Abstract
In the present study, Hu-Mikβ1, a humanized mAb directed at the shared IL-2/IL-15Rβ subunit (CD122) was evaluated in patients with T-cell large granular lymphocytic (T-LGL) leukemia. Hu-Mikβ1 blocked the trans presentation of IL-15 to T cells expressing IL-2/IL-15Rβ and the common γ-chain (CD132), but did not block IL-15 action in cells that expressed the heterotrimeric IL-15 receptor in cis. There was no significant toxicity associated with Hu-Mikβ1 administration in patients with T-LGL leukemia, but no major clinical responses were observed. One patient who had previously received murine Mikβ1 developed a measurable Ab response to the infused Ab. Nevertheless, the safety profile of this first in-human study of the humanized mAb to IL-2/IL-15Rβ (CD122) supports its evaluation in disorders such as refractory celiac disease, in which IL-15 and its receptor have been proposed to play a critical role in the pathogenesis and maintenance of disease activity. The protocol is registered with www.clinicaltrials.gov as number NCT 00076180.
Key Points
Hu-Mikβ1 monoclonal antibody was evaluated in patients with LGL leukemia.
Hu-Mikβ1 blocks the transpresentation of IL-15.
Introduction
IL-15 is a proinflammatory cytokine that stimulates natural killer (NK) and memory CD8+ T-cell activity and induces the expression of IL-2 receptorα (IL-2Rα), IL-18, TNFα, IL-6, IL-1β, and IFNγ.1-7 IL-15 signals through heterotrimeric receptors that include the IL-15–specific receptor subunit, IL-15Rα, IL-2/IL-15Rβ (CD122) that is shared with IL-2, and the common γ-chain (γc; CD132) that is shared with the IL-2, IL-4, IL-7, IL-9, and IL-21 receptors.3,7,8 Unlike most other cytokines, little IL-15 is secreted into the circulation.9 Rather, after stimulation by type I or II IFNs after ligation of TLRs or activation by CD40, monocytes and dendritic cells coordinately express IL-15 and its IL-15Rα.9 The associated IL-15 and IL-15Rα recycle from the cell surface through endosomal vesicles and are then reexpressed on the cell surface. As part of an immunologic synapse, IL-15Rα presents IL-15 in trans to NK cells, intraepithelial lymphocytes, and memory T cells that express IL-2/IL-15Rβ and the common γc, but not IL-15α.9-12 Interference with self-tolerance mediated by IL-2–induced activation-induced cell death and regulatory T cells and the facilitation of the maintenance of CD8+ memory T-cell survival that include self-reactive memory T cells suggests that disordered IL-15 expression might contribute to the development of autoimmune disease.13 Indeed, dysregulated IL-15 expression has been reported in patients with a range of autoimmune diseases including rheumatoid arthritis,14 multiple sclerosis,15 ulcerative colitis,16 refractory celiac disease,17,18 type 1 diabetes,19 and diseases associated with the retrovirus HTLV-1.20
To address such putative IL-15–mediated disorders, agents that inhibit IL-15 activity have been developed. These include soluble IL-15Rα, a dominant-negative IL-15 molecule, and antibodies specific for IL-15 or IL-2/IL-15Rβ.21,22 The approach used in the present study involved a humanized Ab, Hu-Mikβ1, which is specific for IL-2/IL-15Rβ.23-25 This Ab blocks trans presentation of IL-15, thus inhibiting IL-15–mediated effects.13 Hu-Mikβ1 has also been shown to prolong cardiac allograft survival in cynomolgus monkeys.26 We previously translated these observations into a phase 1 clinical trial in patients with T-cell large granular lymphocytic (T-LGL) leukemia with associated hematocytopenias23 using the murine mAb Mu-Mikβ1.23
The present study evaluated the safety, pharmacokinetics, and ability to saturate the IL-2/IL-15Rβ and the immunogenicity of the humanized mAb Hu-Mikβ1 in patients with monoclonal T-LGL with hematocytopenias.
The majority of patients with T-LGL have a clinically indolent course.27 However, a significant fraction is known to develop neutropenia, anemia, symptomatic splenomegaly, recurrent bacterial infections, and autoimmune disorders including rheumatoid arthritis.27
Several have studies suggested that IL-15 might play a role in the pathogenesis of T-LGL leukemia, including a network model of survival signaling in LGL.28,29 In addition, increased serum-soluble IL-15Rα levels were demonstrated previously in patients with T-LGL leukemia.30 These studies provided the scientific basis for evaluating IL-2/IL-15Rβ (CD122) blockade with Hu-Mikβ1 in patients with T-LGL leukemia and hematocytopenias.13,31
Previously, we conducted a phase 1 clinical trial that involved IL-15 blockade in T-LGL leukemia using the murine Mikβ1 mAb,23 but no efficacy was observed. Several factors may underlie this lack of efficacy. One factor is that the duration of blockade of IL-2/IL-15 receptors may have been insufficient because the murine mAb had a very short in vivo survival. Furthermore, in contrast to Hu-Mikβ1, murine Mikβ1 does not fix complement nor manifest Ab-dependent cellular cytotoxicity with human mononuclear cells.24 To address these issues of pharmacokinetics and function, a humanized form of Mikβ1 was generated.24 These characteristics of Hu-Mikβ1, but not the murine form, were associated with greater efficacy in prolonging cardiac allograft survival in a cynomolgus monkey model.26 A reason for performing initial studies of Hu-Mikβ1 in patients with T-LGL leukemia rather than in patients with autoimmune disorders was that the T-LGL group provides an excellent opportunity to achieve classic phase 1 goals to define toxicity, immunogenicity, pharmacokinetics, and pharmacodynamics because T-LGL leukemia, a very indolent stable disorder, does not usually require urgent treatment with chemotherapy. Furthermore, T-LGL leukemia cells express high levels of IL-2/IL-15Rβ, the target of Hu-Mikβ1.
Methods
Hu-Mikβ1 (anti-CD122)
Patient population and treatment plan
Eligibility requirements were as follows: histologically confirmed T-LGL leukemia; an absolute C3+CD8+CD4−CD57+ T-LGL cell count ≥ 1000/μL; ≥ 50% of T cells must express CD122 (IL-2R/IL-15Rβ); hematocytopenia defined as an absolute granulocyte count of < 1000/μL, platelet count < 100 000/μL, or hemoglobin < 10 g/dL, or a requirement of transfusion of 3 or more units of blood in the last 6 months for LGL-related anemia; age ≥ 18 years and circulating mononuclear cells containing a monoclonal T-cell population as demonstrated by PCR analysis of the TCR γ-c rearrangement.
Patients were entered at 3 sequential dose levels of Hu-Mikβ1. Groups of 3 patients each received 0.5, 1.0, or 1.5 mg/kg as a single IV dose. Adverse events were assessed using the National Cancer Institute Common Toxicity Criteria Version 3.0. The study was approved by the National Cancer Institute institutional review board and by the Food and Drug Administration, was conducted in accordance with the Declaration of Helsinki protocol, and all patients gave written informed consent.
See Table 1 for a description of the patients. Nine patients with a mean age of 65 years (range, 49-82), 7 males and 2 females, 8 whites and 1 Hispanic were treated. One patient had rheumatoid arthritis and an additional 5 patients had detectable serum rheumatoid factor (range, 32-540 μ/mL). Seven patients were anemic (hemoglobin < 10 g/L) and 2 had thrombocytopenia. Leukocyte counts ranged from 3000-17 000 (geometric mean, 9213/μL), the geometric mean absolute lymphocyte count was 8650/μL (range, 2109-16 016/μL), the neutrophil counts ranged from 57-996/μL (geometric mean, 593/μL). The number of leukemic cells characterized as CD4−CD8+CD122+ ranged from 1625-14 170/μL (geometric mean, 6905/μL; Table 2). The T-LGL phenotype was CD2+CD3+CD4−CD8+CD122+CD16+ and CD57+. The CD4:CD8 ratio ranged from 0.02-0.38 (geometric mean, 0.085). Circulating mononuclear cells of the patients showed high level expression of CD122 (IL-2/IL-15Rβ).
Characteristics of patients with T-LGL before therapy with Hu-Mikβ1
| Patient . | Age, y . | Sex . | Race . | Prior G-CSF before study entry . | Leukocytes, μL . | Absolute granulocyte count, μL . | Anemia (hemoglobin < 10 g/L) . | Thrombo-cytopenia . | RA . | Skin rash . | Prior infection . | TCRγ rearrangement . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | White | Y | 7020 | 442 | N | Y | N | N | Y | Y |
| 2 | 68 | M | White | N | 3080 | 641 | Y | N | RF = 32 | Y | N | Y |
| 3 | 65 | M | White | Y | 16 400 | 787 | Y | N | RA Y | N | Y | Y |
| 4 | 49 | M | White | Y | 15 700 | 958 | Y | N | RF = 111 | N | Y | Y |
| 5 | 59 | M | Hispanic | Y | 3000 | 57 | Y | N | RF = 540 | Y | Y | Y |
| 6 | 64 | F | White | Y | 16 000 | 996 | Y | N | RF = 62 | N | Y | Y |
| 7 | 82 | M | White | N | 17 000 | 880 | Y | N | N | N | Y | Y |
| 8 | 73 | M | White | N | 12 000 | 600 | Y | N | N | N | N | Y |
| 9 | 74 | F | White | N | 8170 | 413 | N | Y | RF = 49 | N | N | Y |
| Patient . | Age, y . | Sex . | Race . | Prior G-CSF before study entry . | Leukocytes, μL . | Absolute granulocyte count, μL . | Anemia (hemoglobin < 10 g/L) . | Thrombo-cytopenia . | RA . | Skin rash . | Prior infection . | TCRγ rearrangement . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | White | Y | 7020 | 442 | N | Y | N | N | Y | Y |
| 2 | 68 | M | White | N | 3080 | 641 | Y | N | RF = 32 | Y | N | Y |
| 3 | 65 | M | White | Y | 16 400 | 787 | Y | N | RA Y | N | Y | Y |
| 4 | 49 | M | White | Y | 15 700 | 958 | Y | N | RF = 111 | N | Y | Y |
| 5 | 59 | M | Hispanic | Y | 3000 | 57 | Y | N | RF = 540 | Y | Y | Y |
| 6 | 64 | F | White | Y | 16 000 | 996 | Y | N | RF = 62 | N | Y | Y |
| 7 | 82 | M | White | N | 17 000 | 880 | Y | N | N | N | Y | Y |
| 8 | 73 | M | White | N | 12 000 | 600 | Y | N | N | N | N | Y |
| 9 | 74 | F | White | N | 8170 | 413 | N | Y | RF = 49 | N | N | Y |
RA indicates rheumatoid arthritis; and RF, rheumatoid factor.
Baseline immunophenotype of T-LGL leukemia patients treated Hu-Mikβ1
| Patient . | CD2 . | CD3 . | CD4 . | CD8 . | CD4/8 ratio . | CD16 . | CD56 . | CD57 . | CD122+ . | Leukocytes, μL . | Lymphocytes, μL . | CD8+CD122+ cells, μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | 0.33 | + | − | + | 76% | 7020 | 6128 | 4658 |
| 2 | + | + | − | + | 0.23 | + | − | +/− | 77% | 3080 | 2109 | 1625 |
| 3 | + | + | − | + | 0.20 | + | − | + | 78% | 16 400 | 15 367 | 11 986 |
| 4 | + | + | − | + | 0.13 | + | − | + | 86% | 15 700 | 13 345 | 11 476 |
| 5 | + | + | − | + | 0.38 | +/− | − | + | 71% | 3000 | 2880 | 2049 |
| 6 | + | + | − | + | 0.09 | + | − | +/− | 97% | 16 600 | 14 608 | 14 170 |
| 7 | + | + | − | + | 0.02 | + | − | − | 99% | 17 600 | 16 016 | 15 356 |
| 8 | + | + | − | + | 0.04 | + | − | +/− | 100% | 12 000 | 10 560 | 10 560 |
| 9 | + | + | − | + | 0.10 | + | − | − | 69% | 8170 | 6160 | 4250 |
| Patient . | CD2 . | CD3 . | CD4 . | CD8 . | CD4/8 ratio . | CD16 . | CD56 . | CD57 . | CD122+ . | Leukocytes, μL . | Lymphocytes, μL . | CD8+CD122+ cells, μL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | 0.33 | + | − | + | 76% | 7020 | 6128 | 4658 |
| 2 | + | + | − | + | 0.23 | + | − | +/− | 77% | 3080 | 2109 | 1625 |
| 3 | + | + | − | + | 0.20 | + | − | + | 78% | 16 400 | 15 367 | 11 986 |
| 4 | + | + | − | + | 0.13 | + | − | + | 86% | 15 700 | 13 345 | 11 476 |
| 5 | + | + | − | + | 0.38 | +/− | − | + | 71% | 3000 | 2880 | 2049 |
| 6 | + | + | − | + | 0.09 | + | − | +/− | 97% | 16 600 | 14 608 | 14 170 |
| 7 | + | + | − | + | 0.02 | + | − | − | 99% | 17 600 | 16 016 | 15 356 |
| 8 | + | + | − | + | 0.04 | + | − | +/− | 100% | 12 000 | 10 560 | 10 560 |
| 9 | + | + | − | + | 0.10 | + | − | − | 69% | 8170 | 6160 | 4250 |
Molecular analysis of T-cell antigen receptor
PCR analysis of TCR γ-c gene rearrangements demonstrating monoclonality were performed on the 9 patients.32
Hu-Mikβ1 as an inhibitor of IL-15– and IL-2–induced proliferation of cytokine-dependent cell lines
To examine specificity of the antibodies, proliferation assays were performed with the IL-2 and IL-15 cytokine–dependent cell line 32Dβ, a mouse hematopoietic cell line transfected with human IL-2/IL-15Rβ chain, Kit 225, a chronic T-cell leukemia line, and Kit 225 7.10, the Kit 225 cell line transfected with IL-15Rα. Cells were seeded for tissue culture without cytokine or with exogenous IL-2 (a gift of Hoffmann-La Roche) or IL-15 (PeproTech); the concentration of IL-2 used with 32Dβ that does not express IL-2Rα was 1.0 ng/mL, whereas that with Kit 225 that expresses the high-affinity IL-2R was 0.5 ng/mL. The concentration of IL-15 with 32Dβ cells was 20 ng/mL, for Kit 225 it was 3.0 ng/mL, and for Kit 7.10 with IL-15Rα transfected it was 1.0 ng/mL. Hu-Mikβ1 was added to cell lines supplemented with IL-2 or IL-15. After a 16- to 21-hour culture of cells with cytokine, l uCi [3H] thymidine was added during the final 4 hours and proliferation was measured in the presence and absence of Hu-Mikβ1.
Receptor saturation with Hu-Mikβ1
The IL-2/IL-15Rβ saturation by Hu-Mikβ1 was examined before and after therapy by flow cytometry using 2 different fluorochrome-labeled antibodies, Mikβ1 and Mikβ3. The 2 antibodies identified noncompeting epitopes on the IL-2/IL-15R β-chain. If saturation were achieved after the Hu-Mikβ1 infusions, the occupancy of the receptor by infused Hu-Mikβ1 would block subsequent binding of fluorochrome-labeled Mikβ1, but not Mikβ3, on circulating LGL cells. Saturation of receptor by infused Hu-Mikβ1 was assessed by comparing relative mean fluorescence intensity of binding of that with fluorochrome-labeled Hu-Mikβ1 observed after therapy and with Hu-Mikβ1 binding observed before and with that obtained with Mikβ3.
Measurement of Hu-Mikβ1 serum levels
Serum concentration of Hu-Mikβ1 was determined by a competitive inhibition ELISA described in supplemental Methods.
Immune responses to Hu-Mikβ1
Development of antibodies against Hu-Mikβ1 was examined using an antigen-bridging ELISA as described in supplemental Methods.
Detection of IL-2 and IL-15 mRNA and protein by T-LGL cells
Patient PBMCs were examined ex vivo by real time PCR using TaqMan to quantitate mRNA encoding IL-2 and IL-15.
Total RNA was isolated from normal and LGL PBMCs using the Purescript RNA Purification Kit (Gentra Systems) according to the manufacturer's instructions. RT reactions were carried out for each sample (250 ng) using the Superscript II First-Strand Synthesis System (Invitrogen). The TaqMan Universal PCR Master Mix, human IL-2 (Hs99999150_mH), human IL-15 (Hs99999039_ml), and human HPRT1 (catalog number 4333768T) primer probes were from Applied Biosystems. The detection of human IL-2, IL-15, and HPRT1 was performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions. The copy numbers of IL-2 and IL-15 mRNA were normalized to the copy number of human HPRT1 mRNA.
Human IL-2 and human IL-15 ELISA
IL-2 and IL-15 secreted by patient cells after 6 days of ex vivo culture in RPMI medium 1640 and 10% FBS were evaluated using human IL-2 and IL-15 Quantikine ELISA Kit (R&D Systems).
Spontaneous PBMC proliferation in patients with T-LGL leukemia
Spontaneous proliferation of PBMCs from patients with T-LGL leukemia was assayed as described in supplemental Methods.
Results
Specificity of Hu-Mikβ1
Hu-Mikβ1 is a humanized IgG1κ mAb produced in the human-mouse myeloma cell line Sp2/0-Ag14. Hu-Mikβ1 was constructed using molecular techniques to insert the complementarity-determining regions from an anti–human IL-2/IL-15Rβ (CD122)–specific mouse mAb, Mikβ1.24 This Ab binds to CD122 that is involved in both IL-2– and IL-15–stimulatory signals.
Tissue specificity and distribution analysis of Hu-Mikβ1 was performed by IMPATH Laboratories. No binding of Hu-Mikβ1 to normal human adrenal, bladder, BM, brain, breast, uterine, cervix, esophagus, eye, heart, kidney, large intestine, liver, lung, lymph node, ovary, pancreas, parathyroid, parotid, pituitary, prostate, skin, small intestine, spinal cord, spleen, stomach, testis, thymus, thyroid, tonsil, or uterus was detected. Three skeletal muscle specimens showed 1 to 2+ reactivity. To explore this muscle reactivity, mRNA was extracted from skeletal muscle of a cynomolgus monkey. No IL-2R/IL-15Rβ mRNA was demonstrated by Northern analysis, although it was easily demonstrable in positive control mRNA from the YTS NK cell line.
As part of the 5th International Leukocyte Workshop, the reactivity of Mikβ1 against an array of cells and cell lines was assessed.33 Mikβ1 did not react with 16 endothelial (SUVEC) cell lines, erythrocytes, pre-erythrocyte cell lines, myeloid cells, platelets, resting T cells, or a variety of stromal cell lines. Mikβ1 did react with select EBV-transformed B-cell lines, phytohemagglutinin-activated lymphocytes, and the majority of NK cell lines. Therefore, IL-2R/IL-15Rβ identified by Mikβ1 was limited to expression in NK cells, monocytes and activated B and T-lymphocytes.
Preclinical Hu-Mikβ1 toxicology
All studies were approved by the Animal Care and Use Committee of the NCI. 5 groups of cynomolgus monkeys (N = 3) received Hu-Mikβ1 10 mg/kg every 3 weeks × 9 doses, 1 mg/kg every 3 weeks × 9 doses, 10 mg/kg every 3 weeks × 5 doses, 1 mg/kg every 3 weeks × 5 doses, and normal saline every 3 weeks × 9 doses. No clinical or laboratory abnormalities in routine hematologic or chemistry tests were observed. No major abnormalities were identified on flow cytometry of peripheral blood mononuclear cells. Unlike previous observations with murine Mikβ1 in mice, there was no decline in CD16+56+CD3− NK cell numbers. Examinations performed by certified veterinary pathologists (Charles River Laboratories) demonstrated no pathologic abnormalities, with the exception of 2 animals with multifocal myocardial degeneration on microscopic examination. No abnormalities of troponin I levels were observed. Comparable cardiac abnormalities have been reported by 5 groups in studies that involved up to 510 normal, untreated cynomolgus monkeys, suggesting that these changes were unrelated to Hu-Mikβ1.34-37
Functional analyses
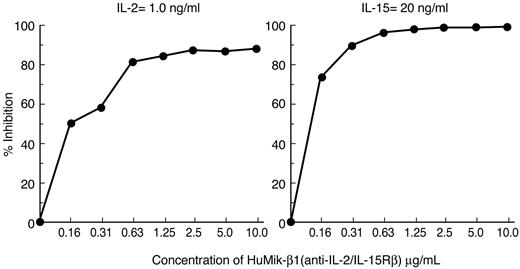
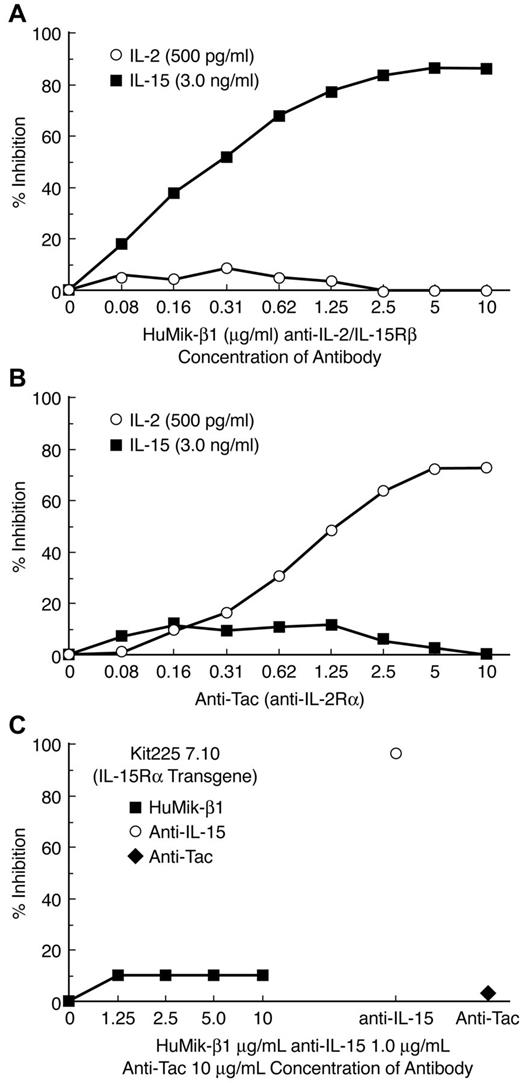
Hu-Mikβ1 is a specific inhibitor of IL-15– and IL-2–induced cell proliferation. Both high-affinity receptors for IL-2 and IL-15 are heterotrimeric and express IL-2-/IL-15Rβ, the γc, and IL-2– or IL-15–specific α-receptors. However, both IL-2 and IL-15 can induce proliferation of cells that express only an intermediate-affinity receptor that involves IL-2/IL-15Rβ and the common γ-c. To examine specificity and target receptor requirements of the Hu-Mikβ1 proliferation, assays were performed with both the cytokine-dependent 32Dβ cell line, a murine hematopoietic cell line transfected with human IL-2/IL-15Rβ and the Kit 225 wild-type T-cell line that expressed both the common γ-c and IL-2/IL-15Rβ.1,38 In addition, the effect of Hu-Mikβ1 addition on cytokine responses was studied in Kit 225 7.10, a Kit 225 cell line that was transfected with IL-15Rα. Both IL-2– and IL-15–mediated proliferation of 32Dβ were inhibited by addition of 10 μg/mL of Hu-Mikβ1 (Figure 1). Furthermore, showing cytokine specificity, Hu-Mikβ1 did not inhibit the proliferation of 32Dβ mediated by IL-3 (data not shown). In the case of wild-type Kit 225, a cell that expresses IL-2Rα, IL-2/IL-15Rβ, and γc, but not functional IL-15Rα, Mikβ1 at the 10 μg/mL dose used did not inhibit (Figure 2A) IL-2–mediated proliferation, whereas anti-Tac (anti–IL-2Rα) inhibited its proliferation. Anti-Tac (IL-2Rα) also inhibited IL-2–mediated proliferation of Kit 225 cells (Figure 2B). IL-15–induced proliferation of Kit 225, which does not express a functional IL-15Rα, was inhibited by Mikβ1 (Figure 2A). In contrast, Hu-Mikβ1 did not block IL-15–mediated proliferation in the Kit 225 7.10 cell line that expresses IL-15Rα (Figure 2C). In summary, Hu-Mikβ1 showed Ab fidelity and blocked only proliferation mediated by IL-2 and IL-15. Hu-Mikβ1 efficiently blocked both IL-2– and IL-15–mediated proliferation, as was also true in the cytokine trans presentation when cells expressed only the IL-2/IL-15Rβ and the common γ-c, but did not block cytokine-mediated proliferation of cells that express in cis high-affinity IL-2– or IL-15–specific heterotrimeric receptors for that cytokine.
Hu-Mikβ1 inhibits IL-2– and IL-15–induced proliferation of 32Dβ cells that express human IL-2/IL-15Rβ and the murine common γ-c. The dose-response effects of addition of Hu-Mikβ1 Ab were evaluated on IL-2 (1.0 ng/mL)– and IL-15 (20 ng/mL)–induced proliferation of 32Dβ cells. In these cells, which express only human IL-2/IL-15Rβ and the common γ-c, but not IL-2Rα or IL-15Rα, IL-2– and IL-15–induced proliferations were inhibited by the addition of Hu-Mikβ1.
Hu-Mikβ1 inhibits IL-2– and IL-15–induced proliferation of 32Dβ cells that express human IL-2/IL-15Rβ and the murine common γ-c. The dose-response effects of addition of Hu-Mikβ1 Ab were evaluated on IL-2 (1.0 ng/mL)– and IL-15 (20 ng/mL)–induced proliferation of 32Dβ cells. In these cells, which express only human IL-2/IL-15Rβ and the common γ-c, but not IL-2Rα or IL-15Rα, IL-2– and IL-15–induced proliferations were inhibited by the addition of Hu-Mikβ1.
Hu-Mikβ1 inhibits IL-15–induced proliferation of wild-type Kit 225 cells but not Kit 225 7.10 cells that had been transfected with IL-15Rα. (A) Hu-Mikβ1 inhibited IL-15– but not IL-2–mediated proliferation of wild-type Kit 225 cells that express IL-2Rα, IL-2/IL-15Rβ, and γc but not IL-15Rα. (B) Anti-Tac (anti–IL-2Rα) inhibited IL-2– but not IL-15–mediated proliferation of wild-type Kit 225 cells. (C) In contrast neither anti-Tac (♦) nor Hu-Mikβ1 (■) inhibited the IL-15 (1 ng/mL)–induced proliferation of Kit 225 7.10 cells that had been transfected with IL-15Rα and that expressed IL-15Rα, IL-2/IL-15Rβ, and γc chains in cis. Anti–IL-15 (○) did inhibit IL-15–mediated proliferation in these cells.
Hu-Mikβ1 inhibits IL-15–induced proliferation of wild-type Kit 225 cells but not Kit 225 7.10 cells that had been transfected with IL-15Rα. (A) Hu-Mikβ1 inhibited IL-15– but not IL-2–mediated proliferation of wild-type Kit 225 cells that express IL-2Rα, IL-2/IL-15Rβ, and γc but not IL-15Rα. (B) Anti-Tac (anti–IL-2Rα) inhibited IL-2– but not IL-15–mediated proliferation of wild-type Kit 225 cells. (C) In contrast neither anti-Tac (♦) nor Hu-Mikβ1 (■) inhibited the IL-15 (1 ng/mL)–induced proliferation of Kit 225 7.10 cells that had been transfected with IL-15Rα and that expressed IL-15Rα, IL-2/IL-15Rβ, and γc chains in cis. Anti–IL-15 (○) did inhibit IL-15–mediated proliferation in these cells.
Trial design
This was a phase 1 open-label study of the Hu-Mikβ1 mAb administered on a single occasion to patients with T-LGL leukemia and hematocytopenia. Hu-Mikβ1 was administered IV at doses of 0.5, 1.0, and 1.5 mg/kg over 90 minutes with a subsequent 42-day observation period. Primary end points were to determine the safety of Hu-Mikβ1 in patients with T-LGL leukemia at the administered doses to determine the dose of Hu-Mikβ1 required to saturate IL-2/IL-15Rβ on circulating T-LGL cells and the ability to maintain this saturation for 3 weeks. Secondary end points were to define the pharmacokinetics and immunogenicity of Hu-Mikβ1.
Hu-Mikβ1 pharmacokinetics and pharmacodynamics
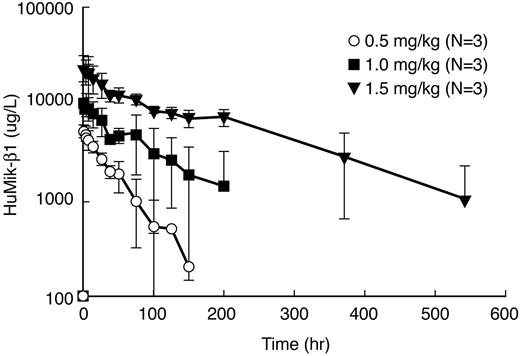
The pharmacokinetics of Hu-Mikβ1 was evaluated in 9 T-LGL leukemia patients. A single IV dose of Hu-Mikβ1 at 0.5, 1.0, and 1.5 mg/kg was administered to patients with T-LGL leukemia, and serum levels were quantitated using an Ab-specific ELISA from samples obtained before and at serial time points after infusion. Analyzed pharmacokinetic parameters are shown in Table 3. The mean of the various parameters for different groups for patients receiving 0.5 mg/kg were Cmax = 5.35 μg/mL (95% confidence interval [CI], 3.9-7.40 μg/mL), for 1.0 mg/kg, Cmax = 10.6 (95% CI, 6.3-17.9 μg/mL), and for 1.5 mg/kg, Cmax = 23.4 (95% CI, 10.6-51.4 μg/mL). Biologic half-life (T1/2) was 16.9 hours (95% CI, 8.5-33.5 hours) for the 0.5 mg/kg group, 32.0 hours (95% CI, 11.5-89.0 hours) for 1.0 mg/kg group, and 91.0 hours (95% CI, 31.4-264.0 hours) for the 1.5 mg/kg treatment group.
Pharmacokinetic properties of Hu-Mikβ1 in T-LGL
| . | 0.5 mg/kg . | 1.0 mg/kg . | 1.5 mg/kg . | |||
|---|---|---|---|---|---|---|
| Geometric mean (n = 3) . | 95% CI . | Geometric mean (n = 3) . | 95% CI . | Geometric mean (n = 3) . | 95% CI . | |
| Biologic t1/2, h | 16.9 | 8.5-33.5 | 32.0 | 11.5-89.0 | 91.0 | 31.4-264 |
| Cmax, μg/mL | 5.4 | 3.9-7.3 | 10.6 | 6.3-17.9 | 23.4 | 10.6-51.4 |
| Cmax/D, /mL | 10.7 | 7.8-14.7 | 10.6 | 6.3-17.9 | 15.6 | 7.1-34.2 |
| AUCinf, mg Hg/L | 206.6 | 106.0-402.9 | 692.2 | 166.01-2885.9 | 3358.7 | 1683.0-6702.9 |
| AUCinf/D, h/L | 413.3 | 213.0-805.9 | 692.1 | 166.0-2885.9 | 2239.1 | 1122.0-4468.6 |
| Volume of distribution, L/kg | 0.059 | 0.029-0.120 | 0.066 | 0.036-0.124 | 0.0596 | 0.029-0.12 |
| Clearance, L/h/kg × 10−3 | 2.44 | 1.26-4.72 | 1.47 | 0.39-5.56 | 0.448 | 0.19-1.09 |
| . | 0.5 mg/kg . | 1.0 mg/kg . | 1.5 mg/kg . | |||
|---|---|---|---|---|---|---|
| Geometric mean (n = 3) . | 95% CI . | Geometric mean (n = 3) . | 95% CI . | Geometric mean (n = 3) . | 95% CI . | |
| Biologic t1/2, h | 16.9 | 8.5-33.5 | 32.0 | 11.5-89.0 | 91.0 | 31.4-264 |
| Cmax, μg/mL | 5.4 | 3.9-7.3 | 10.6 | 6.3-17.9 | 23.4 | 10.6-51.4 |
| Cmax/D, /mL | 10.7 | 7.8-14.7 | 10.6 | 6.3-17.9 | 15.6 | 7.1-34.2 |
| AUCinf, mg Hg/L | 206.6 | 106.0-402.9 | 692.2 | 166.01-2885.9 | 3358.7 | 1683.0-6702.9 |
| AUCinf/D, h/L | 413.3 | 213.0-805.9 | 692.1 | 166.0-2885.9 | 2239.1 | 1122.0-4468.6 |
| Volume of distribution, L/kg | 0.059 | 0.029-0.120 | 0.066 | 0.036-0.124 | 0.0596 | 0.029-0.12 |
| Clearance, L/h/kg × 10−3 | 2.44 | 1.26-4.72 | 1.47 | 0.39-5.56 | 0.448 | 0.19-1.09 |
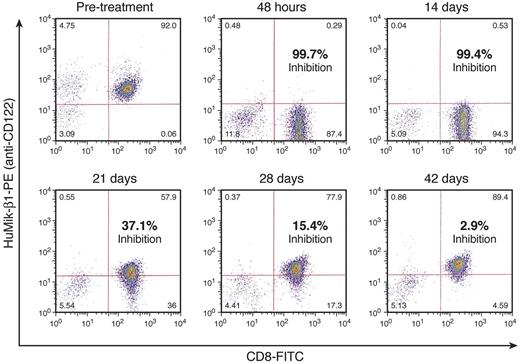
Saturation of the IL-2/IL-15Rβ is required to block the IL-15 and IL-2 binding that is the presumed mode of action of Hu-Mikβ1. Patients were evaluated for saturation of surface CD122 receptors before therapy, at 6-72 hours, and at 7, 14, 21, 28, and 42 days after infusion of Hu-Mikβ1. Flow cytometry was performed at these times with fluorochrome-labeled Hu-Mikβ1 and with fluorochrome-labeled Mikβ3 that define non-cross-competing epitopes on the IL-2/IL-15Rβ (Figure 3). The receptor was deemed to be saturated when the reaction of PBMCs with fluorochrome-labeled Mikβ1 in direct immunofluorescence analysis was reduced by ≥ 95% compared with both pretherapy reactivity with Hu-Mikβ1 of that patient and with reactivity of simultaneously administered Mikβ3. The quantity of Hu-Mikβ1 administered was deemed to be sufficient to yield and maintain saturation if such CD122 saturation was achieved at 6-72 hours and was maintained at the 3-week time point. As noted in Figure 3A, Hu-Mikβ1 saturation kinetics in T-LGL was not sustained for even 1 week with the 0.5 mg/kg dose. However, as shown in Figure 3B and Figure 4, at 6-72 hours and 14 days after administration of 1.5 mg/kg, there was 99% saturation of CD122 by administration of 1.5 mg/kg. Saturation diminished to 37% at 21 days, 15% at 28 days, and was negligible at 42 days after infusion. In agreement with these studies, Hu-Mikβ1 was not demonstrable in the serum 2 weeks after 0.5 mg/kg administration, but was still present in the serum at concentrations of 160, 400, and 2530 ng/mL at the day 22 in the 3 patients who received the 1.5 mg/kg dose (Figure 5). Therefore, a dose of 1.5 mg/kg was deemed to be required to maintain adequate saturation of IL-2R/IL-15Rβ for a 3-week period between doses.
A 0.5 mg/kg dose of Hu-Mikβ1 does not maintain saturation of the IL-2/IL-15Rβ, whereas 1.5 mg/kg is sufficient to maintain saturation for 3 weeks. (A) Three patients with T-LGL leukemia were evaluated for saturation of their IL-2/IL-15R between 6 and 72 hours after administration of 0.5 mg/kg of Hu-Mikβ1 and were shown to have saturated receptors. However this receptor saturation was not maintained in 2 patients at 7, 14, and 21 days. To evaluate saturation, FACS analysis was performed at these time points with fluorochrome-labeled Hu-Mikβ1 and fluorochrome-labeled Mikβ3, which define non-cross-competing epitopes on IL-2/IL-15Rβ, as described in “Methods.” Different symbols represent the results for different patients. (B) Three patients with T-LGL leukemia were evaluated for saturation of their receptors after a 1.5 mg/kg IV infusion of Hu-Mikβ1. This dose of Hu-Mikβ1 was sufficient to provide prolonged saturation of the IL-2/IL-15R. In particular, saturation of CD122 was achieved at the 6- to 72-hour time point and was maintained at 100% saturation at the 7- and 14-day time points and at 80% saturation for the cells at the 21-day time point. Different symbols reflect the results for different patients.
A 0.5 mg/kg dose of Hu-Mikβ1 does not maintain saturation of the IL-2/IL-15Rβ, whereas 1.5 mg/kg is sufficient to maintain saturation for 3 weeks. (A) Three patients with T-LGL leukemia were evaluated for saturation of their IL-2/IL-15R between 6 and 72 hours after administration of 0.5 mg/kg of Hu-Mikβ1 and were shown to have saturated receptors. However this receptor saturation was not maintained in 2 patients at 7, 14, and 21 days. To evaluate saturation, FACS analysis was performed at these time points with fluorochrome-labeled Hu-Mikβ1 and fluorochrome-labeled Mikβ3, which define non-cross-competing epitopes on IL-2/IL-15Rβ, as described in “Methods.” Different symbols represent the results for different patients. (B) Three patients with T-LGL leukemia were evaluated for saturation of their receptors after a 1.5 mg/kg IV infusion of Hu-Mikβ1. This dose of Hu-Mikβ1 was sufficient to provide prolonged saturation of the IL-2/IL-15R. In particular, saturation of CD122 was achieved at the 6- to 72-hour time point and was maintained at 100% saturation at the 7- and 14-day time points and at 80% saturation for the cells at the 21-day time point. Different symbols reflect the results for different patients.
FACS analyses of Hu-Mikβ1 saturation was performed onex vivoCD8+ T cells afteradministration of 1.5 mg/kg of Hu-Mikβ1.
FACS analyses of Hu-Mikβ1 saturation was performed onex vivoCD8+ T cells afteradministration of 1.5 mg/kg of Hu-Mikβ1.
Pharmacokinetic analysis of Hu-Mikβ1 was performed after administration of 0.5, 1.0, and 1.5 mg/kg of Hu-Mikβ1 Ab to patients with T-LGL leukemia. After administration of 0.5 (○), 1.0 (■), and 1.5 (▾) mg/kg of Hu-Mikβ1, the serum concentration of the Ab was quantitated at subsequent time points in patients with T-LGL leukemia. Hu-Mikβ1 was not demonstrable in the serum after the 2-week time point after 0.5 mg/kg administration, but was still present in the circulation at day 22 at concentrations of 160, 400, and 2530 pg/mL in the 3 patients receiving 1.5 mg/kg of Hu-Mikβ1.
Pharmacokinetic analysis of Hu-Mikβ1 was performed after administration of 0.5, 1.0, and 1.5 mg/kg of Hu-Mikβ1 Ab to patients with T-LGL leukemia. After administration of 0.5 (○), 1.0 (■), and 1.5 (▾) mg/kg of Hu-Mikβ1, the serum concentration of the Ab was quantitated at subsequent time points in patients with T-LGL leukemia. Hu-Mikβ1 was not demonstrable in the serum after the 2-week time point after 0.5 mg/kg administration, but was still present in the circulation at day 22 at concentrations of 160, 400, and 2530 pg/mL in the 3 patients receiving 1.5 mg/kg of Hu-Mikβ1.
Toxicity of Hu-Mikβ1
Acute toxicities associated with Hu-Mikβ1 administration, excluding the entry requirement of hematocytopenia, were limited to a single patient who manifested a progressive CK-MB elevation associated with autoimmune myositis associated with CD8 LGL infiltration in muscles that was subsequently confirmed to be a preexisting condition. This patient had had cardiac surgery associated with Dressler syndrome, arthritis, and a pre–Hu-Mikβ1 administration elevation of CK-MB. In addition, one patient died 22 months after intensive antileukemic chemotherapy for acute myeloid leukemia, an outcome though not to be a consequence of Hu-Mikβ1 therapy.
Immunogenicity of Hu-Mikβ1
Immunogenicity was assessed with a Hu-Mikβ1 Ab–specific ELISA assay. HAHA (human anti–human Ab) developed in 1 of 9 patients studied in a subject who had received murine Mikβ1 previously.
Response to Hu-Mikβ1 in patients with T-LGL
Nine patients were evaluated after a single IV administration, 3 at each dose of Hu-Mikβ1 (Table 1). All had T-LGL leukemia with associated hematocytopenias; 9 patients had neutropenia, 7 anemia, and 2 thrombocytopenia (Table 1). After administration of Hu-Mikβ1, all patients showed stable disease without a reduction in peripheral leukemic cell counts. However, 3 patients demonstrated transient increases in their neutrophil counts (supplemental Figure 1) and, in one case each, a transient increase in the number of circulating reticulocytes or platelets.
T-LGL did not demonstrate IL-2– and IL-15–mediated autocrine/paracrine proliferation
Several reports suggest that IL-15 might play a role in the pathogenesis of T-LGL leukemia,28-30 specifically that survival of T-LGL cells might depend on either IL-2 or IL-15 and that their proliferation would be inhibited by Hu-Mikβ1. Ex vivo proliferation of T-LGL cells was evaluated by [3H]-thymidine uptake by culturing 105 PBMCs for 6 days in absence of added antigen, mitogen, or cytokine. Little spontaneous proliferation of PBMCs from any patient was observed (Figure 6). This contrasts with the much higher level of thymidine uptake observed in comparable 6-day cultures of PBMCs from 11 patients with smoldering and chronic adult T-cell leukemia/lymphoma,39 whose leukemic cells manifested 2 autocrine (IL-2/IL-2R and IL-15/IL-15R) and 1 paracrine (IL-9/IL-9R) stimulatory loop.
PBMCs from patients with T-LGL leukemia do not manifestex vivospontaneous proliferation in a 6-dayex vivoculture. PBMCs from patients with T-LGL leukemia were placed in a 6-day culture in1640 media containing 10% FCS and proliferation was assessed by the evaluation of the uptake of thymidine added during the last 4 hours of culture. There was no meaningful thymidine uptake, indicating that there was essentially no ex vivo proliferation of PBMCs from the 6 patients studied with monoclonal T-LGL leukemia. When 20 000 pg/mL of IL-15 was added to the T-LGL of 5 patients, there was an increase in the geometric mean proliferation from 472-5 565 cpm per 1 millicurie of 3H thymidine added. This proliferation was inhibited to baseline levels of 728 cpm on addition of 10 μg/mL of Hu-Mikβ1 at the onset of the cultures.
PBMCs from patients with T-LGL leukemia do not manifestex vivospontaneous proliferation in a 6-dayex vivoculture. PBMCs from patients with T-LGL leukemia were placed in a 6-day culture in1640 media containing 10% FCS and proliferation was assessed by the evaluation of the uptake of thymidine added during the last 4 hours of culture. There was no meaningful thymidine uptake, indicating that there was essentially no ex vivo proliferation of PBMCs from the 6 patients studied with monoclonal T-LGL leukemia. When 20 000 pg/mL of IL-15 was added to the T-LGL of 5 patients, there was an increase in the geometric mean proliferation from 472-5 565 cpm per 1 millicurie of 3H thymidine added. This proliferation was inhibited to baseline levels of 728 cpm on addition of 10 μg/mL of Hu-Mikβ1 at the onset of the cultures.
Expression of IL-2 and IL-15 mRNA was evaluated in PBMCs from 10 patients with T-LGL leukemia, including 4 from the present study, using real-time PCR. Little or no IL-2 and IL-15 mRNA expression was observed (Figure 7). Furthermore, little or no IL-2 (mean, 5 pg/mL) or IL-15 (mean, 1 pg/mL) was detected by ELISA in the 6-day culture supernatants of PBMCs of 6 patients with T-LGL leukemia, including 4 from the present study.
The expression of IL-2 and IL-15 mRNA was evaluated in the PBMCs from 6 patients with LGL. There was either no or only minimal IL-2 and IL-15 mRNA expression observed in the patients examined. This contrasts with the high IL-15 expression in the adult T-cell leukemia/lymphoma cell line HuT-102 or in normal PBMCs after ionomycin/phorbol 12-myristate 13-acetate addition.
The expression of IL-2 and IL-15 mRNA was evaluated in the PBMCs from 6 patients with LGL. There was either no or only minimal IL-2 and IL-15 mRNA expression observed in the patients examined. This contrasts with the high IL-15 expression in the adult T-cell leukemia/lymphoma cell line HuT-102 or in normal PBMCs after ionomycin/phorbol 12-myristate 13-acetate addition.
Recently, we demonstrated that in 2 of 5 cases examined, T-LGL cells expressed IL-15Rα as well as IL-2/IL-15Rβ and γc.30 Although Hu-Mikβ1 blocks trans presentation of IL-15 to cells that express IL-2/IL-15Rβ and common γ-c alone, it was not effective at inhibiting proliferation of cells that express high-affinity heterotrimeric IL-15 receptors in cis. We investigated whether 10 μg/mL of Hu-Mikβ1 would inhibit the proliferation mediated by the addition of 20 000 pg/mL of IL-15 to T-LGL cells of 5 patients in the present study (Figure 6). When IL-15 was added to the PBMCs of 5 of these patients, proliferation was increased from the baseline geometric mean of 472 to 15 565 cpm per 1 millicurie of 3H-thymidine added. This proliferation was inhibited back to a baseline level of 724 on addition of 10 μg/mL of Hu-Mikβ1 at the onset of the culture, thereby demonstrating that the Ab could inhibit IL-15–mediated proliferation of the leukemic cells of the patients studied.
Discussion
This study was a first-in-human phase 1 trial to evaluate Hu-Mikβ, a humanized mAb that targets the IL-2/IL-15Rβ. Hu-Mikβ1 targets this shared coreceptor for IL-2 and IL-15, and is shown to inhibit the trans presentation of IL-15 to cells that express IL-2/IL-15Rβ and the common γ-c. However, Hu-Mikβ1 was not effective at inhibiting IL-2– or IL-15–mediated proliferation of cells that expressed high-affinity heterotrimeric IL-2R or IL-15R, which also include each cytokine receptor's specific α-chain. The pharmacokinetic and pharmacodynamic studies indicate that the 0.5 mg/kg dose of Hu-Mikβ1 was inadequate, whereas the 1.5 mg/kg dose was sufficient to maintain unbound Ab in the circulation for 3 weeks. Furthermore, 1.5 mg/kg, but not 0.5 mg/kg, was sufficient to maintain saturation of surface CD122 on T-LGL cells for 3 weeks. Most importantly, no dose-limiting or other toxicities because of Hu-Mikβ1 were observed after administration of single doses of up to 1.5 mg/kg. Furthermore, Hu-Mikβ1 did not elicit an immune response in recipients, with the exception of a single patient who had previously received murine Mikβ1. Therefore, Hu-Mikβ1 appears to have a good safety profile at doses that effectively block in vivo IL-15 trans presentation in human subjects.
Despite the capacity to block IL-15 trans presentation and thereby block IL-2 and IL-15 stimulation of IL-2/IL-15Rβ and γc-expressing T-LGL cells, little efficacy other than a transient increase in neutrophils was observed after a single administration of Hu-Mikβ1 to patients with T-LGL and hematocytopenia. Several factors may be responsible for this lack of efficacy, including that the duration of receptor blockade achieved by a single-dose administration of Hu-Mikβ1 may not be sufficient. In addition, we previously demonstrated that select T-LGL cells coexpress heterotrimeric IL-15Rs that include IL-15Rα as well as IL-2/IL-15Rβ and the common γ-c in cis.30 Hu-Mikβ1 may not block IL-2– or IL-15–induced stimulation of such cells. To address this, we induced ex vivo proliferation of the LGL cells and PBMCs of 5 patients by the addition of 20 000 ng/mL of IL-15. Cytokine-induced proliferation was inhibited from 15 565 to baseline by the addition of Hu-Mikβ1. This observation does not support the hypothesis that the expression of heterotrimeric cytokine receptors on the same target cells caused the failure to see efficacy in vivo with Hu-Mikβ1. The recent observation that 40% of patients with T-LGL leukemia have mutated STAT3 genes that are downstream of IL-15/IL-15R interaction that show increased transcriptional activity and up-regulated expression of downstream genes provides an alternative explanation for the pathogenesis of T-LGL leukemia and an explanation for the lack of activity of Mikβ1, at least in some patients. Patients with T-LGL leukemia with normal STAT3 genes may be a more appropriate subgroup for testing this approach.40
The LGL cells in the monoclonal phase of disease did not produce nor require IL-2 or IL-15 for their survival. In particular, T-LGL cells did not manifest spontaneous proliferation, a hallmark of leukemic cells that are in a cytokine-mediated autocrine phase of the disease (Figure 6). Furthermore, the leukemic cells did not express mRNA encoding IL-2 or IL-15, nor did they secrete these cytokines into media (Figure 7). Therefore, patient leukemic cells appear to be in a cytokine-independent phase. Although Hu-Mikβ1 demonstrated Ab-dependent cellular cytotoxicity in vitro, in the present study, this characteristic was not sufficient to yield a meaningful reduction in the number of circulating leukemic cells.
Potentially more favorable targets for Hu-Mikβ1 might be in the therapy of autoimmune diseases that result from disorders of IL-15 expression and action.14-20 For example, IL-15 has been proposed to be a required element in the pathogenesis of rheumatoid arthritis, inflammatory bowel disease, psoriasis, multiple sclerosis, type 1 diabetes, and refractory celiac disease,14-19 as well as diseases associated with infection with the retrovirus HTLV-1 that overexpress IL-15 and its receptor that are transactivated by the protein Tax encoded by HTLV-1.20
PBMCs from patients with HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) demonstrate spontaneous proliferation in the absence of exogenous cytokine.41-43 IL-15 and IL-15Rα mRNA and protein expression are elevated in HAM/TSP as a consequence of HTLV-1–associated Tax trans activation.20,44 Moreover, this spontaneous proliferation of PBMCs from patients with HAM/TSP could be inhibited by addition of Hu-Mikβ1.20 Furthermore, the addition of Hu-Mikβ1 to PBMCs from HAM/TSP patients led to a rapid decline by more than 50% in the number of circulating MHC-A2–restricted Tax-specific CD8+ T cells that have been proposed as participants in the damage to the CNS.20,44 A clinical trial has been initiated in patients with HAM/TSP to examine the impact of IL-15 blockade on antigen-specific CD8+ T cells.
Disordered expressions of IL-15 and IL-15Rα have also been proposed to be critical to the development and maintenance of refractory celiac disease, an autoimmune inflammatory disorder observed predominantly in persons carrying HLA-DQ alleles.18,19,45-48 In such patients, gluten peptides are presented by APCs to cognate gluten-specific CD4+ T-lymphocytes. Furthermore, extensive intraepithelial infiltration of CD8+ T-lymphocytes is a hallmark. Emerging evidence has implicated a pivotal role for IL-15 in celiac disease.45-48 IL-15 induces proliferation and activity of CD8+ T cells in part by coordinate induction of the NKG2D signaling pathway. In completing the cycle of tissue damage, IL-15 also induced expression of the cognate ligand of NKG2D, a MHC class I related antigen A (MICA).46,47 We evaluated blockade of IL-15 action in IL-15 transgenic mice that express human IL-15 in intestinal cells using the enterocyte-specific T3b promoter to drive the transgene.49 These mice developed florid inflammation of the duodenal region with extensive villus atrophy and massive accumulation of NK cell–like CD8+ T cell, a phenomenon that recapitulates pathognomic lesions of celiac disease. Administration of TM-β-1, a murine equivalent of Hu-Mikβ1 that blocks IL-15 trans presentation to IL-2/Il-15Rβ in mice, was associated with a reversal of pathologic changes in the intestines of T3b-hIL-15 Tg mice.49
To translate these insights, a clinical trial has been initiated involving evaluation of 5 doses of Hu-Mik-Beta-1 separated by 3-week intervals in patients with refractory celiac disease to define the clinical impact of this therapy on the disappearance of diarrhea, histologic improvement defined by significant recovery of villi, and normalization of intraepithelial lymphocyte phenotype, and loss of T-cell monoclonality. A secondary objective of the study is to define the impact of Hu-Mikβ1 on the development of enteropathy-associated T-cell lymphoma (EATL), a complication of refractory celiac disease, and mortality. This trial of Hu-Mikβ1 would provide a direct in vivo test in humans of the hypothesis that uncontrolled expression of IL-15 and its receptor are critical in the pathogenesis of refractory celiac disease and EATL and may prevent transition in patients from a monoclonal nonmalignant CD8+ proliferation that characterizes refractory celiac disease to EATL. Because of its lack of toxicity and little immunogenicity and the capacity of Hu-Mikβ1 to saturate IL-2/IL-15Rβ and thereby block trans presentation of IL-15, Hu-Mikβ1 has therapeutic potential in the treatment of autoimmune diseases such as HAM/TSP and celiac disease. Clinical trials have been initiated to assess the efficacy of Hu-Mikβ1 in patients with these clinical disorders.
In the present study, we have demonstrated that Hu-Mikβ1 blocks IL-15 trans presentation to cell lines that express IL-15Rβ and common γ-subunits, but does not effectively block IL-15 action on cells that express heterotrimeric IL-15 receptors in cis. Administration of Hu-Mikβ1 to patients was not associated with significant toxicity. However, no patients with T-LGL leukemia were seen to have a significant improvement in their clinical abnormalities in response to a single infusion of Hu-Mikβ1. Nevertheless, the results of this first in-human study of Hu-Mikβ1 support its evaluation in other clinical disorders such as refractory celiac disease, in which abnormalities of IL-15 and its receptor have been proposed to play a critical role in the pathogenesis and maintenance of disease activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: T.A.W. designed the research, analyzed and interpreted the data, and wrote the manuscript; K.C.C., D.M.S., T.A.W., and J.E.J. provided patient care and collected patient samples; T.A.F., C.K.G., B.R.B., and M.N.P. performed the research; P.S.A. performed the statistical analyses; W.D.F., S.D.S., and J.R.D. performed the data analyses; M.R. performed the PCR; S.P.C. provided critical reagents; and J.C.M. designed the research and provided patient care.
Conflict-of-interest disclosure: T.A.W. has been granted a patent for the use of antibodies for the IL-2/IL-15Rβ. The remaining authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, National Institutes of Health, Metabolism Branch, 10 Center Dr, MSC 1374, Bldg 10, Rm 4N115, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal