a mechanism whereby defective p38MAPK signaling is selected for in high ROS AML.
Materials and methods
Primary cell material and cell culture
Peripheral blood or bone marrow from AML patients and normal cord blood from healthy donors were collected with informed consent and approval from the South East Wales Research Ethics Committee in accordance with the 1964 Declaration of Helsinki (supplemental Table 1, available on the Blood Web site). Only peripheral blood mononuclear cell/bone marrow aspirate mononuclear cell samples that contained >80% blasts (determined by CD45 expression vs side-scatter characteristics) and were >80% viable were resuspended in Iscove’s modified Dulbecco medium (IMDM) containing 10% fetal calf serum (FCS) and assayed after a 3-hour incubation period at 37°C in a 5% CO2 atmosphere. CD34+ cord blood hematopoietic cells were selected using the miniMACS, as previously described.23 THP-1, MV4-11, and KG-1 cell lines were obtained from ATCC and cultured under the recommended conditions.
Detection of superoxide
Extracellular superoxide was measured using Diogenes (National Diagnostics, Atlanta, GA), as previously described,22 using a Chameleon V instrument (Hidek, Finland) in the presence of vehicle control or: 10 to 500 nM diphenyleneiodonium (DPI), 100 to 500 nM 2-acetylphenothiazine (2-AP), 1 μM 2-O-tetradecanoylphorbol-13-acetate (TPA),1 μM rotenone, 1 μM antimycin A (Sigma-Aldrich), or 5 μM MitoTempol (Enzo Life Sciences, Exeter, United Kingdom).
Mitochondrial superoxide was measured by flow cytometry using MitoSOX (Life Technologies, Paisley, UK), as previously described.22 For some experiments, cells were preincubated with 1 μM antimycin A for 10 minutes at 37°C.
Measurement of glutathione
Total glutathione (GSH) content was measured using the GSH-Glo Assay kit (Promega, Madison, WI) according to the manufacturer’s instructions, in the presence of 500 μM Tris (2-carboxyethyl) phosphine (TCEP; Sigma-Aldrich). TCEP allows measurement of total GSH by converting oxidized GSH dimers or GS-R conjugates into reduced GSH monomers. Measurements were performed in duplicate at 25°C using a Chameleon V. GSH concentration in each sample was calculated using a GSH standard curve and normalized to total protein concentration (measured using Bradford’s assay as previously described).22
Measurement of cell proliferation by MTS assay
Proliferation was measured by MTS assay (Promega). Normal CD34+ cells, AML cell lines (9 × 103 cells/well), or primary AML blasts (50 × 103 cells/well) were seeded in a volume of 90 μL of IMDM supplemented with FCS in a 96-well plate and incubated for 48 to 72 hours at 37°C in a 5% CO2 atmosphere in the presence of 0 to 100 mU/mL GOX. Some experiments were carried out with the addition of 100 μg/mL catalase (Sigma-Aldrich) or 50 nM UR13756 (provided by Dr Terence Davis, Cardiff University) or dimethyl sulfoxide vehicle control. After incubation, cells were incubated for 4 hours with 20 μL MTS reagent and absorbance at 492 nm measured.
Detection of H2O2 using Amplex UltraRed
Amplex UltraRed (Life Technologies) was prepared according to the manufacturer’s instructions and was used to detect H2O2 production by GOX and primary AML blasts. GOX (0-100 mU/mL) or primary AML blasts (5 × 104 cells/well) were prepared in phenol red-free IMDM supplemented with 10% FCS and added in triplicate to a 96-well plate. Amplex UltraRed reagent was added, and fluorescence (excitation/emission maxima 570/585 nm) was measured at 37°C for 4 hours. The addition of catalase (100 μg/mL) was used to ascertain background fluorescence. The H2O2 production rate was determined using an H2O2 standard curve.
Western blotting
Western blotting was carried out as previously described22 with the following antibodies: total p38MAPK, phospho-p38MAPK (Thr180/182), thioredoxin, peroxiredoxin-1, total MAPKAPK-2 (Cell Signaling, Hertfordshire, United Kingdom), NOX1 (Abcam, Cambridge, United Kingdom), NOX4, and GSTM1 (Santa Cruz, Biotechnology, CA). Equal protein loading was verified by expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology) or β-actin (Abcam).
Flow cytometric analysis of NOX2 expression, MitoSOX fluorescence, and DNA content
To establish NOX2 expression, cells were incubated for 30 minutes at 4°C with an anti-NOX2 antibody conjugated to PE (clone 7D5; Caltag Medsystems, Buckingham, United Kingdom). Background fluorescence was established using manufacturer- and isotype-matched controls conjugated to the appropriate fluorochrome. For MitoSOX analysis, background fluorescence was established using unstained samples. For cell cycle analysis, MV4-11 cells were cultured in growth medium containing 5 mU/mL GOX for 18 hours, and 1 × 105 cells were harvested and DNA content was examined by flow cytometry as previously described.24
Flow cytometric data were acquired using an Accuri C6 instrument (Becton Dickinson, Oxford, United Kingdom) and analyzed using FCS Express v4.03 (De Novo Software, Los Angeles, CA).
Statistical analysis
Significance of difference was determined using the Mann-Whitney U test, the Kruskal-Wallis test with Dunn’s posttest, or 1-way analysis of variance with Tukey’s honestly significant differences; strength of correlation was analyzed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient. Tests were performed using Prism v5 (GraphPad Software Inc., La Jolla, CA).
Results
Primary AML blasts constitutively generate excess superoxide
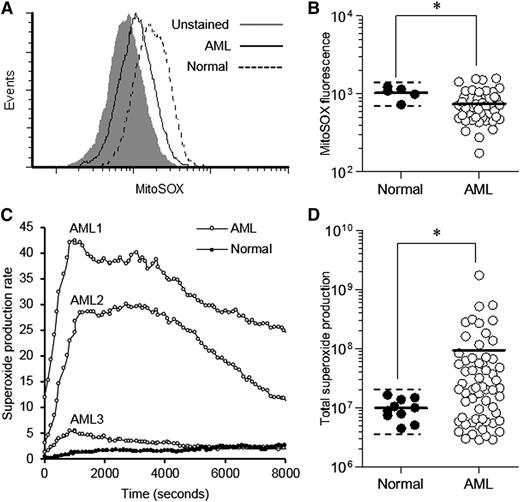
Previous studies indicate that primary cancer cells and cancer cell models frequently generate ROS (superoxide) via the mitochondria25 and/or NOX oxidases.11 To establish whether primary human AML blasts generate this form of ROS, we examined the extent and source of superoxide production in these cells. To measure mitochondrial ROS, we used the mitochondrial superoxide-specific probe, MitoSOX, and compared superoxide production in AML blasts with that of normal human CD34+ hematopoietic cells. To exclude the influence of apoptotic cells (which we found to be associated with very high MitoSOX signal), we dual-labeled test samples with annexin V to exclude this population (supplemental Figure 1A). The specificity of the MitoSOX probe was demonstrated using the mitochondrial electron transport–chain inhibitor antimycin A, which promotes mitochondrial superoxide production and induced a clear increase in MitoSOX signal in normal CD34+ cells (supplemental Figure 1B). In a cohort of 49 AML patients, we observed a ∼10-fold range of mitochondrial superoxide levels in AML blasts (Figure 1A-B). Overall, AML blasts displayed significantly lower levels than normal cells, which may be related to the lower mitochondrial gene expression that has been observed in AML patients.26 Four patients (8%) showed a twofold increase in mitochondrial superoxide compared with normal cells, suggesting that mitochondrial dysfunction may occur in a small subset of patients (Figure 1B). Furthermore, we observed only homogeneous MitoSOX signals (as exemplified in Figure 1A), suggesting an absence of subpopulations with differing mitochondrial superoxide levels.
Primary AML blasts generate significantly elevated extracellular superoxide. (A) Representative histograms showing fluorescence of unstained primary AML blasts, MitoSOX-labeled primary AML blasts and MitoSOX-labeled normal human CD34+ cells. (B) Summary of MitoSOX mean fluorescence of normal human CD34+ cells (n = 5) compared with primary AML blasts (n = 49). The solid horizontal bar represents the mean cohort fluorescence; the dotted horizontal bar indicates the mean + 2 SD boundary. (C) Representative time-course of constitutive superoxide production (×103 photons/s) of 3 different unstimulated primary AML cell samples compared with that of unstimulated normal human CD34+ cells. (D) Summary of total constitutive superoxide production (total photons) by primary AML blasts (n = 61) compared with normal CD34+ cells (n = 10). The solid horizontal bar represents the cohort mean; the dotted horizontal bar indicates the mean + 2 SD boundary. Significance of difference was determined using the Mann-Whitney U test. *P < .05.
Primary AML blasts generate significantly elevated extracellular superoxide. (A) Representative histograms showing fluorescence of unstained primary AML blasts, MitoSOX-labeled primary AML blasts and MitoSOX-labeled normal human CD34+ cells. (B) Summary of MitoSOX mean fluorescence of normal human CD34+ cells (n = 5) compared with primary AML blasts (n = 49). The solid horizontal bar represents the mean cohort fluorescence; the dotted horizontal bar indicates the mean + 2 SD boundary. (C) Representative time-course of constitutive superoxide production (×103 photons/s) of 3 different unstimulated primary AML cell samples compared with that of unstimulated normal human CD34+ cells. (D) Summary of total constitutive superoxide production (total photons) by primary AML blasts (n = 61) compared with normal CD34+ cells (n = 10). The solid horizontal bar represents the cohort mean; the dotted horizontal bar indicates the mean + 2 SD boundary. Significance of difference was determined using the Mann-Whitney U test. *P < .05.
Plasma membrane–bound NOX proteins are a significant source of extracellular superoxide. To determine whether NOX-derived superoxide was elevated in AML, we determined constitutive extracellular superoxide production using the chemiluminescent probe Diogenes, which gives a linear and specific response to superoxide (supplemental Figure 2). In our cohort of 61 AML patients, we observed a four-log range of superoxide production by AML blasts (compared with the fourfold range seen in normal cells). In contrast to the mitochondrial ROS, 65% of AML patients showed significantly elevated superoxide production compared with normal controls, with some patients exhibiting levels 100-fold greater (Figures 1C-D). Elevated constitutive superoxide production was observed in samples representing nearly all developmental subtypes tested: M1, M2, M4, and M5 (M3 patients were unavailable for testing). We observed no correlation between elevated superoxide production and mitochondrial ROS, common molecular abnormalities, or prognostic risk group in this cohort of patients (supplemental Table 1). Taken together, these results suggest that extracellular superoxide generation is frequently elevated in AML patients but cannot be ascribed to mitochondrial ROS production.
Primary AML cells frequently generate excess superoxide via NOX family oxidases
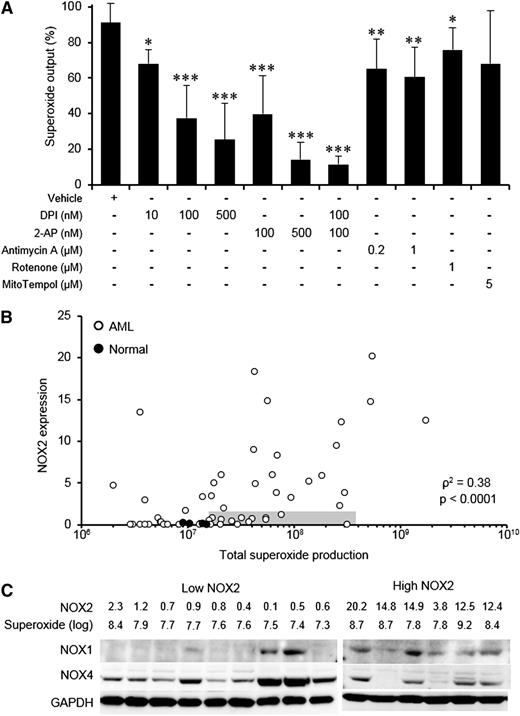
To determine whether NOX family members played a role in superoxide production by primary AML blasts, we examined the effect of DPI and 2-AP (NOX inhibitors) on superoxide production. These inhibitors suppressed superoxide production at nanomolar concentrations in a dose-dependent manner by as much as 90% (Figure 2A). In contrast, treatment with antimycin A, rotenone (mitochondrial electron transport–chain inhibitors), or MitoTempol (a mitochondria-targeted SOD mimetic) had only modest effects on superoxide production, demonstrating that the assay was insensitive to mitochondrially-derived ROS. Taken together, the data suggest that NOX oxidases are an important source of extracellular superoxide in primary AML blasts, consistent with previous observations in AML cell lines.27 In support of this, treatment with TPA (which indirectly activates NOX) significantly increased superoxide production as much as 50-fold (data not shown). To establish whether the superoxide was generated by a specific NOX protein family member, NOX protein expression was examined. Flow cytometric analysis showed that NOX2 expression in primary AML blasts correlated with superoxide production (Figure 2B), and in this context NOX2 knockdown was able to inhibit superoxide production (supplemental Figure 3A-B). Knockdown of NOX2 also inhibited proliferation, an observation consistent with previously published data in AML cell lines (supplemental Figure 3C).28 However, a minority (18%) of patients showed high levels of superoxide production despite expressing low levels of NOX2 (shaded region in Figure 2B), suggesting that other NOX family members may be responsible for superoxide production. Because expression of NOX3 and NOX5 is restricted to nonmyeloid tissues,5,29 we examined the expression of remaining NOX family members NOX1 and NOX4. We found that both these proteins were frequently expressed in AML blasts regardless of NOX2 expression level (Figure 2C), although all NOX2-low patients showed expression of NOX4. These data suggest that NOX1 and NOX4 may also contribute to excess ROS production in AML.
Superoxide production in primary AML blasts is driven by NOX family oxidases. (A) Superoxide produced by primary AML blasts (n ≥ 10) was measured using Diogenes in the presence of NOX family inhibitors (DPI, 2-AP) or modulators of mitochondrial ROS (antimycin A, rotenone, MitoTempol). Data were normalized to superoxide output of cells alone (without additions). (B) Expression of NOX2 in normal CD34+ cells or primary AML blasts (background subtracted mean fluorescence) as a function of superoxide production (total photons) detected by Diogenes. The shaded gray region indicates AML samples in the lowest quartile for NOX2 expression and overproducing ROS (> mean + 2 SD of control cell superoxide production). (C) Expression of NOX1, NOX2, and NOX4 in NOX2-low, superoxide-high samples (shaded region in B) was compared with that of samples with high NOX2 expression. NOX2 values represent the background subtracted mean fluorescence (scale = ×103); superoxide values represent log10 of the total superoxide production measured by Diogenes. GAPDH expression indicates relative protein loading. The significance of difference was determined by the Mann-Whitney U test; the significance of correlation was analyzed using Spearman’s rank correlation test. *P < .05, **P < .01, ***P < .001.
Superoxide production in primary AML blasts is driven by NOX family oxidases. (A) Superoxide produced by primary AML blasts (n ≥ 10) was measured using Diogenes in the presence of NOX family inhibitors (DPI, 2-AP) or modulators of mitochondrial ROS (antimycin A, rotenone, MitoTempol). Data were normalized to superoxide output of cells alone (without additions). (B) Expression of NOX2 in normal CD34+ cells or primary AML blasts (background subtracted mean fluorescence) as a function of superoxide production (total photons) detected by Diogenes. The shaded gray region indicates AML samples in the lowest quartile for NOX2 expression and overproducing ROS (> mean + 2 SD of control cell superoxide production). (C) Expression of NOX1, NOX2, and NOX4 in NOX2-low, superoxide-high samples (shaded region in B) was compared with that of samples with high NOX2 expression. NOX2 values represent the background subtracted mean fluorescence (scale = ×103); superoxide values represent log10 of the total superoxide production measured by Diogenes. GAPDH expression indicates relative protein loading. The significance of difference was determined by the Mann-Whitney U test; the significance of correlation was analyzed using Spearman’s rank correlation test. *P < .05, **P < .01, ***P < .001.
High superoxide production is associated with reduced antioxidant expression
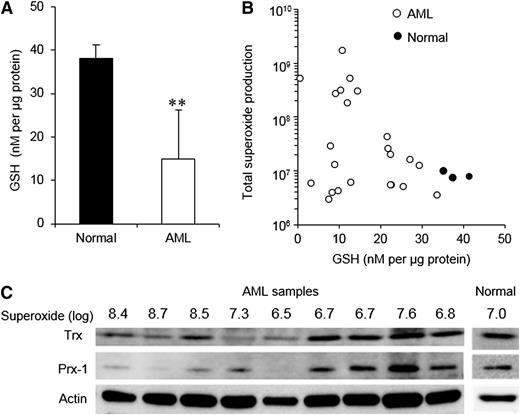
We next set out to determine the consequences of excess ROS production in AML blasts. Extracellular superoxide rapidly dismutates to H2O2,30 which is a membrane-permeable and relatively long-lived ROS; therefore, we reasoned that if ROS is constitutively produced at high levels by AML blasts, their intracellular antioxidant capacity would be affected. To investigate this, we first examined expression of GSH, which plays an important role in the defense against oxidative stress.12,31 GSH was significantly reduced by an average of 60% in our cohort of AML blasts when compared with normal cells (Figure 3A). A ∼10-fold range of GSH levels was observed among AML patients; however, high ROS patients had consistently low levels of GSH (Figure 3B).
Glutathione, thioredoxin, and peroxiredoxin-1 are depleted in high superoxide–producing AML blasts. (A) GSH levels in normal human CD34+ cells (n = 3) compared with primary AML blasts (n = 22). (B) Superoxide production by normal CD34+ cells and primary AML blasts in relation to GSH levels. (C) Thioredoxin (Trx) and peroxiredoxin-1 (Prx-1) expression in primary AML blasts in relation to their superoxide production. Expression levels in normal CD34+ cells are also indicated. Actin expression indicates relative protein loading. The significance of difference was determined by the Mann-Whitney U test. **P < .01.
Glutathione, thioredoxin, and peroxiredoxin-1 are depleted in high superoxide–producing AML blasts. (A) GSH levels in normal human CD34+ cells (n = 3) compared with primary AML blasts (n = 22). (B) Superoxide production by normal CD34+ cells and primary AML blasts in relation to GSH levels. (C) Thioredoxin (Trx) and peroxiredoxin-1 (Prx-1) expression in primary AML blasts in relation to their superoxide production. Expression levels in normal CD34+ cells are also indicated. Actin expression indicates relative protein loading. The significance of difference was determined by the Mann-Whitney U test. **P < .01.
The expression of antioxidant molecules thioredoxin and peroxiredoxin-1 was also examined. We found that expression in low-superoxide AML samples for both proteins was generally comparable with that observed in normal cells, whereas the expression was reduced in AML samples with the highest levels of superoxide (Figure 3C). The mechanisms by which antioxidant molecules are depleted in AML are not clear; however, several mechanisms may contribute (see Discussion). Taken together, these data demonstrate that reduced antioxidant capacity is a general feature of AML, with antioxidant depletion being most acute in AML samples generating high levels of superoxide.
Overproduction of superoxide is associated with suppression of p38MAPK stress signaling
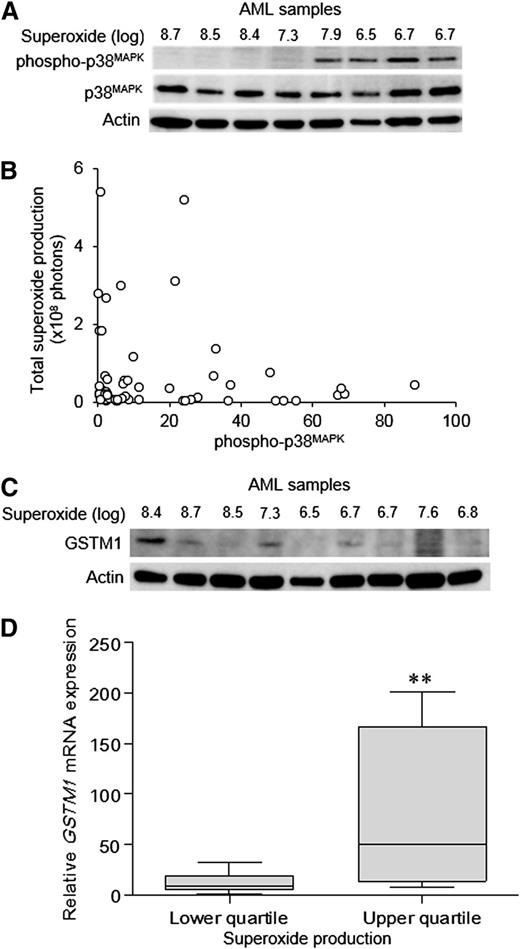
High ROS production combined with reduced antioxidant capacity would be expected to increase the levels of stress signaling in AML blasts. p38MAPK is a central mediator of the oxidative stress response and is known to become activated in response to ROS in hematopoietic cells.32 We therefore determined whether the degree of constitutive p38MAPK phosphorylation was related to ROS production in our cohort of AML patients. Contrary to expectation, elevated superoxide production in primary AML blasts was associated with low or undetectable constitutive p38MAPK phosphorylation (Figure 4A-B).33 These patients also showed attenuated p38MAPK phosphorylation, even when treated with super-physiological H2O2 (supplemental Figure 4), indicating a constitutively defective p38MAPK response. Previously, attenuated p38MAPK responses have been reported to be associated with overexpression of GSTM1 or GSTM2, which act as inhibitors of oxidative stress–induced p38MAPK activation.34 We therefore examined whether this was also the case for AML blasts. We observed no correlation of GSTM2 protein with ROS expression (data not shown), but there was an association with its close family member GSTM1 (Figure 4C-D), suggesting that GSTM1 overexpression may indeed mediate suppression of p38MAPK. To test this hypothesis, we overexpressed GSTM1 in 4 AML cell lines and in normal human CD34+ hematopoietic cells; however, in normal cells and in 3 of the 4 AML cell lines overexpressing GSTM1, we observed no reduction of p38MAPK activation in response to oxidative stress (data not shown). Overall, these data suggest that AML blasts can evade the oxidative stress responses arising from high levels of ROS by inactivating or attenuating the activation of p38MAPK, but that this is not mediated through GSTM1 overexpression.
High ROS levels in AML patients are associated with reduced p38MAPKphosphorylation. (A) Representative Western blot of primary AML blasts showing phospho-p38MAPK and total p38MAPK protein expression in relation to superoxide production (total photons). (B) Summary data showing phospho-p38MAPK in relation to total superoxide production in a cohort of 49 AML patients. (C) Western blot of AML blasts showing GSTM1 protein expression in relation to superoxide production. (D) Relative GSTM1 mRNA expression (normalized to a calibrator sample) in samples from the upper and lower quartiles of superoxide production in a separate cohort of 39 AML patients was analyzed by quantitative real-time polymerase chain reaction using the following GSTM1-specific primers as previously described33 : 5′-TGATGTCCTTGACCTCCACCGT-3′ (forward), 5′-GCTGCACTTCATGTAGGCAGAG-3′ (reverse). The significance of difference was determined by the Mann-Whitney U test. **P < .01.
High ROS levels in AML patients are associated with reduced p38MAPKphosphorylation. (A) Representative Western blot of primary AML blasts showing phospho-p38MAPK and total p38MAPK protein expression in relation to superoxide production (total photons). (B) Summary data showing phospho-p38MAPK in relation to total superoxide production in a cohort of 49 AML patients. (C) Western blot of AML blasts showing GSTM1 protein expression in relation to superoxide production. (D) Relative GSTM1 mRNA expression (normalized to a calibrator sample) in samples from the upper and lower quartiles of superoxide production in a separate cohort of 39 AML patients was analyzed by quantitative real-time polymerase chain reaction using the following GSTM1-specific primers as previously described33 : 5′-TGATGTCCTTGACCTCCACCGT-3′ (forward), 5′-GCTGCACTTCATGTAGGCAGAG-3′ (reverse). The significance of difference was determined by the Mann-Whitney U test. **P < .01.
H2O2 promotes the proliferation of AML cell lines and primary AML cells
In the context of hematopoietic cells, we and others have previously shown that NOX-derived superoxide dismutates to H2O2, which plays an important role in proliferation in response to growth factors10 and oncogene expression.22 Using the H2O2-sensitive probe Amplex UltraRed, we observed that primary AML cells constitutively generate nanomolar H2O2 (supplemental Figure 5A-B). Using GOX to mimic these levels of H2O2 in cell culture medium (supplemental Figure 5C), we investigated whether H2O2 could drive proliferation in MV4-11 and KG-1 cells (which exhibit low constitutive levels of extracellular ROS). Both cell lines showed a dose-dependent increase in proliferation with GOX (Figure 5A-B). Catalase (which eliminates H2O2) negated the effect of GOX, demonstrating that the effect was specifically caused by H2O2 (Figure 5A-B). Increasing the GOX concentration further resulted in apoptosis, which was also prevented by the addition of catalase (data not shown). H2O2-induced proliferation was also observed in primary AML blasts and to a lesser extent in normal human CD34+ cells (Figure 5C). Using Amplex UltraRed, we were able to estimate that proliferation of AML cell lines in response to GOX treatment typically occurred at nanomolar H2O2 concentrations, similar to that generated by primary AML blasts (Figure 5D). To confirm that H2O2 promoted cell cycle progression under these conditions, we carried out cell cycle analysis of MV4-11 cells treated with an optimal GOX concentration for proliferation. As expected, we observed a significant 11% increase in the number of cells in the S+G2M phase compared with untreated cells, which were blocked by the addition of catalase (Figure 5E). In summary, these data indicate that overproduction of extracellular superoxide results in increased levels of H2O2, which in turn are able to promote the proliferation of both primary AML blasts and AML cell lines.
H2O2induces proliferation in hematopoietic cells. (A) Proliferation of MV4-11 cells treated with GOX for 48 hours in the absence or presence of catalase (determined by MTS assay; n = 6). The graph shows MTS reagent absorbance at 492 nm as a percentage of untreated control absorbance (normalized to 100%). (B) KG-1 cell proliferation measured as in (A). (C) Proliferation of primary AML blasts and normal human CD34+ cells treated with GOX in the absence of growth factors (n = 3). (D) The rate of GOX-derived H2O2 production in culture medium (determined by Amplex UltraRed) as a function of GOX concentration. (E) Cell cycle analysis of MV4-11 cells treated with vehicle control or 5 mU/mL GOX in the presence or absence of catalase (n = 5). Significance of difference was determined by the Mann-Whitney U test or the Kruskal-Wallis test followed by Dunn’s multiple comparison test. *P < .05, **P < .01, ***P < .001.
H2O2induces proliferation in hematopoietic cells. (A) Proliferation of MV4-11 cells treated with GOX for 48 hours in the absence or presence of catalase (determined by MTS assay; n = 6). The graph shows MTS reagent absorbance at 492 nm as a percentage of untreated control absorbance (normalized to 100%). (B) KG-1 cell proliferation measured as in (A). (C) Proliferation of primary AML blasts and normal human CD34+ cells treated with GOX in the absence of growth factors (n = 3). (D) The rate of GOX-derived H2O2 production in culture medium (determined by Amplex UltraRed) as a function of GOX concentration. (E) Cell cycle analysis of MV4-11 cells treated with vehicle control or 5 mU/mL GOX in the presence or absence of catalase (n = 5). Significance of difference was determined by the Mann-Whitney U test or the Kruskal-Wallis test followed by Dunn’s multiple comparison test. *P < .05, **P < .01, ***P < .001.
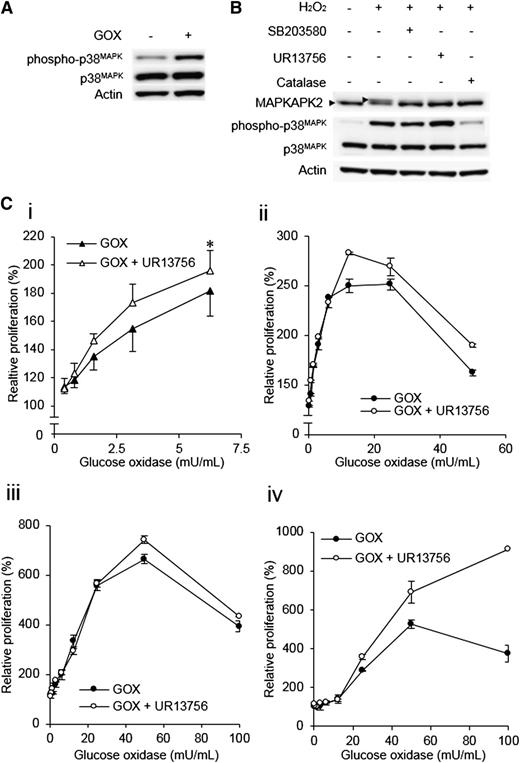
p38MAPK activation limits the proliferative response to ROS
Because activation of p38MAPK normally acts to limit cell cycle progression,34 we reasoned that the attenuated p38MAPK responses in high ROS AML may be driven through a selective process in which cells with defective p38MAPK activation would be the most responsive to the proliferative influence of ROS. To investigate this, we first confirmed that GOX induced p38MAPK phosphorylation at the low nanomolar concentrations that induced proliferation. We found that MV4-11 cells showed increased p38MAPK phosphorylation under these conditions, suggesting that the proliferative response of these cells could be limited by activation of p38MAPK (Figure 6A). To address this, we examined the effect of p38MAPK inhibitors on their proliferative response to ROS. We assessed 2 inhibitors for their capacity to inhibit p38MAPK in this context, SB203580 and a highly selective inhibitor, UR13756.35 In the presence of these inhibitors, p38MAPK is phosphorylated by upstream kinases but is unable to phosphorylate downstream targets such as MAPKAPK2.35 Both inhibitors were able to potently block phosphorylation of MAPKAPK2 in H2O2-treated MV4-11 cells (Figure 6B). UR13756 was selected for further experiments because of its superior specificity for p38MAPK.
p38MAPKlimits H2O2-induced proliferation. (A) p38MAPK phosphorylation was induced in MV4-11 cells treated with 12.5 mU/mL GOX for 3 hours. (B) Demonstration of inhibitor efficacy. MV4-11 cells were pretreated with either vehicle control, UR13756, SB203580 (p38MAPK inhibitors), or catalase before treatment with 1 mM H2O2. Phosphorylation of p38MAPK and its downstream target MAPKAPK2 were then examined by Western blot. Phosphorylation of MAPKAPK2 causes a retardation of electrophoretic migration (arrows). (C) MV4-11 cells (n = 4) (i) or 3 primary AML blast samples (ii-iv) were incubated for 48 hours with GOX and either vehicle control or 50 nM UR13756. The chart shows MTS reagent absorbance at 492 nm as a percentage of untreated control absorbance, which was set at 100%. Significance of difference determined by one-way analysis of variance followed by Tukey’s honestly significant difference test. *P < .05.
p38MAPKlimits H2O2-induced proliferation. (A) p38MAPK phosphorylation was induced in MV4-11 cells treated with 12.5 mU/mL GOX for 3 hours. (B) Demonstration of inhibitor efficacy. MV4-11 cells were pretreated with either vehicle control, UR13756, SB203580 (p38MAPK inhibitors), or catalase before treatment with 1 mM H2O2. Phosphorylation of p38MAPK and its downstream target MAPKAPK2 were then examined by Western blot. Phosphorylation of MAPKAPK2 causes a retardation of electrophoretic migration (arrows). (C) MV4-11 cells (n = 4) (i) or 3 primary AML blast samples (ii-iv) were incubated for 48 hours with GOX and either vehicle control or 50 nM UR13756. The chart shows MTS reagent absorbance at 492 nm as a percentage of untreated control absorbance, which was set at 100%. Significance of difference determined by one-way analysis of variance followed by Tukey’s honestly significant difference test. *P < .05.
If activation of p38MAPK was limiting the proliferative response to exogenous ROS, p38MAPK inhibition would be predicted to promote the proliferative response to ROS. Consistent with this, UR13756-treated MV4-11 cells showed a significant augmentation of the proliferative response induced by GOX (Figure 6Ci), and a similar effect was observed in several p38MAPK-responsive primary AML samples (Figure 6Cii-iv). In summary, these data demonstrate that H2O2 promotes proliferative responses in AML cells, but this proliferative response is limited by the induction of p38MAPK, suggesting an explanation for p38MAPK suppression in high-ROS AML patients.
Discussion
ROS are emerging as an important class of second messengers in normal hematopoiesis and are also implicated in a variety of cancer phenotypes. Our previous studies using a mutant Ras-expressing human CD34+ model suggested a role for ROS in cell proliferation22 ; however, the relevance of these observations to primary AML was unknown. Here we show that unstimulated AML blasts from 60% of patients constitutively produce large amounts of extracellular superoxide, primarily via NOX family oxidases. NOX-derived ROS are known to play several key roles in normal hematopoiesis including hematopoietic stem cell (HSC) motility,36 growth factor signaling,9,10 and differentiation.37 Other studies demonstrate a key role for ROS in regulating HSC self-renewal32,38,39 ; however, the role of NOX oxidases in this process is currently unclear. Given the association of NOX proteins with the control of normal hematopoietic function, we propose that increased NOX activity in AML may represent subversion of normal NOX activity in leukemic cells. Specifically, NOX2 was the primary source of superoxide in primary AML, consistent with our previous observations22 and with other studies implicating NOX2 in promoting survival and proliferation in leukemic cell lines.28,40 Furthermore, we have identified a subset of high ROS patients who show strong expression of NOX4.
Having defined the source of ROS, we next investigated the consequences of ROS overproduction in AML. Examination of antioxidant molecule expression revealed that depletion of the antioxidant defense molecule GSH was frequent in AML and most acute in patients with elevated ROS. Given that GSH depletion is frequently associated with chronic oxidative stress in a variety of diseases,12 it is likely that GSH depletion in AML patients is primarily caused by their elevated ROS production. Similarly, we commonly observed depletion of the antioxidant proteins Trx and Prx-1 in AML blasts with elevated ROS. Because regeneration of oxidized Trx and Prx-1 requires a sufficient pool of NADPH,14 the loss of these proteins may be a direct consequence of NADPH depletion by NADPH oxidases and subsequent degradation of oxidized proteins. Alternatively, because p38MAPK signaling has been reported to promote Prx-1 gene expression,41 the attenuation of stress response signaling (discussed later) may contribute to the loss of antioxidant expression. We also noted a subset of AML samples that exhibited loss of antioxidant molecules in the absence of increased extracellular or mitochondrial ROS. The cause of antioxidant depletion in these samples is currently unclear; however, epigenetic silencing of antioxidant genes has previously been reported in AML,42,43 suggesting a possible mechanistic explanation for these observations. The functional significance of antioxidant depletion also remains unclear, but it is reasonable to suggest that lowered antioxidant defenses in these cells may render them vulnerable to therapies that introduce additional oxidative stress.3
p38MAPK is a stress-activated protein kinase known to become activated in response to ROS in hematopoietic cells.22,32 Surprisingly, we observed that, despite overproduction of ROS and loss of antioxidant capacity, AML blasts with elevated ROS exhibited attenuated activation of the ROS-responsive stress protein p38MAPK. Because ROS-induced p38MAPK activation drives cell cycle arrest, apoptosis and loss of self-renewal,44 reduced p38MAPK activity in these patients may reflect a mechanism to evade the negative effects of p38MAPK signaling. In support of this, INK4 family proteins (that mediate p38MAPK-induced cell cycle arrest) are frequently silenced in AML,45,46 suggesting that this stress response pathway may be targeted at multiple points. Attenuated p38MAPK signaling in response to ROS has previously been observed in murine fibroblasts expressing mutant Ras,34 mediated by overexpression of GSTM1 or GSTM2. However, despite observing a correlation between GSTM1 expression and superoxide production, GSTM1 had little effect on p38MAPK activation, suggesting that in hematopoietic cells, an alternative mechanism of p38MAPK suppression operates, and we are currently investigating this.
The high frequency of overproduction of ROS in cancers and leukemia strongly suggests that they are integral to the pathogenesis of these diseases.3 Previously it has been shown that ROS promotes oxidative DNA damage in leukemia and so may promote disease progression though mutagenesis.47 It has also been shown to mediate suppression of antileukemic immune responses.48 Here we show that in AML, ROS can also act by promoting proliferation of leukemic blasts. Using GOX, we were able to continuously generate H2O2 at concentrations similar to that constitutively produced by primary AML blasts and were able to show for the first time that these concentrations promoted the proliferation of both AML cell lines and primary AML blasts, and to a far greater extent than that seen in normal blasts, suggesting that ROS overproduction in AML may drive proliferation in an autocrine manner. Together these observations suggest that AML blasts become adapted to high ROS and that attenuated p38MAPK responses may be part of this adaptation. In support of this, we also showed that p38MAPK activation accompanies and restricts H2O2-induced proliferation in AML cells, suggesting that proliferative responses to ROS may act as a driver for the selection of leukemic clones with impaired p38MAPK activation. Given that excessive ROS production negatively affects normal hematopoiesis,38,49,50 our data suggest that extracellular ROS generated by AML blasts could also act in a paracrine manner to confer a competitive advantage over normal cells.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by Leukaemia and Lymphoma Research, United Kingdom. Dr Chinmay Munje is funded by the National Institute for Social Care and Health Research, Wales, United Kingdom.
Authorship
Contribution: P.H. and J.Z. co-wrote the manuscript, designed and executed experiments, and analyzed data; C.M., Z.N., L.P., P.W., and N.A. provided technical assistance; R.K.H. provided statistical support; A.K.B. provided resources and clinical insight; and R.L.D. and A.T. designed experiments, provided project direction, and co-wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Richard Darley, Department of Haematology, Main Building, 7th floor, School of Medicine, Cardiff University, Heath Park, Cardiff, United Kingdom, CF14 4XN; e-mail: darley@cf.ac.uk.
References
Author notes
P.S.H. and J.Z. contributed equally to this study.
A.T. and R.L.D. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal