Abstract
NOTCH1 and SF3B1 mutations have been previously reported to have prognostic significance in chronic lymphocytic leukemia but to date they have not been validated in a prospective, controlled clinical trial. We have assessed the impact of these mutations in a cohort of 494 patients treated within the randomized phase 3 United Kingdom Leukaemia Research Fund Chronic Lymphocytic Leukemia 4 (UK LRF CCL4) trial that compared chlorambucil and fludarabine with and without cyclophosphamide in previously untreated patients. We investigated the relationship of mutations in NOTCH1 (exon 34) and SF3B1 (exon 14-16) to treatment response, survival and a panel of established biologic variables. NOTCH1 and SF3B1 mutations were found in 10% and17% of patients, respectively. NOTCH1 mutations correlated with unmutated IGHV genes, trisomy 12, high CD38/ ZAP-70 expression and were associated with reduced overall (median 54.8 vs 74.6 months, P = .02) and progression-free (median 22.0 vs 26.4 months, P = .02) survival. SF3B1 mutations were significantly associated with high CD38 expression and with shorter overall survival (median 54.3 vs 79.0 months, P < .001). Furthermore, multivariate analysis, including baseline clinical variables, treatment, and adverse prognostic factors demonstrated that although TP53 alterations remained the most informative marker of dismal survival in this cohort, NOTCH1 (HR 1.58, P = .03) and SF3B1 (HR 1.52, P = .01) mutations have added independent prognostic value.
Key Points
This is the first study to validate the importance of NOTCH1 and SF3B1 gene mutations in the context of a randomized, prospective clinical trial.
Mutations in both genes are independent prognostic biomarkers, and therefore have clinical utility in the accurate risk-adapted stratification of CLL patients.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participationin the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 566.
Disclosures
Associate Editor John G. Gribben served as an advisor or consultant for Celgene and Roche and as a speaker or a member of a speakers bureau for Roche, Jensen, and Celgene. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the frequency of NOTCH1 and SF3B1 mutations in patients with chronic lymphocytic leukemia (CLL), and their correlations with other genetic markers.
Describe survival in CLL patients with NOTCH1 mutations, and the prognostic value of this mutation.
Describe survival in CLL patients with SF3B1 mutations, and the prognostic value of this mutation.
Release date: January 17, 2013; Expiration date: January 17, 2014
Introduction
Deletions and duplications of genomic DNA are detectable in more than 80% of patients with chronic lymphocytic leukemia (CLL).1 Together with mutations of TP53 and ATM genes located within minimally deleted regions on 17p and 11q, respectively, they constitute the most powerful predictors of the natural history and response to therapy in CLL. However, neither these genomic abnormalities, nor biomarkers that reflect the ability of leukemic cells to respond to their environment, identify all cases with early disease destined to progress. Nor do they identify ∼ 50% of the cases with primary or acquired resistance to chemo-immunotherapy.
The recent application of whole genome sequencing to discovery CLL cohorts followed by targeted resequencing of recurring variants in larger cohorts has identified clinically significant mutations in genes not affected by copy number alterations.2-6 Mutations within the PEST domain of NOTCH1 have been found in 8% to 12% of patients at diagnosis, in 21% of patients with alkylating agent or purine analog-refractory disease and in 30% of cases with the diffuse large B-cell variant of Richter syndrome (RS).4,5,7 They have been correlated with advanced stage at diagnosis, an increased risk of RS, unmutated IGHV genes, trisomy 12 (especially in unmutated IGHV cases), but are under-represented in patients with del13q or a TP53 abnormality.7,8 To date, NOTCH1 mutations have been associated with a shorter time to first treatment and shorter overall survival independent of other prognostic factors, such as TP53 abnormalities and IGHV gene mutation status.4,5,7,8 Mutations in RNA splicing genes have been recently discovered in myeloid malignancies9 with a strong association between SF3B1 gene mutations and cases of the myelodysplastic syndrome with increased ringed sideroblasts.10 Subsequently, SF3B1 mutations have also been found in CLL but not in other chronic B-cell lymphoproliferative disorders.2,3,6 Mutations were documented in 5% to 17% of patients and were associated with advanced stage, fludarabine-refractory disease in cases with no TP53 abnormality, 11q23 deletions and with short time to first treatment and overall survival independent of other prognostic variables.2,3,6
These data suggest that these mutations represent novel independent prognostic factors that may influence clinical practice. However, mutations in neither gene have been evaluated within the context of a large clinical trial. To address this we have screened patients entered into the United Kingdom Leukaemia Research Fund Chronic Lymphocytic Leukemia 4 (UK LRF CCL4) trial for NOTCH1 and SF3B1 mutations and correlated their presence with a comprehensive panel of established biomarkers and with clinical outcome. With this approach, we show that although TP53 alterations remained the most informative predictor of poor outcome in our study, NOTCH1 and SF3B1 mutations are independent markers of shorter overall survival.
Methods
Patients and biomarker data
Between 1999 and 2004, the UK LRF CLL4 trial randomly assigned 777 patients to first-line treatment with chlorambucil (CHL), fludarabine (FDR), or fludarabine plus cyclophosphamide (FC).11 Up to 494 patients with DNA available were included in the current study and their characteristics did not differ significantly from the entire trial cohort, in terms of treatment allocation, cytogenetics, age, gender, disease stage, ZAP-70 and CD38 expression, or IGHV status (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All samples were taken at trial entry before initiation of treatment and, on average, were more than 80% tumor cells. The assessment of established biomarkers was performed as previously described,12 and a combined TP53 deletion and/or mutation variable was used.13 Because of sample availability from United Kingdom DNA repositories, the number screened for NOTCH1 and SF3B1 varied slightly. This study was conducted in accordance with the Declaration of Helsinki and was ethically approved by the local REC.
NOTCH1 and SF3B1 mutational screening and sequencing
NOTCH1 and SF3B1 mutations were successfully ascertained in 466 and 437 patients, respectively. Each genomic DNA sample was subjected to whole genome amplification (WGA) using the Illustra GenomiPhi V2 Amplification Kit (GE Healthcare), before mutational screening using high-resolution melt (HRM) analysis as previously reported.14 For each gene we targeted our screening approach to capture the majority of previously reported CLL-specific somatic variation; for NOTCH1 we screened a 399bp section of exon 34 (amino acids 2405-2525, where all of the PEST domain mutations have been found)4,5,7,15 and for SF3B1 we screened exons 14-16 (capturing all the previously reported disease-specific variation2,3,6 ; see supplemental information for primer sequences). Products showing abnormal melt patterns were sequenced. All mutations were sequence validated on genomic DNA (gDNA) from the archival sample. Furthermore, we sequenced the aforementioned regions of NOTCH1 and SF3B1 in gDNA samples from 58 cases that exhibited normal HRM melt profiles on WGA material. In doing so, we found no additional mutations present in the gDNA.
To further assess the sensitivity of the HRM approach, we identified cases with the P2515Rfs*4 (c.7544_7545delCT, referred to as “delCT”) variant using PCR-based fragment analysis [PCR-FA, n = 372] and allele-specific PCR [n = 213]. For the PCR-FA, the delCT variant was identified using PEST2 domain-specific primers (FW: GTGACCGCAGCCCAGTTC, RV: AAAGGAAGCCGGGGTCTC) as previously described.16 PCR products were sized using the LT3500 (Life Technologies) and the 271bp wild-type and 269bp mutant fragments were identified using GeneMapper Version 4.1 software (Life Technologies). Allele-specific PCR for the delCT variant was performed by KBioscience (http://www.kbiosciences.co.uk) using their own novel fluorescence-based competitive allele-specific PCR (KASPar). Duplicates (n = 106) were included and the concordance between duplicates was > 99%. Sample processing using the PCR-FA and KASPar technologies were performed blindly in independent laboratories. Using these 2 approaches, we confirm the sensitivity of our HRM approach which showed a 100% concordance with our PCR-FA and KASPar analysis.
Statistical analysis
Statistical analysis was performed using STATA Version 9.2 and SPSS Version 19. We used univariate logistic regression and dependent variables examined included the presence/absence of a NOTCH1/SF3B1 mutation in relation to a range of biomarkers and clinical data. We included age at trial entry and gender as covariates in all models. Overall response status (ORS) was defined as complete (CR), nodular partial (nPR), and partial response (PR). Overall survival (OS) was defined as time from randomization to death, or to the follow-up date (August 2012) for survivors. Progression free survival (PFS) was defined as time from randomization to relapse needing further therapy, progression, or death; or to the follow-up date (October 2010; final LRF CLL4 PFS update) for those with no progression/death. Kaplan-Meier analysis and log-rank test or Cox regression were undertaken for survival analyses examining the impacts of mutation on OS and PFS. Fixed covariates included in the Cox regression models were: age at trial entry, gender, Binet stage (A versus B/C), IGHV mutation status, 11q deletion, and TP53 deletion and/or mutation (TP53 del/mut). Based on previous NOTCH1 survival studies,7 with a 10% mutational prevalence, a mean survival time of 3.5 years for mutated individuals and a follow-up of 10 years, our study had 90% power with a significance level = 0.05 to detect a minimum hazard ratio (HR) of 1.35. This HR is lower than that observed previously.7 Results were determined to be statistically significant at the 5% level.
Results
NOTCH1 mutations are associated with the presence of unmutated IGHV genes, trisomy 12, CD38 expression, and with shorter progression-free and overall survival
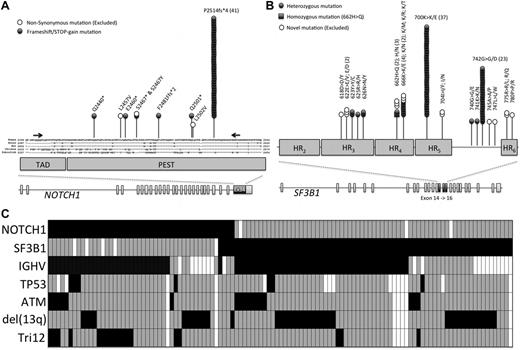
We identified 49 patients with putative mutations within exon 34 of NOTCH1 (Figure 1A). Mutations were most represented (41/49, 84%) by the recurrent 2-bp frameshift deletion (delCT, Figure 1A). 63% (5/8) of the remaining mutations were previously reported and/or are predicted to disrupt the NOTCH1 PEST domain (supplemental Table 2). As patients were randomized 8 to 13 years ago, germ-line material was not available to confirm the somatic-nature of our novel mutations. Therefore, to ensure that likely pathogenic mutations were included in subsequent analysis, only PEST domain-truncating mutations were analyzed further in this study; providing a NOTCH1 mutational frequency of 10% (46/466, Table 1) in the LRF CLL4 trial. Of the 348 deaths in patients with NOTCH1 data, 12 (3%) were because of Richter syndrome (RS). Only 1 of 12 patients (8%) had a NOTCH1 mutation. The incidence of death because of RS was not significantly different in the mutated and the wild-type groups.
NOTCH1 and SF3B1 mutational analysis in the LRF CLL4 patients. (A) The distribution of mutations in NOTCH1. The NOTCH1 gene contains 34 exons and encodes a protein with a C-terminal TAD-PEST domain, which is a hotspot for mutation in CLL. Part of exon 34 is magnified and the location of each mutation is shown, along with evolutionary conservation of amino acids. (B) The distribution of missense mutations in SF3B1. The SF3B1 gene contains 25 exons and encodes a protein with a C-terminal domain consisting of 22 HEAT domains. Exons 14, 15, and 16 are magnified and the location of each mutation is shown. (C) The mutual relationship between NOTCH1 and SF3B1 mutations and other gene lesions in CLL. Rows correspond to specific genes and columns represent individual patients (only patients with either a NOTCH1 or SF3B1 mutation are shown). Boxes colored black and gray show the presence or absence of a mutation of NOTCH1 or SF3B1, with deletion of 11q, deletion or mutation of TP53, an unmutated IGHV sequence, deletion of 13q, or the presence of trisomy 12. A white box denotes that no data were available.
NOTCH1 and SF3B1 mutational analysis in the LRF CLL4 patients. (A) The distribution of mutations in NOTCH1. The NOTCH1 gene contains 34 exons and encodes a protein with a C-terminal TAD-PEST domain, which is a hotspot for mutation in CLL. Part of exon 34 is magnified and the location of each mutation is shown, along with evolutionary conservation of amino acids. (B) The distribution of missense mutations in SF3B1. The SF3B1 gene contains 25 exons and encodes a protein with a C-terminal domain consisting of 22 HEAT domains. Exons 14, 15, and 16 are magnified and the location of each mutation is shown. (C) The mutual relationship between NOTCH1 and SF3B1 mutations and other gene lesions in CLL. Rows correspond to specific genes and columns represent individual patients (only patients with either a NOTCH1 or SF3B1 mutation are shown). Boxes colored black and gray show the presence or absence of a mutation of NOTCH1 or SF3B1, with deletion of 11q, deletion or mutation of TP53, an unmutated IGHV sequence, deletion of 13q, or the presence of trisomy 12. A white box denotes that no data were available.
Frequencies of NOTCH1 and SF3B1 mutations and clinical characteristics of the 494 LRF CLL4 patients
| Variable . | NOTCH1 (all PEST terminating mutations) . | SF3B1 (previously shown as somatic in CLL) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N (%) . | Wild-type N (%) . | Mutated N (%) . | P . | Total N (%) . | Wild-type N (%) . | Mutated N (%) . | P . | |
| Screened cases | 466 (94) | 420 (90) | 46 (10) | 437 (88) | 364 (83) | 73 (17) | ||
| Male | 344 (74) | 307 (89) | 37 (11) | NS | 319 (73) | 263 (82) | 56 (18) | NS |
| Female | 122 (26) | 113 (93) | 9 (7) | 118 (27) | 101 (86) | 17 (14) | ||
| Age (range)* | 63 (38-86) | 66 (48-83) | 63 (38-83) | 64 (42-82) | ||||
| Binet stage* | NS | NS | ||||||
| A | 106 (23) | 96 (91) | 10 (9) | 97 (22) | 85 (88) | 12 (12) | ||
| B | 218 (47) | 192 (88) | 26 (12) | 209 (48) | 172 (82) | 37 (18) | ||
| C | 142 (30) | 132 (93) | 10 (7) | 131 (30) | 107 (82) | 24 (18) | ||
| IGHV unmutated | 240 (63) | 209 (87) | 31 (13) | .01 | 228 (64) | 184 (81) | 44 (19) | NS |
| mutated | 144 (37) | 137 (95) | 7 (5) | 131 (36) | 115 (88) | 16 (12) | ||
| CD38 −ve | 212 (55) | 203 (96) | 9 (4) | .0001 | 193 (55) | 173 (90) | 20 (10) | .007 |
| +ve | 170 (44) | 139 (82) | 31 (18) | 160 (45) | 126 (79) | 34 (21) | ||
| ZAP70 −ve | 164 (47) | 155 (95) | 9 (5) | .008 | 154 (47) | 132 (86) | 22 (14) | NS |
| +ve | 186 (53) | 159 (85) | 27 (15) | 174 (53) | 141 (81) | 33 (19) | ||
| TP53 normal | 408 (93) | 368 (90) | 40 (10) | NS | 388 (94) | 323 (83) | 65 (17) | NS |
| del/mut | 32 (7) | 28 (87) | 4 (13) | 24 (6) | 21 (88) | 3 (12) | ||
| del(11q) −ve | 352 (80) | 314 (89) | 38 (11) | NS | 330 (80) | 273 (83) | 57 (7) | NS |
| +ve | 89 (20) | 83 (93) | 6 (7) | 83 (20) | 72 (87) | 11 (13) | ||
| del(13q) −ve† | 272 (62) | 240 (88) | 32 (12) | NS | 254 (62) | 214 (85) | 40 (15) | NS |
| +ve | 166 (38) | 154 (93) | 12 (7) | 156 (38) | 128 (82) | 28 (18) | ||
| Tri12 −ve | 369 (84) | 340 (92) | 29 (8) | .002 | 343 (83) | 278 (81) | 65 (19) | .001 |
| +ve | 72 (16) | 57 (79) | 15 (21) | 70 (17) | 67 (96) | 3 (4) | ||
| Variable . | NOTCH1 (all PEST terminating mutations) . | SF3B1 (previously shown as somatic in CLL) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N (%) . | Wild-type N (%) . | Mutated N (%) . | P . | Total N (%) . | Wild-type N (%) . | Mutated N (%) . | P . | |
| Screened cases | 466 (94) | 420 (90) | 46 (10) | 437 (88) | 364 (83) | 73 (17) | ||
| Male | 344 (74) | 307 (89) | 37 (11) | NS | 319 (73) | 263 (82) | 56 (18) | NS |
| Female | 122 (26) | 113 (93) | 9 (7) | 118 (27) | 101 (86) | 17 (14) | ||
| Age (range)* | 63 (38-86) | 66 (48-83) | 63 (38-83) | 64 (42-82) | ||||
| Binet stage* | NS | NS | ||||||
| A | 106 (23) | 96 (91) | 10 (9) | 97 (22) | 85 (88) | 12 (12) | ||
| B | 218 (47) | 192 (88) | 26 (12) | 209 (48) | 172 (82) | 37 (18) | ||
| C | 142 (30) | 132 (93) | 10 (7) | 131 (30) | 107 (82) | 24 (18) | ||
| IGHV unmutated | 240 (63) | 209 (87) | 31 (13) | .01 | 228 (64) | 184 (81) | 44 (19) | NS |
| mutated | 144 (37) | 137 (95) | 7 (5) | 131 (36) | 115 (88) | 16 (12) | ||
| CD38 −ve | 212 (55) | 203 (96) | 9 (4) | .0001 | 193 (55) | 173 (90) | 20 (10) | .007 |
| +ve | 170 (44) | 139 (82) | 31 (18) | 160 (45) | 126 (79) | 34 (21) | ||
| ZAP70 −ve | 164 (47) | 155 (95) | 9 (5) | .008 | 154 (47) | 132 (86) | 22 (14) | NS |
| +ve | 186 (53) | 159 (85) | 27 (15) | 174 (53) | 141 (81) | 33 (19) | ||
| TP53 normal | 408 (93) | 368 (90) | 40 (10) | NS | 388 (94) | 323 (83) | 65 (17) | NS |
| del/mut | 32 (7) | 28 (87) | 4 (13) | 24 (6) | 21 (88) | 3 (12) | ||
| del(11q) −ve | 352 (80) | 314 (89) | 38 (11) | NS | 330 (80) | 273 (83) | 57 (7) | NS |
| +ve | 89 (20) | 83 (93) | 6 (7) | 83 (20) | 72 (87) | 11 (13) | ||
| del(13q) −ve† | 272 (62) | 240 (88) | 32 (12) | NS | 254 (62) | 214 (85) | 40 (15) | NS |
| +ve | 166 (38) | 154 (93) | 12 (7) | 156 (38) | 128 (82) | 28 (18) | ||
| Tri12 −ve | 369 (84) | 340 (92) | 29 (8) | .002 | 343 (83) | 278 (81) | 65 (19) | .001 |
| +ve | 72 (16) | 57 (79) | 15 (21) | 70 (17) | 67 (96) | 3 (4) | ||
Age and disease stage were both assessed at trial entry. NS indicates nonsignificant (significance level = .05). TP53 abnormalities defined by deletion and/or mutation (del/mut).
The presence of a 13q deletion as a sole abnormality using a standard FISH panel. P values were calculated from 2 × 2 or 2 × 3 χ2 tests (Fisher exact test was used when observations were < 5).
The clinical and biologic features of NOTCH1 mutated CLL are summarized in Tables 1 and 2. Importantly, mutations showed a significant association with unmutated IGHV genes (OR 2.91, P = .014, Table 2) and positive expression of ZAP-70 (OR 2.92, P = .007) and CD38 (OR 5.03, P < .001, Table 2). In contrast, the presence of a mutation was not associated with DNA damage response biomarkers such as deletion of 11q or 17p or mutation of TP53. The presence of a NOTCH1 mutation was significantly associated with the presence of trisomy 12 (OR 3.09, P = .001); within those cases, 13 of 15 NOTCH1 mutated-trisomy 12 cases were IGHV unmutated (Figure 1C).
Association between NOTCH1/SF3B1 gene mutation status and clinical characteristics of the LRF CLL4 patients
| Variable . | NOTCH1 mutated cases (all PEST terminating mutations) . | SF3B1 mutated cases (previously shown as somatic in CLL) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | SE . | P . | n = * . | OR . | 95% CI . | SE . | P . | n = * . | |
| Age at diagnosis | 1.04 | 0.99-1.07 | 0.02 | .06 | 466 | 1.01 | 0.98-1.04 | 0.02 | .46 | 437 |
| Sex | 0.66 | 0.31-1.41 | 0.26 | .29 | 466 | 0.79 | 0.44-1.43 | 0.30 | .43 | 437 |
| Binet stage (A, B, or C) | 0.85 | 0.56-1.29 | 0.18 | .45 | 466 | 1.23 | 0.86-1.75 | 0.18 | .26 | 437 |
| CD38 positive | 5.03 | 2.32-10.90 | 1.98 | < .001 | 382 | 2.33 | 1.28-4.25 | 0.71 | .005 | 353 |
| ZAP70 positive | 2.92 | 1.32-6.42 | 1.17 | .007 | 350 | 1.40 | 0.78-2.53 | 0.42 | .26 | 328 |
| IGHV unmutated | 2.91 | 1.24-6.80 | 0.15 | .014 | 384 | 1.72 | 0.93-3.19 | 0.18 | .09 | 359 |
| TP53 del/mut | 1.31 | 0.44-3.94 | 0.56 | .63 | 440 | 0.71 | 0.21-2.45 | 0.63 | .59 | 412 |
| del(11q) | 0.60 | 0.24-1.46 | 0.27 | .26 | 441 | 0.73 | 0.37-1.47 | 0.26 | .38 | 413 |
| del(13q)† | 0.54 | 0.27-1.09 | 0.19 | .09 | 441 | 1.08 | 0.63-1.83 | 0.28 | .79 | 413 |
| Tri12 | 3.09 | 1.56-6.11 | 1.08 | .001 | 441 | 0.19 | 0.06-0.63 | 0.12 | .006 | 413 |
| Response to treatment‡ | 0.88 | 0.57-1.35 | 0.19 | .58 | 441 | 0.95 | 0.67-1.36 | 0.18 | .79 | 413 |
| LRF CLL4 treatment arm§ | 0.68 | 0.45-1.03 | 0.14 | .07 | 466 | 1.15 | 0.84-1.56 | 0.16 | .38 | 437 |
| Variable . | NOTCH1 mutated cases (all PEST terminating mutations) . | SF3B1 mutated cases (previously shown as somatic in CLL) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | SE . | P . | n = * . | OR . | 95% CI . | SE . | P . | n = * . | |
| Age at diagnosis | 1.04 | 0.99-1.07 | 0.02 | .06 | 466 | 1.01 | 0.98-1.04 | 0.02 | .46 | 437 |
| Sex | 0.66 | 0.31-1.41 | 0.26 | .29 | 466 | 0.79 | 0.44-1.43 | 0.30 | .43 | 437 |
| Binet stage (A, B, or C) | 0.85 | 0.56-1.29 | 0.18 | .45 | 466 | 1.23 | 0.86-1.75 | 0.18 | .26 | 437 |
| CD38 positive | 5.03 | 2.32-10.90 | 1.98 | < .001 | 382 | 2.33 | 1.28-4.25 | 0.71 | .005 | 353 |
| ZAP70 positive | 2.92 | 1.32-6.42 | 1.17 | .007 | 350 | 1.40 | 0.78-2.53 | 0.42 | .26 | 328 |
| IGHV unmutated | 2.91 | 1.24-6.80 | 0.15 | .014 | 384 | 1.72 | 0.93-3.19 | 0.18 | .09 | 359 |
| TP53 del/mut | 1.31 | 0.44-3.94 | 0.56 | .63 | 440 | 0.71 | 0.21-2.45 | 0.63 | .59 | 412 |
| del(11q) | 0.60 | 0.24-1.46 | 0.27 | .26 | 441 | 0.73 | 0.37-1.47 | 0.26 | .38 | 413 |
| del(13q)† | 0.54 | 0.27-1.09 | 0.19 | .09 | 441 | 1.08 | 0.63-1.83 | 0.28 | .79 | 413 |
| Tri12 | 3.09 | 1.56-6.11 | 1.08 | .001 | 441 | 0.19 | 0.06-0.63 | 0.12 | .006 | 413 |
| Response to treatment‡ | 0.88 | 0.57-1.35 | 0.19 | .58 | 441 | 0.95 | 0.67-1.36 | 0.18 | .79 | 413 |
| LRF CLL4 treatment arm§ | 0.68 | 0.45-1.03 | 0.14 | .07 | 466 | 1.15 | 0.84-1.56 | 0.16 | .38 | 437 |
Shows the number of observations included in each logistic regression analysis.
The presence of a 13q deletion as a sole abnormality using a standard FISH panel.
Defined as response (CR/nPR, PR, NR) to any treatment.
Any treatment (CHL, FC, FDR). TP53 abnormalities defined by deletion and/ or mutation (del/mut).
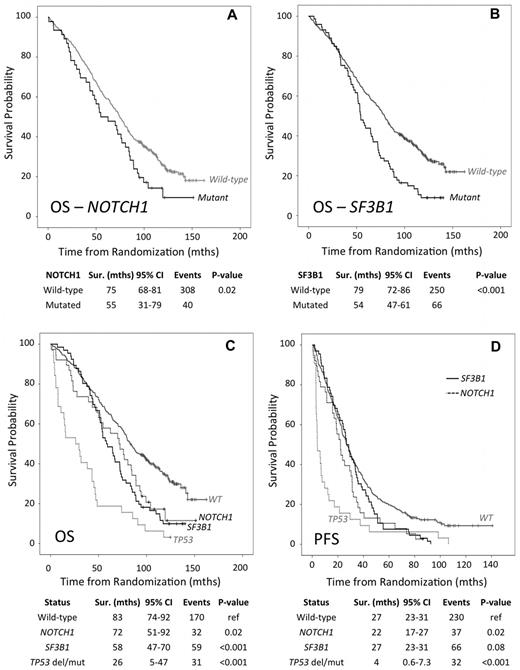
Next, we determined the impact of a NOTCH1 mutation on overall response status (ORS), overall survival (OS) and progression-free survival (PFS). Mutations were not associated with ORS within any of the 3 treatment arms (data not shown). However, univariate analysis revealed that the presence of a mutation was associated with a significant reduction in median OS of 54.8 versus 74.6 months (HR 1.47, 95% CI 1.06-2.05, P = .02) and median PFS of 22.0 versus 26.4 months (HR 1.46, 95% CI 1.07-1.99, P = .02), for mutant and wild-type, respectively (Figure 2A, Table 3).
Survival analysis of LRF CLL4 cases accounting for NOTCH1, SF3B1 and TP53 gene status. (A-B) Overall survival for NOTCH1 and SF3B1 mutational status, respectively. (C-D) Overall survival and progression free survival, respectively. Patient subgroups with mutations in NOTCH1 (but wild-type for SF3B1 and TP53), SF3B1 (but wild-type for NOTCH1 and TP53), and TP53 del/mut cases (but wild-type for SF3B1 and NOTCH1); highlighted by a solid black, dashed black, and dashed gray line, respectively. The solid-gray line identifies the WT subgroup that is wild-type for NOTCH1, SF3B1, and TP53 (that lack either a deletion or mutation). The P values are derived from Kaplan-Meier analysis with a log-rank test and median survival times with 95% confidence intervals.
Survival analysis of LRF CLL4 cases accounting for NOTCH1, SF3B1 and TP53 gene status. (A-B) Overall survival for NOTCH1 and SF3B1 mutational status, respectively. (C-D) Overall survival and progression free survival, respectively. Patient subgroups with mutations in NOTCH1 (but wild-type for SF3B1 and TP53), SF3B1 (but wild-type for NOTCH1 and TP53), and TP53 del/mut cases (but wild-type for SF3B1 and NOTCH1); highlighted by a solid black, dashed black, and dashed gray line, respectively. The solid-gray line identifies the WT subgroup that is wild-type for NOTCH1, SF3B1, and TP53 (that lack either a deletion or mutation). The P values are derived from Kaplan-Meier analysis with a log-rank test and median survival times with 95% confidence intervals.
Univariate Cox proportional hazard analysis of OS and PFS in the LRF CLL4 patients
| . | Mutation/Biomarkers . | . | Overall Survival (mouths) . | Progression-free survival (months) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | Events . | Median . | 95% CI . | HR . | 95% CI . | P . | Total . | Events . | Median (mean) . | 95% CI . | HR . | 95% CI . | P . | |||
| Gene mutation | NOTCH1 | Wild-type | 420 | 308 | 74.6 | 68.4-80.9 | 420 | 387 | 26.4 | 23.6-29.3 | ||||||
| Mutated | 46 | 40 | 54.8 | 31.0-78.5 | 1.47 | 1.06-2.05 | .02 | 46 | 45 | 22.0 | 17.2-26.9 | 1.46 | 1.07-1.99 | .02 | ||
| SF3B1 | Wild-type | 364 | 250 | 79.0 | 71.8-86.3 | 364 | 326 | 26.5 (39.1) | 23.1-29.9 | |||||||
| Mutated | 73 | 66 | 54.3 | 47.3-61.4 | 1.71 | 1.30-2.25 | < .001 | 73 | 73 | 26.5 (29.4) | 22.4-30.7 | 1.32 | 1.02-1.71 | .03 | ||
| Clinical feature | Age | 1.05 | 1.04-1.07 | < .001 | 1.00 | 0.99-1.01 | .87 | |||||||||
| Sex | Male | 365 | 280 | 70.3 | 61.4-79.2 | 365 | 340 | 25.2 | 22.3-28.2 | |||||||
| Female | 129 | 86 | 79.7 | 66.5-93.0 | 0.77 | 0.61-0.98 | .04 | 129 | 115 | 29.4 | 25.5-33.3 | 0.79 | 0.64-0.98 | .03 | ||
| Binet Stage (A/B/C) | A | 112 | 76 | 80.6 | 63.4-97.7 | 112 | 104 | 27.2 | 23.8-30.7 | |||||||
| B | 231 | 174 | 76.7 | 70.2-83.3 | 231 | 213 | 26.5 | 22.5-30.6 | ||||||||
| C | 151 | 116 | 54.3 | 44.8-63.8 | 1.21 | 1.04-1.39 | .01 | 151 | 138 | 24.4 | 18.4-30.4 | 1.02 | 0.89-1.16 | .77 | ||
| Binet Stage (A vs. B/C) | A | 112 | 76 | 80.6 | 63.4-97.7 | 112 | 104 | 27.2 | 23.8-30.7 | |||||||
| B/C | 382 | 290 | 71.5 | 64.8-78.2 | 1.29 | 1.01-1.67 | .04 | 382 | 351 | 26.1 | 23.0-29.1 | 1.00 | 0.81-1.25 | .98 | ||
| Biomarker | IGHV status | Mutated | 115 | 91 | 104.2 | 93.3-115.1 | 115 | 128 | 34.8 | 29.3-40.4 | ||||||
| Unmutated | 254 | 215 | 60.6 | 52.5-68.7 | 2.18 | 1.70-2.79 | < .001 | 254 | 249 | 22.7 | 20.2-25.2 | 1.86 | 1.49-2.31 | < .001 | ||
| ZAP70 positive | Negative | 177 | 117 | 84.7 | 69.6-99.8 | 177 | 154 | 30.5 | 25.5-35.4 | |||||||
| Positive | 197 | 162 | 69.0 | 59.7-78.2 | 1.47 | 1.16-1.86 | .002 | 197 | 188 | 23.0 | 20.2-25.8 | 1.42 | 1.15-1.76 | .001 | ||
| CD38 positive | Negative | 223 | 149 | 84.7 | 73.8-95.6 | 223 | 199 | 28.5 | 24.4-32.7 | |||||||
| Positive | 177 | 145 | 64.5 | 54.2-74.9 | 1.54 | 1.22-1.94 | < .001 | 177 | 169 | 21.6 | 17.6-25.7 | 1.39 | 1.13-1.70 | .002 | ||
| TP53 del/mut | Normal | 431 | 313 | 75.9 | 69.7-82.1 | 431 | 395 | 26.7 | 23.9-29.5 | |||||||
| Del/mut | 32 | 31 | 26.1 | 4.9-47.3 | 3.19 | 2.20-4.62 | < .001 | 32 | 32 | 3.9 | 0.57-7.3 | 2.70 | 1.88-3.89 | < .001 | ||
| del(11q) | Undeleted | 372 | 266 | 75.0 | 67.5-82.6 | 372 | 340 | 28.5 | 26.0-31.0 | |||||||
| Deleted | 92 | 79 | 57.7 | 42.4-73.0 | 1.62 | 1.26-2.09 | < .001 | 92 | 88 | 17.9 | 13.4-22.5 | 1.49 | 1.18-1.88 | .001 | ||
| del(13q)sole | Undeleted | 284 | 227 | 58.4 | 48.7-68.1 | 284 | 270 | 23.0 | 20.6-25.4 | |||||||
| Deleted | 177 | 115 | 88.9 | 73.2-104.5 | 0.59 | 0.47-0.74 | < .001 | 177 | 155 | 32.3 | 28.0-36.5 | 0.67 | 0.56-84.0 | < .001 | ||
| Trisomy12 | Absent | 388 | 284 | 73.9 | 67.3-80.4 | 388 | 357 | 26.5 | 23.5-29.5 | |||||||
| Present | 76 | 61 | 59.4 | 38.9-80.1 | 1.30 | 0.98-1.71 | .07 | 76 | 71 | 19.4 | 11.9-26.8 | 1.28 | 0.99-1.65 | .06 | ||
| . | Mutation/Biomarkers . | . | Overall Survival (mouths) . | Progression-free survival (months) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | Events . | Median . | 95% CI . | HR . | 95% CI . | P . | Total . | Events . | Median (mean) . | 95% CI . | HR . | 95% CI . | P . | |||
| Gene mutation | NOTCH1 | Wild-type | 420 | 308 | 74.6 | 68.4-80.9 | 420 | 387 | 26.4 | 23.6-29.3 | ||||||
| Mutated | 46 | 40 | 54.8 | 31.0-78.5 | 1.47 | 1.06-2.05 | .02 | 46 | 45 | 22.0 | 17.2-26.9 | 1.46 | 1.07-1.99 | .02 | ||
| SF3B1 | Wild-type | 364 | 250 | 79.0 | 71.8-86.3 | 364 | 326 | 26.5 (39.1) | 23.1-29.9 | |||||||
| Mutated | 73 | 66 | 54.3 | 47.3-61.4 | 1.71 | 1.30-2.25 | < .001 | 73 | 73 | 26.5 (29.4) | 22.4-30.7 | 1.32 | 1.02-1.71 | .03 | ||
| Clinical feature | Age | 1.05 | 1.04-1.07 | < .001 | 1.00 | 0.99-1.01 | .87 | |||||||||
| Sex | Male | 365 | 280 | 70.3 | 61.4-79.2 | 365 | 340 | 25.2 | 22.3-28.2 | |||||||
| Female | 129 | 86 | 79.7 | 66.5-93.0 | 0.77 | 0.61-0.98 | .04 | 129 | 115 | 29.4 | 25.5-33.3 | 0.79 | 0.64-0.98 | .03 | ||
| Binet Stage (A/B/C) | A | 112 | 76 | 80.6 | 63.4-97.7 | 112 | 104 | 27.2 | 23.8-30.7 | |||||||
| B | 231 | 174 | 76.7 | 70.2-83.3 | 231 | 213 | 26.5 | 22.5-30.6 | ||||||||
| C | 151 | 116 | 54.3 | 44.8-63.8 | 1.21 | 1.04-1.39 | .01 | 151 | 138 | 24.4 | 18.4-30.4 | 1.02 | 0.89-1.16 | .77 | ||
| Binet Stage (A vs. B/C) | A | 112 | 76 | 80.6 | 63.4-97.7 | 112 | 104 | 27.2 | 23.8-30.7 | |||||||
| B/C | 382 | 290 | 71.5 | 64.8-78.2 | 1.29 | 1.01-1.67 | .04 | 382 | 351 | 26.1 | 23.0-29.1 | 1.00 | 0.81-1.25 | .98 | ||
| Biomarker | IGHV status | Mutated | 115 | 91 | 104.2 | 93.3-115.1 | 115 | 128 | 34.8 | 29.3-40.4 | ||||||
| Unmutated | 254 | 215 | 60.6 | 52.5-68.7 | 2.18 | 1.70-2.79 | < .001 | 254 | 249 | 22.7 | 20.2-25.2 | 1.86 | 1.49-2.31 | < .001 | ||
| ZAP70 positive | Negative | 177 | 117 | 84.7 | 69.6-99.8 | 177 | 154 | 30.5 | 25.5-35.4 | |||||||
| Positive | 197 | 162 | 69.0 | 59.7-78.2 | 1.47 | 1.16-1.86 | .002 | 197 | 188 | 23.0 | 20.2-25.8 | 1.42 | 1.15-1.76 | .001 | ||
| CD38 positive | Negative | 223 | 149 | 84.7 | 73.8-95.6 | 223 | 199 | 28.5 | 24.4-32.7 | |||||||
| Positive | 177 | 145 | 64.5 | 54.2-74.9 | 1.54 | 1.22-1.94 | < .001 | 177 | 169 | 21.6 | 17.6-25.7 | 1.39 | 1.13-1.70 | .002 | ||
| TP53 del/mut | Normal | 431 | 313 | 75.9 | 69.7-82.1 | 431 | 395 | 26.7 | 23.9-29.5 | |||||||
| Del/mut | 32 | 31 | 26.1 | 4.9-47.3 | 3.19 | 2.20-4.62 | < .001 | 32 | 32 | 3.9 | 0.57-7.3 | 2.70 | 1.88-3.89 | < .001 | ||
| del(11q) | Undeleted | 372 | 266 | 75.0 | 67.5-82.6 | 372 | 340 | 28.5 | 26.0-31.0 | |||||||
| Deleted | 92 | 79 | 57.7 | 42.4-73.0 | 1.62 | 1.26-2.09 | < .001 | 92 | 88 | 17.9 | 13.4-22.5 | 1.49 | 1.18-1.88 | .001 | ||
| del(13q)sole | Undeleted | 284 | 227 | 58.4 | 48.7-68.1 | 284 | 270 | 23.0 | 20.6-25.4 | |||||||
| Deleted | 177 | 115 | 88.9 | 73.2-104.5 | 0.59 | 0.47-0.74 | < .001 | 177 | 155 | 32.3 | 28.0-36.5 | 0.67 | 0.56-84.0 | < .001 | ||
| Trisomy12 | Absent | 388 | 284 | 73.9 | 67.3-80.4 | 388 | 357 | 26.5 | 23.5-29.5 | |||||||
| Present | 76 | 61 | 59.4 | 38.9-80.1 | 1.30 | 0.98-1.71 | .07 | 76 | 71 | 19.4 | 11.9-26.8 | 1.28 | 0.99-1.65 | .06 | ||
The double SF3B1 mutated (700 and 742) cases (n = 2) remain in this analysis. We performed the analysis excluding these cases and obtained similar results. TP53 abnormalities defined by deletion and/or mutation (del/mut).
SF3B1 mutations are associated with high CD38 expression and overall survival
Of the 92 putative mutations identified in exon 14, 15, and 16 of SF3B1, 73 patients harbored 75 mutations that were previously shown to be somatically acquired in CLL,2,3,6 providing a frequency of confirmed somatic mutations in SF3B1 of 17% (73/437; Table 1) in the LRF CLL4 trial. In addition to these 75 mutations, we identified 17 sequence variants that have not been previously identified in CLL, 6 of which have been previously observed in myeloid disease9,10 (supplemental Table 3) and the remaining 11 have been predicted to be functional deleterious using PolyPhen and SIFT analysis. However, as these could not be somatically confirmed in the context of CLL they were precluded from the main statistical analysis. SF3B1 was altered by missense mutations that clustered in the HEAT3 to HEAT6 repeats of the SF3B1 protein (Figure 1B). Six codons were targeted by recurrent mutations, including codons 666, 700, and 742 in 6, 37, and 23 patients, respectively. The clinical and biologic features of SF3B1 mutated CLL are summarized in Tables 1 and 2. Mutations occurred irrespective of the IGHV status, ZAP-70 expression, deletion of 11q or 17p and mutation of TP53. However, mutations were enriched in CD38 positive patients (OR 2.33, P = .005) and those without trisomy 12 (OR 0.19, P = .006, Table 2).
With univariate analysis we found that SF3B1 mutations were significantly associated with a reduced median OS of 54.3 versus 79.0 months for mutant and wild-type alleles, respectively (HR 1.71; 95% CI 1.30-2.25, P < .001), which was independent of treatment arm (Figure 2B). The presence of a SF3B1 mutation was associated with a reduced mean PFS (29.4 versus 39.1mths) in the LRF CLL4 cohort (HR 1.32, 95% CI 1.02-1.71, P = .03; Table 3). However, wild-type and mutant alleles had a similar median PFS of 26.5 months. By investigating the impact of mutations in each trial arm, we show that, after controlling for TP53 del/mut cases, a mutant SF3B1 gene was most associated with reduced PFS for FC-treated patients (HR 2.08; 95% CI 1.29-3.34, P = .002), with a median survival of 46.0 and 29.4 months for a wild-type and mutant SF3B1 allele, respectively. SF3B1 mutations were not associated with ORS in any of the 3 treatment arms (data not shown).
NOTCH1 and SF3B1 mutations identify additional patients with poor outcome after treatment with chemotherapy
We estimated the adjusted impact of NOTCH1 and SF3B1 mutations on PFS and OS after controlling for confounding variables using multivariate Cox proportional hazard analysis. Along with NOTCH1 and SF3B1 mutations other variables included in the analysis were age, gender, stage, deletion of 11q, IGHV mutation status, and deletion and/or mutation of TP53. We developed this model for OS and PFS as it is comparable with those that were previously used to show the prognostic independence of both genes in other studies.6,7 We confirmed the independent prognostic significance of several established biomarkers, including IGHV mutational status and deletion and/or mutation of TP53 in our patients (Table 4). Moreover, multivariate analysis selected NOTCH1 (HR 1.58, 95% CI 1.05-2.38, P = .03) and SF3B1 (HR 1.52, 95% CI 1.10-2.12, P = .01) mutations as independent risk factors of OS but not PFS (Table 4).
Multivariate Cox proportional hazard analysis of OS and PFS in the LRF CLL4 patients
| Variable . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| NOTCH1 | 1.58 | 1.05-2.38 | .03 | 1.29 | 0.87-1.90 | .21 |
| SF3B1 | 1.52 | 1.10-2.12 | .01 | 1.31 | 0.97-1.78 | .08 |
| Age | 1.05 | 1.03-1.07 | < .001 | 0.99 | 0.98-1.01 | .29 |
| Sex | 0.72 | 0.53-0.99 | .04 | 0.82 | 0.63-1.08 | .15 |
| Binet stage (A vs B/C) | 1.45 | 1.02-2.04 | .04 | 1.02 | 0.77-1.36 | .90 |
| del(11q) | 1.39 | 1.04-1.87 | .03 | 1.61 | 1.22-2.11 | .001 |
| IGHV unmutated | 1.85 | 1.38-2.50 | < .001 | 1.86 | 1.43-2.42 | < .001 |
| TP53 del/mut | 2.48 | 1.50-4.08 | < .001 | 2.09 | 1.29-3.37 | .003 |
| LRF CLL4 arm (Chl vs FDR/FC) | 1.01 | 0.78-1.30 | .97 | 0.50 | 0.40-0.63 | < .001 |
| Variable . | Overall survival . | Progression-free survival . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| NOTCH1 | 1.58 | 1.05-2.38 | .03 | 1.29 | 0.87-1.90 | .21 |
| SF3B1 | 1.52 | 1.10-2.12 | .01 | 1.31 | 0.97-1.78 | .08 |
| Age | 1.05 | 1.03-1.07 | < .001 | 0.99 | 0.98-1.01 | .29 |
| Sex | 0.72 | 0.53-0.99 | .04 | 0.82 | 0.63-1.08 | .15 |
| Binet stage (A vs B/C) | 1.45 | 1.02-2.04 | .04 | 1.02 | 0.77-1.36 | .90 |
| del(11q) | 1.39 | 1.04-1.87 | .03 | 1.61 | 1.22-2.11 | .001 |
| IGHV unmutated | 1.85 | 1.38-2.50 | < .001 | 1.86 | 1.43-2.42 | < .001 |
| TP53 del/mut | 2.48 | 1.50-4.08 | < .001 | 2.09 | 1.29-3.37 | .003 |
| LRF CLL4 arm (Chl vs FDR/FC) | 1.01 | 0.78-1.30 | .97 | 0.50 | 0.40-0.63 | < .001 |
OS Multivariate: 330 cases with 244 events; 164 cases with missing data. PFS Multivariate: 330 cases with 306 events; 164 cases with missing data. TP53 abnormalities defined by deletion and/or mutation (del/mut).
It has been suggested that mutations in NOTCH1 and SF3B1 identify patients with dismal outcome comparable with those with TP53 del/mut.6 However, in LRF CLL4, TP53 del/mut patients exhibited a considerably shorter OS and PFS than wild-type TP53 patients with either mutations of NOTCH1 or SF3B1 (Table 3, Figure 2C-D). Specifically, TP53 del/mut patients, within the subgroup of patients analyzed for NOTCH1 and SF3B1, exhibited a median OS of 26.1 months compared with 75.9 months for wild-type individuals (HR 3.19, P < .001; Table 3). This shows that in the context of patients receiving first-line chemotherapy, TP53 abnormalities remain the most significant marker of reduced survival.
Discussion
Initial whole-genome sequencing studies have identified a panel of novel recurrently mutated genes in CLL including NOTCH1 and SF3B1.2-6 Although these lesions have been linked to clinical outcome, these associations have largely been defined in heterogeneous retrospective CLL cohorts. However, given the highly heterogeneous natural history of CLL and the often-serendipitous date of initial diagnosis, it is important to confirm the prognostic relevance of novel biomarkers in the context of randomized clinical trials. Herein, we present just such a study of our findings from the LRF CLL4 trial, where we show that NOTCH1 and SF3B1 mutations occur at a frequency of 10% and 17%, respectively, in samples taken at trial entry.
The incidence of NOTCH1 mutations is greater than that previously seen in MBL (3.2%)17 and comparable with the frequency found at CLL diagnosis (12%).4-6 Our data support previously published findings, that showed a significant enrichment of NOTCH1 mutations in IGHV unmutated, ZAP-70 positive patients,4-6 and between NOTCH1 mutations and trisomy 12 as defined by others.3,8 The explanation for this association between NOTCH1 mutations and other biomarkers is currently unclear. In contrast to previous findings,7 we show that 22% of NOTCH1 mutations (10/46) occurred concomitantly with alterations of ATM/TP53. We did not detect a difference in the incidence of death from RS between NOTCH1 mutated and wild-type individuals. However, we did not have the necessary data to further examine the relationship between NOTCH1 mutated CLL and subsequent Richter transformation.4,5,7 We confirm previous observations that NOTCH1 mutational status impacts on OS as an isolated variable in univariate analysis.4,5,7 We also show a novel association between NOTCH1 mutations and shorter PFS. The multivariate survival analysis identified NOTCH1 mutation as an independent marker of poor prognosis (OS not PFS) when we controlled for a comprehensive panel of covariates, including deletion of ATM and TP53. However, our data suggest that in a homogeneous population of CLL patients requiring first-line treatment, NOTCH1 mutations identify patients with intermediate survival, comparable with 11q loss, rather than the poor survival exhibited by TP53 deleted or mutated individuals. Taken together, our data demonstrate that NOTCH1 mutational status is an independent marker that identifies patients with intermediate outcome after initial therapy with DNA damaging agents.
SF3B1 is a critical component of the RNA splicing machinery that achieves successful transcription and guarantees the functional diversity of protein species using alternative spicing. The precise consequences of SF3B1 mutations are not known but it is possible that differences in the transcriptome of lymphoid and myeloid cells would result in distinct functional consequences. This may be relevant to the observation that mutations are associated with poorer overall survival in CLL,2,3,6 wheres in the myelodysplastic syndrome (MDS) they correlate with a more favorable disease subtype and improved survival even though the strikingly similar distribution of mutations suggest similar mechanisms of action.10,18-20 It was previously demonstrated that chemo-refractory CLL is enriched for SF3B1 mutations (17%) compared with 4% at diagnosis.6 In contrast, we observed a similar high frequency (17%) in previously untreated patients and no correlation between the presence of a mutation and response to treatment. In univariate analysis, we have confirmed the previous association between mutation of SF3B1 and shorter overall survival. We did observe a weak association between SF3B1 mutation and PFS, which was most evident in the FC arm of the trial. Critically, our multivariate survival analysis demonstrated that SF3B1 mutations are an independent marker for intermediate OS similar to 11q loss or NOTCH1 mutation, but they are not an independent marker for PFS in the LRF CLL4 trial.
In conclusion, using NOTCH1 and SF3B1 as examples, we present the first study to validate the importance of novel mutated cancer genes identified by ongoing CLL sequencing initiatives in the context of a randomized, prospective clinical trial. Given the treatment modalities used in the LRF CLL4 trial, it will be important to validate this observation in the context of chemo-immunotherapy trials and in studies of novel agents, such as B-cell receptor (BCR) signaling inhibitors. Importantly, we show the independent prognostic importance of NOTCH1 and SF3B1 mutations, thereby providing valuable validation of the clinical utility of these gene mutations in the accurate risk-adapted stratification of CLL patients. As important, is our observation that alterations of the TP53 gene remains the most informative marker of dismal survival in this trial, supporting strategies for interrogating the P53 pathway for the identification of poor prognosis patients. However, our data add to a growing body of evidence demonstrating the value of mutational analysis of other key genes. This will ultimately establish a stratified and individualized approach to care, including the potential for targeted therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and clinicians who participated in the trial.
This study was funded by The Kay Kendall Leukemia Fund, Leukemia and Lymphoma Research, Cancer Research UK, and Wessex Medical Research. The LRF CLL4 trial was funded by a core grant from Leukemia and Lymphoma Research. D.G. and D.C. acknowledge the support by The Royal Marsden Hospital and The Institute of Cancer Research National Institute of Health Research Biomedical Research Center. N.W. and M.E. acknowledge the support of Dr Mildred Scheel Stiftung for Cancer Research (Bonn, Germany) and the Arbib Foundation, respectively.
Authorship
Contribution: N.W., M.J.J.R.-Z., D.G.d.C., J.F., H.P., and B.G. performed the experimental work; A.G., D.G.d.C., and A.P. performed the molecular diagnostic assays; M.J.J.R.-Z., A.P., M.E., and A.C. conducted the statistical analyses; D.G.O. and D.C. contributed patient samples and data; D.G.O., N.C.P.C., and J.C.S. initiated and designed the study; M.J.J.R.-Z., D.G.O., and J.C.S. wrote the paper with contributions from N.C.P.C., D.G.d.C., and N.W; and all authors critically reviewed the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan C. Strefford, Cancer Genomics Group, Cancer Sciences Unit, Somers Cancer research Bldg, Southampton General Hospital, Tremona Road, Southampton SO16 6YD, United Kingdom; e-mail: JCS@soton.ac.uk.
References
Author notes
D.G.O., M.J.J.R.-Z., and N.W. share first authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal