Key Points
The MYD88 locus is altered in 91% of patients with WM.
MYD88 might be new target for therapeutic in WM.
Abstract
Mutation of the MYD88 gene has recently been identified in activated B-cell–like diffuse cell lymphoma and enhanced Janus kinase/signal transducer and activator of transcription (JAK-STAT) and nuclear factor κB (NF-κB) signaling pathways. A whole exome–sequencing study of Waldenstrom macroglobulinemia (WM) suggested a high frequency of MYD88 L265P mutation in WM. The genetic background is not fully deciphered in WM, although the role of NF-κB and JAK-STAT has been demonstrated. We analyzed MYD88 mutation in exon 5 and characterized the clinical significance of this genetic alteration in 67 WM patients. Clinical features; immunophenotypic markers; and conventional cytogenetic, fluorescence in situ hybridization, and single nucleotide polymorphism array data were analyzed. MYD88 L265P mutation was acquired in 79% of patients. Overall, we have identified alteration of the MYD88 locus in 91% of WM patients, including 12% with gain on chromosome 3 at the 3p22 locus that included the MYD88 gene. Patients with absence of MYD88 mutation were WM characterized with a female predominance, a splenomegaly, gain of chromosome 3, and CD27 expression. Importantly, inhibition of MYD88 signaling induced cytotoxicity and inhibited cell growth of cell lines issued from patients with WM. In conclusion, these results confirm a high frequency of MYD88 L265P mutation in WM. The discovery of MYD88 L265P mutation may contribute to a better understanding of the physiopathogeny of WM.
Introduction
MYD88 is an adaptor protein through which most of the Toll-like receptors (TLRs) and receptors for interleukin (IL)-1 and IL-18 cytokines signal.1,2 MYD88 coordinates the assembly of a multisubunit signaling complex, including IL-1 receptor-associated kinase (IRAK) 1 and IRAK4,3 and also signals to Janus kinase/signal transducer and activator of transcription (JAK-STAT) 3 signaling and to activation pathways and transcription factors, such as the canonical nuclear factor-κB (NF-κB), activator protein 1, and interferon regulatory factor.2 Recently, Ngo et al4 reported that gain-of-function mutation in MYD88 (MYD88 L265P) drove nearly 30% of activated B-cell–like diffuse B-cell lymphomas (ABC-DLBCLs), implicating deregulation of TLR signaling in pathogenesis of ABC lymphoma. Experimentally, the MYD88 L265P mutation enhanced cell survival through increase in NF-κB activity, JAK-STAT3 signaling, and consequently cytokine production.4
The genetics and pathogenesis of Waldenstrom macroglobulinemia (WM), a low-grade B-cell lymphoplasmacytic lymphoma associated with monoclonal immunoglobulin M (IgM) secretion in the serum, are not fully understood.5,6 Constitutive activation of the NF-κB pathway was suspected,7 but the underlying genetic basis remains to be demonstrated. Furthermore, deletion of tumor necrosis factor, α-induced protein 3 (TNFAIP3/A20) in the context of the deletion 6q is the most frequent cytogenetic event described in WM.8 However, previous studies ruled out gene mutation of key regulators of both the canonical (TNFAIP3/A20) and noncanonical (TNF receptor-associated factor 3) NF-κB signaling pathways in WM. However, deletion of TNFAIP3/A20 in the context of the deletion 6q is the most frequent cytogenetic event in WM.8 A whole exome–sequencing study of 31 WM patients suggested a high frequency of MYD88 L265P mutation in WM.9 Our aim was to analyze MYD88 mutation in WM and to characterize the clinical significance of this genetic alteration in a cohort of 67 WM patients.
Patients and methods
Patients
Sixty-seven patients (42 males, 25 females) diagnosed with WM were included in this study.10 The diagnosis criteria of WM were (1) a lymphoplasmacytic bone marrow (BM) involvement; (2) any level of IgM paraprotein6,10,11 ; and (3) exclusion of other low-grade lymphomas. Patients were untreated at the time of BM collection and gave informed consent prior to research sampling. No familial form of WM was included. In addition, patients with various B lymphoid disorders were included as follows: 5 patients with myeloma/plasma cell leukemia (MM), 9 patients with chronic lymphocytic leukemia (CLL), 6 patients with hairy cell leukemia, 23 patients with follicular lymphoma, 10 patients with mantle cell lymphoma, 2 patients with IgM monoclonal gammopathy of undetermined significance, 1 patient with non-IgM lymphoplasmacytic lymphoma, and 16 patients with marginal zone lymphoma (MZL). The approval of this protocol was obtained from the local Institutional Review Board of the Centre Hospitalier Régional Universitaire of Lille (CSTMT045), and the study was conducted in accordance with the Declaration of Helsinki.
Cell selection
Research sampling consisted of BM samples for all WM patients. T lymphocytes from blood samples were used as an intraindividual reference to distinguish acquired somatic aberrations in WM from germline polymorphisms. Mononuclear cells from BM and blood cells were isolated by Ficoll-Paque gradient centrifugation. The samples were enriched for cell populations (tumoral and T lymphocytes) of interest using immunomagnetic beads (B-cell isolation kit and pan T-cell isolation kit; Myltenyi-Biotec, Paris, France). The purity of the sample was confirmed by flow cytometry on tumor and T-cell fractions. DNA was extracted from samples using QuiAmp kit (Sigma-Aldrich Co., France) according to the manufacturer’s recommendations.
Cell lines and reagents
BCWM1, MWCL1, MEC-1, RL, MM.1S, and OCI-Ly3 cell lines were used in this study. BCWM1 and MWCL1 are B-cell lines developed in patients with WM; BCWM1 was a gift from Dr S.P. Treon from the Dana-Farber Cancer Institute (Boston, MA),12 and MWCL1 was a gift from Dr S. Ansell (Mayo Clinic, Rochester, MN).13 Both cell lines harbor MYD88 L265P mutation. OCI-Ly3 is derived from a patient with non-Hodgkin lymphoma and is homozygous for the MYD88 L265P mutation (kind gift from Dr Marc D. Minden, Ontario Cancer Institute, Toronto, ON, Canada).4 MM.1S, a myeloma cell line (kind gift from Dr S. Rosen, Northwestern University, Chicago); RL, a non-Hodgkin lymphoma (kind gift from Dr C. Bastard, center H Becquerel, Rouen, France); and MEC-1, a B-cell line derived from CLL, are not mutated on MYD88 (ACC-497; Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). BCWM1, MEC-1, RL, and MM.1S were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Gibco, Carlsbad, CA) and 100 U/mL of penicillin and streptomycin (Invitrogen, Carlsbad, CA). MWCL1 and OCI-Ly3 were cultured in Iscove modified Dulbecco medium complemented with 10% and 20% fetal bovine serum, respectively, and 100 µg/mL of penicillin/streptomycin. Cells were kept at 37°C 5% CO2 in humid atmosphere. The MYD88 inhibitor and its control peptide were provided by Invitrogen and were diluted in endotoxin-free water (vehicle) and stored at −20°C. The maximum final concentration of vehicle did not induce any cytotoxicity on the cell lines tested (data not shown). The pan-caspase inhibitor Z-VAD-fmk was purchased from Promega (Fitchburg, WI).
Proliferation and viability assay
Viability and cell growth of treated cells were determined using the MTS assay: CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) and the Click-iT EdU Flow Cytometry Assay kit (Invitrogen) according to the manufacturer’s protocol, respectively. Cells were analyzed on a CyAn ADP Analyzer flow cytometer (Beckman Coulter Inc.). Apoptosis was quantitated using annexin V–propidium iodide staining using flow cytometric analysis (Beckman Coulter, Brea, CA). Study of mitochondrial membrane potential was performed using MitoTracker Red CMXRos-Special Packaging (Invitrogen). Study of caspases activity was performed using staining CaspaLux (OncoImmunin, Gaithersburg, MD).
Immunoblotting
Whole-cell lysates were subjected to 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Invitrogen), followed by incubation with primary antibodies. The following antibodies were used: anti-STAT3, antiphospho STAT3 (Tyr705), anti-IRAK4, antiphospho IRAK4 (Thr345/Ser346) (Cell Signaling Technology, Danvers, MA), and anti-Hsc70 (Santa Cruz Biotechnology, Santa Cruz, CA).
DNA sequencing of exon 5 of MYD88
Exon 5 of MYD88 gene was amplified from genomic DNA by polymerase chain reaction (PCR) using the HotStar HiFidelity Polymerase Kit (Qiagen, CA). The following forward and reverse primers were used for MYD88: 5′GTTGAAGACTGGGCTTGTCC 3′ and 5′AGGAGGCAGGGCAGAAGTA3′.14 The purified PCR products were directly sequenced in both directions using BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, CA) and analyzed on the Applied Biosystems 3130xl Genetic Analyzer. Data were analyzed with SeqScape software version 2.5 (Applied Biosystems).
Cytogenetic analysis and fluorescence in situ hybridization (FISH)
Conventional cytogenetic analysis and/or FISH was available in 63 patients. Conventional cytogenetic analysis was performed on CpG-oligonucleotide DSP30 in combination with IL-2–stimulated BM cells. FISH was performed to detect deletion 6q23 (MYB deletion; CytoCell Aquarius, Cambridge, UK), 17p12 and 11q22 (LSI p53/LSI ATM; Abbott, Paris, France), 13q14, trisomy 12 (LSI D13S318/LSI13q14/CEP 12; Abbott), and trisomy 4 (FIPIL1/CHIC2/PDGFRA 4q12; Abbott).
Single nucleotide polymorphism (SNP) array analysis
Genome-Wide Human SNP Array 6.0 (Affymetrix chips) was used for delineation of the location and size of submicroscopic chromosomal defects, copy-neutral loss of heterozygosity (LOH), and germline copy number variations in 46 patients. LOH and copy number aberration (CNA) were identified using genotyping console 3.02 software (Affymetrix) and Partek genomic suite. LOH without copy number changes was also referred as uniparental disomy (UPD). These regions were confirmed as copy number polymorphism using the copy number variants database described at http://projects.tcag.ca/variation and the reference genotyping data from the HapMap project. Size, position, and location of genes were identified with UCSC Genome Browser HG18 mars 2006 assembly. The co-occurrence of MYD88 L265P mutation with other genetic alterations analyzed in our series was represented using MeV software (TM4 microarray software suite).15,16
Immunophenotypic studies
Immunologic characterization of a large panel of markers was performed by flow cytometry (FC500; Beckman Coulter, Villepinte, France), including determination of the Matutes’s score, of CD27 (n = 47), CD80 (n = 49), CD138 (n = 33), and CD38 expression (n = 65) gating on CD19+ cells (all clones from Immunotech, France).
IL-6 expression
Quantification of messenger RNA of IL-6 was performed by real-time quantitative PCR against an endogenous control gene TBP (ABI Prism 7500; Applied Biosystems). Relative IL-6 expression values were calculated using the mean of ΔCt (threefold cycle) from the 2 duplicates and expressed as 2ΔCt IL-6 – ΔCt TBP.
Statistical analysis
Statistical associations were assessed using χ2 and Fisher’s exact tests. Pairwise comparisons were performed by Mann-Whitney test or Student t test. Overall survival was defined as the time between diagnosis and death or the last follow-up. Statistics were performed using SPSS 15.0.
Results
High frequency of MYD88 L265P mutation in WM
Fifty-three (79%) patients were identified to harbor the MYD88 L265P mutation (MYDmut) (Figure 1A) in our cohort of 67 patients with WM, including homozygous mutation in 2 patients (3%). In the remaining patients, we detected both the normal and mutated alleles, consistent with heterozygous mutation. We confirmed that MYD88 was an acquired mutation as no aberration was identified in the mutational hot spots of MYD88 in T lymphocytes isolated from WM patients (n = 4). We have not observed any other mutation on exon 5 of MYD88 in our cohort. In addition, we have investigated MYD88 L265P mutation across a spectrum of mature tumor B-cell histologies (n = 72). The MYD88 L265P mutation was absent in hairy cell leukemia, MM, mantle cell lymphoma, and CLL, whereas it was found sporadically in MZL (1/16; 6%) and follicular lymphoma (1/23, 4%). MYD88 mutation was observed in 1 of the 2 cases of IgM monoclonal gammopathy of undetermined significance and in 1 case of non-IgM lymphoplasmacytic lymphoma.
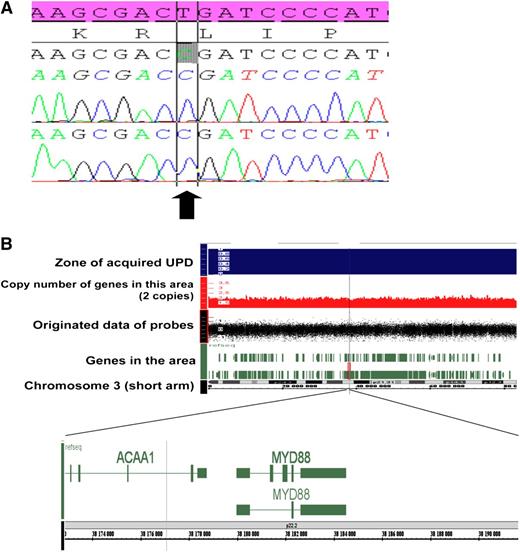
Mechanisms of MYD88 gene alteration. (A) Sanger sequence of MYD88 exon 5 in WM patient characterized with UPD on the 3p22 locus. The electrophoregram shows the recurrent homozygous L265P MYD88 mutation (arrow) detected in tumoral B cell of 1 patient. The reference sequence is reported in pink. (B) UPD (uniparental disomy, LOH without variation of copy number) of the short arm of chromosome 3 including MYD88 gene observed in 1 WM with MYD88 mutation using SNP array.
Mechanisms of MYD88 gene alteration. (A) Sanger sequence of MYD88 exon 5 in WM patient characterized with UPD on the 3p22 locus. The electrophoregram shows the recurrent homozygous L265P MYD88 mutation (arrow) detected in tumoral B cell of 1 patient. The reference sequence is reported in pink. (B) UPD (uniparental disomy, LOH without variation of copy number) of the short arm of chromosome 3 including MYD88 gene observed in 1 WM with MYD88 mutation using SNP array.
Gain of chromosome 3 or acquired UPD, a new mechanisms of alteration of MYD88 locus in WM
We then sought other mechanisms of MYD88 gene alteration, such as CNA and UPD at MYD88 locus using SNP array (n = 46). We found UPD at the MYD88 locus in only 1 patient (2%) (Figure 1B), and consequently, this patient was homozygous for L265P mutation in tumoral cells (Figure 1A). We have not identified mechanisms of loss of MYD88, such as deletion at 3p22 in our cohort. On the contrary, we observed a gain on chromosome 3 at the 3p22 locus (including MYD88 gene) in 7 of 59 (12%) patients, including 2 (5%) patients with MYD88 mutation. Overall, we found gain on chromosome 3 more frequently in the MYDwild group than in the MYDmut group (P = .006). These data need to be confirmed in a larger series of WM. We noticed that other cytogenetic aberrations were associated with the gain of chromosome 3 in 6 of 7 patients. Taken together, we identified alteration of the MYD88 locus in 91% of patients with WM, by either gain-of-function mutation (79%) or CNA (12%), using cytogenetic analysis, SNP array, and sequencing of MYD88.
MYD88 mutation is related to NF-κB abnormalities
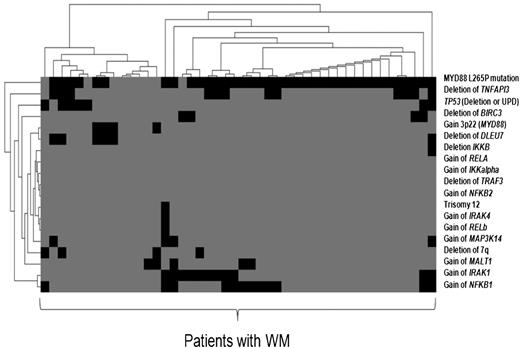
Several previous studies have demonstrated deregulation of NF-κB in WM,7,17 although the underlying mechanisms of deregulation remain to be demonstrated. NF-κB is constitutively active as a consequence of various genomic abnormalities including amplifications and deletions that ultimately subvert the normal function of NF-κB in tumor cells in other lymphoma subtypes.18 Recent studies have described genomic deletions of 2 potent regulators/inhibitors of the NF-κB pathway in WM, TNFAIP3 (located on 6q23),8 and deleted in lymphocytic leukemia 7 (located on 13q14).8 We then sought to demonstrate the association of MYD88 mutation with NF-κB aberrations in WM (Figure 2). We analyzed CNA or UPD that targeted multiple NF-κB genes of the canonical pathway, including inhibitor of NF-κB kinase (a positive regulator) and TNFAIP3 (a negative regulator), and the noncanonical pathway, including MAP3K14/NIK using SNP array and FISH data analysis, in our series. All patients that displayed alteration of MAP3K14 also harbored MYD88 mutation. Overall, 63% of WM had at least 1 additional genetic alteration in the NF-κB pathway in our cohort, but no significant difference was observed according to the MYD88 mutation status.
The heat map represents co-occurrence of MYD88 L265P mutation with other genetic alterations analyzed in 46 patients with WM using SNP array, cytogenetic analysis, and FISH. We have used Tmev software.15 Each row corresponds to key regulator genes of the canonical and noncanonical NF-κB pathways targeted by CNA, UPD, or mutation in WM. The columns represent individual patients color coded on the base of gene status. Each patient is represented by a virtual column (black mutation, deletion or gain of gene; gray, wild-type).
The heat map represents co-occurrence of MYD88 L265P mutation with other genetic alterations analyzed in 46 patients with WM using SNP array, cytogenetic analysis, and FISH. We have used Tmev software.15 Each row corresponds to key regulator genes of the canonical and noncanonical NF-κB pathways targeted by CNA, UPD, or mutation in WM. The columns represent individual patients color coded on the base of gene status. Each patient is represented by a virtual column (black mutation, deletion or gain of gene; gray, wild-type).
Clinical and biological features of WM with MYDwild
Twenty-one percent of the patients with WM had no mutation of MYD (MYDwild). Table 1 summarizes the main clinical and hematologic features of these patients. The median age was 66 years (43-95). We found several characteristics that varied in the MYDwild subgroup as compared with the MYDmut subgroup. Interestingly, there was a female predominance (P = .004), and patients presented more often with a splenomegaly (P = .015) in the MYDwild group. No association was found with regard to age at diagnosis, β2 microglobulin, M component, hematologic values, lymphocytosis, autoimmune hemolytic anemia, cryoglobulinemia, cold agglutinin, and presence of adenopathy. MYD88 mutation was not associated with deletion 6q, gain of 4, deletion 11q, deletion 17p, or deletion 13q14 in our study. Interestingly, deletion 7q, a frequent cytogenetic aberration in MZL,18 was rare in our series (4/57 using SNP array and FISH; 7%) and was independent of MYD88 mutation status (2 in the MYDwild and 2 in the MYDmut) (P = not significant [NS]). We did not observe any difference in terms of survival according to the MYD88 mutation status (data not shown).
Clinical and biological characteristics of patients with WM according to MYD88 L265P mutation status
| . | MYD88wild (n = 14) . | MYD88mut (n = 53) . | P . |
|---|---|---|---|
| Sex ratio (F/M) | 10/4 | 15/38 | .004 |
| Age at diagnosis, median [range], years | 66 [54-73] | 63 [43-95] | NS |
| Age < 65 y, N | 5 | 24 | NS |
| ISS-WM stage III, N (%) | 2 (17) | 8 (15) | NS |
| Symptomatic WM, N (%) | 7 (54) | 32 (61) | NS |
| Anemia, median, g/dL | 11.8 | 12.4 | NS |
| Hb <11.5 g/dL, N (%) | 5 (36) | 15 (28) | NS |
| Thrombopenia, median, ×109/L | 222 | 266 | NS |
| Platelet count <100 × 109/L, N (%) | 0 | 7 (13) | NS |
| β2 microglobulin, median, mg/L | 2.8 | 2.6 | NS |
| β2 microglobulin >3 mg/L, N (%) | 6 (43) | 16 (30) | NS |
| Monoclonal component, median, g/L | 20.6 | 20.1 | NS |
| Lymphocytosis, median, ×109/L | 2.7 | 1.7 | NS |
| Adenopathy, N (%) | 3 (21) | 6 (11) | NS |
| Splenomegaly, N (%) | 5 (36) | 3 (6) | .015 |
| Cryoglobulinemia, N (%) | 4 (33) | 6 (12) | NS |
| Cold agglutinin, N (%) | 4 (27) | 14 (27) | NS |
| Autoimmune hemolytic anemia, N (%) | 0 | 3 (6) | NS |
| . | MYD88wild (n = 14) . | MYD88mut (n = 53) . | P . |
|---|---|---|---|
| Sex ratio (F/M) | 10/4 | 15/38 | .004 |
| Age at diagnosis, median [range], years | 66 [54-73] | 63 [43-95] | NS |
| Age < 65 y, N | 5 | 24 | NS |
| ISS-WM stage III, N (%) | 2 (17) | 8 (15) | NS |
| Symptomatic WM, N (%) | 7 (54) | 32 (61) | NS |
| Anemia, median, g/dL | 11.8 | 12.4 | NS |
| Hb <11.5 g/dL, N (%) | 5 (36) | 15 (28) | NS |
| Thrombopenia, median, ×109/L | 222 | 266 | NS |
| Platelet count <100 × 109/L, N (%) | 0 | 7 (13) | NS |
| β2 microglobulin, median, mg/L | 2.8 | 2.6 | NS |
| β2 microglobulin >3 mg/L, N (%) | 6 (43) | 16 (30) | NS |
| Monoclonal component, median, g/L | 20.6 | 20.1 | NS |
| Lymphocytosis, median, ×109/L | 2.7 | 1.7 | NS |
| Adenopathy, N (%) | 3 (21) | 6 (11) | NS |
| Splenomegaly, N (%) | 5 (36) | 3 (6) | .015 |
| Cryoglobulinemia, N (%) | 4 (33) | 6 (12) | NS |
| Cold agglutinin, N (%) | 4 (27) | 14 (27) | NS |
| Autoimmune hemolytic anemia, N (%) | 0 | 3 (6) | NS |
Hb, hemoglobin; ISS-WM, international staging system for Waldenstrom macroglobulinemia; M/F, male/female.
MYDwild expressed CD27
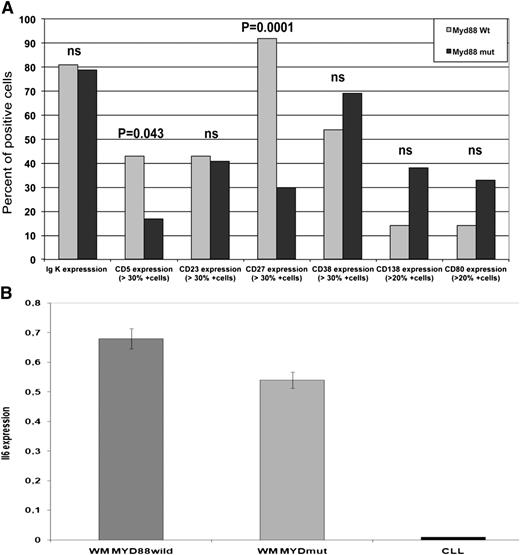
In order to further characterize the MYDwild group as compared with the MYDmut group, we have studied a panel of markers using flow cytometry (Figure 3A). We found that CD27 (positive expression in 49% of malignant B cells from WM in our series) was expressed in a significantly lower proportion in the MYDmut group than in the MYDwild group, 30% and 92%, respectively (P = .0001). CD27, a TNF-family member, is expressed on the cell surface of memory B cells from which WM is thought to derive.19 We have also found a higher frequency of CD5 positive cases (positive expression in 23% of WM in our series) (P = .043) in MYDwild group. However, we found no difference in the Matutes’s score; the K/l expression; and the CD23 (dim positive expression in 43% of patients), CD138, CD38, and the CD10 (dim positive expression in 14% of patients) between MYDwild and MYDmut subgroups. MYD88 knockdown in the ABC-DLBCL cell line affected a number of genes of the NF-κB or JAK-STAT signaling pathways. Among them, CD80 is a membrane receptor that is activated by the binding of CD28 or Cytotoxic T-Lymphocyte Antigen 4.4 CD80 was observed in 26 of 49 (53%) patients in our series but showed no difference in MYDwild vs MYDmut subgroups.
Characteristics of patients of the MYDmut vs MYDwild groups. (A) The histogram depicts the principal immunologic differences observed in the 2 groups of WM with MYDmut vs MYDwild. We observed significant differences based on the CD5 and CD27 expression. CD5, CD23, CD38, CD138, CD80, and CD27 expression were studied on CD19+ cells. (B) IL-6 expression using real-time quantitative PCR in WM compared with CLL. The histogram shows no significant difference in IL-6 expression in patients with MYDwild group (n = 4) as compared with MYDmut group (n = 10); however, patients with WM had significantly higher expression of IL-6 as compared with CLL (P < .0001).
Characteristics of patients of the MYDmut vs MYDwild groups. (A) The histogram depicts the principal immunologic differences observed in the 2 groups of WM with MYDmut vs MYDwild. We observed significant differences based on the CD5 and CD27 expression. CD5, CD23, CD38, CD138, CD80, and CD27 expression were studied on CD19+ cells. (B) IL-6 expression using real-time quantitative PCR in WM compared with CLL. The histogram shows no significant difference in IL-6 expression in patients with MYDwild group (n = 4) as compared with MYDmut group (n = 10); however, patients with WM had significantly higher expression of IL-6 as compared with CLL (P < .0001).
High expression of IL-6 was observed in WM independently of MYD mutation status
MYD88 L265P mutation promotes JAK-STAT3 signaling, which mediates IL-6 and IL-10 production.4 Furthermore, gene expression profiling has identified a high expression of IL-6 in WM.20 We then sought to analyze IL-6 expression according to the MYD88 mutated status in WM. Fourteen patients with WM were analyzed for IL-6 expression, including 10 patients that were MYDmut, and were compared with CLL cases with MYDwild status (n = 9). Previous reports showed that IL-6 expression was greater in WM as compared with CLL.20,21 There was no significant difference in IL-6 expression according to MYD mutation status in our WM series, although a significantly higher expression of IL-6 was observed in WM as compared with CLL (Figure 3B). One might thus consider the increase of IL-6 expression through JAK-STAT3 signaling in patients with MYDmut, whereas other mechanisms of IL-6 expression should be suspected in patients with MYDwild, through the BM microenvironment for example.
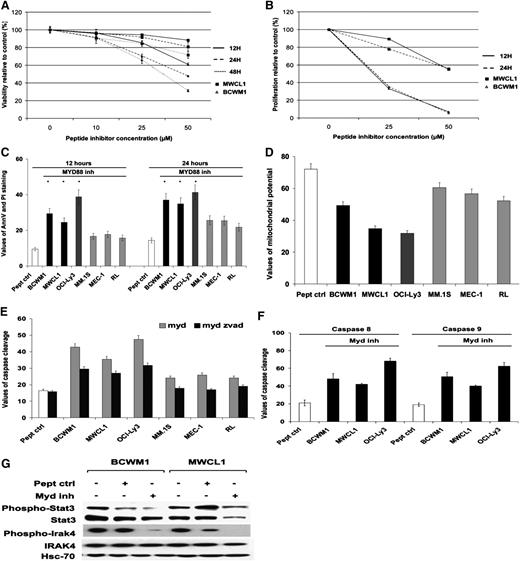
Inhibition of MYD88 signaling induced cytotoxicity and inhibited cell growth in cells expressing MYD88 L265P mutation
We have studied inhibition of MYD88 signaling in BCWM1, MWCL1, OCI-Ly3, MEC-1, and MM.1S cell lines; the former 3 cell lines are MYDmut, OCI-Ly3 being homozygous, and the latter being MYDwild. The cell lines were cultured for various time points, ranging from 6 to 48 hours, and various concentrations, ranging from 10 to 50 µM in the presence of the MYD88 inhibitor and its peptide control. We found a marked induction of cytotoxicity and inhibition of cell growth upon inhibition of MYD88 signaling in BCWM1 and MWCL1 cell lines in a dose-dependent manner and in a time-dependent manner, although to a lesser extent (Figure 4A-B). We next investigated inhibition of MYD88 signaling on induction of apoptosis as evidenced by annexin V–positive and propidium iodide–negative staining by flow cytometric analysis, but also by loss of mitochondrial potential and caspase 3 cleavage. We found that inhibition of MYD88 signaling induced apoptosis as early as 12 hours in cell lines with MYDmut (OCI-Ly3, BCWM1, and MWCL1) to a greater extent than MYDwild (MEC-1, MM.1S, and RL) cell lines, with a maximum difference observed at 24 hours (Figure 4C-E). Overall, no clear difference was demonstrated in OCI-Ly3 homozygous for MYD88 mutation as compared with BCWM1 and MWCL1 that are heterozygous for MYD88 mutation. We noticed that induction of apoptosis through caspase 3 cleavage was partially reversed by the pan-caspase inhibitor Z-VAD-fmk in certain cell lines, leading to the hypothesis that other types of cell death might be involved. Future studies will have to further characterize the various types of cell death induced by MYD88 signaling blockade. As shown in Figure 4F, inhibition of MYD88 signaling induced both intrinsic and extrinsic apoptotic pathways with caspase 9 and 8 cleavage in all 3 cell lines, BCWM1, MWCL1, and OCI-Ly3. Finally, we verified that inhibition of MYD88 homodimerization significantly inhibited MYD88 signaling in BCWM1 and MWCL1, the 2 cell lines derived from patients with WM, as exemplified by the marked downregulation of STAT3 phosphorylation, a downstream target of MYD88 and a key pathway involved in the physiopathogeny of WM (Figure 4G).1,4
Inhibition of MYD88 signaling induced cytotoxicity and inhibited cell growth in cells expressing MYD88 L265P mutation. Data represent the mean of triplicate experiments plus or minus standard deviation. (A-B) Inhibition of MYD88 by the MYD88 inhibitor induced cytotoxicity and inhibition of cell growth on BCWM1 and MWCL1 cell lines developed from patients with WM, characterized with MYD88mut. The data are compared with the control peptide (peptide crtl) provided by the manufacturer. Time points varied from 12 to 48 hours, and concentrations from 10 to 50 µM. (A) Cytotoxicity. (B) Inhibition of cell growth proliferation (*P < .05, significant difference with the control). (C-F) Inhibition of MYD88 induced apoptosis in MYD88 mutated cell lines, BCWM1, MWCL1, and OCI-Ly3, and to a lesser extent in MYD88wild cell lines, MEC-1, RL, and MM.1S. (C) The percentage of cells undergoing apoptosis was studied using annexin V–positive and propidium iodide–negative staining and flow cytometry at 12 and 24 hours, and at 25 µM (BCWM1, MM.1S, MEC-1, and RL) and 50 µM (MWCL1 and OCI-Ly3) of MYD88 inhibitor. (D) Study of mitochondrial membrane potential at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (E) Study of caspase 3 activity (caspase cleavage) in presence of the pan-caspase inhibitor Z-VAD-fmk (50 µM) at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (F) Study of caspase 8 and 9 cleavage in all 3 cell lines that are MYD88 mutated, BCWM1, MWCL1, and OCI-Ly3, at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (G) Inhibition of MYD88 signaling downregulates phosphorylation of STAT3 and IRAK4 in BCWM1 and MWCL1 cell lines. Cell lines were treated for 12 hours at 25 µM and 50 µM of MYD88 inhibitor, respectively.
Inhibition of MYD88 signaling induced cytotoxicity and inhibited cell growth in cells expressing MYD88 L265P mutation. Data represent the mean of triplicate experiments plus or minus standard deviation. (A-B) Inhibition of MYD88 by the MYD88 inhibitor induced cytotoxicity and inhibition of cell growth on BCWM1 and MWCL1 cell lines developed from patients with WM, characterized with MYD88mut. The data are compared with the control peptide (peptide crtl) provided by the manufacturer. Time points varied from 12 to 48 hours, and concentrations from 10 to 50 µM. (A) Cytotoxicity. (B) Inhibition of cell growth proliferation (*P < .05, significant difference with the control). (C-F) Inhibition of MYD88 induced apoptosis in MYD88 mutated cell lines, BCWM1, MWCL1, and OCI-Ly3, and to a lesser extent in MYD88wild cell lines, MEC-1, RL, and MM.1S. (C) The percentage of cells undergoing apoptosis was studied using annexin V–positive and propidium iodide–negative staining and flow cytometry at 12 and 24 hours, and at 25 µM (BCWM1, MM.1S, MEC-1, and RL) and 50 µM (MWCL1 and OCI-Ly3) of MYD88 inhibitor. (D) Study of mitochondrial membrane potential at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (E) Study of caspase 3 activity (caspase cleavage) in presence of the pan-caspase inhibitor Z-VAD-fmk (50 µM) at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (F) Study of caspase 8 and 9 cleavage in all 3 cell lines that are MYD88 mutated, BCWM1, MWCL1, and OCI-Ly3, at 12 hours with 25 µM and 50 µM of MYD88 inhibitor, respectively. (G) Inhibition of MYD88 signaling downregulates phosphorylation of STAT3 and IRAK4 in BCWM1 and MWCL1 cell lines. Cell lines were treated for 12 hours at 25 µM and 50 µM of MYD88 inhibitor, respectively.
Discussion
We have confirmed a high frequency of MYD88 L265P mutation in this study of 67 patients diagnosed with WM, similar to the initial report by Treon and colleagues.22,23 They have described MYD88 L265P mutation in 90% of cases in a first series of 46 patients with WM. MYD88 L265P mutation was originally described in ABC-DLBCL in nearly 30% of cases. This major hot-spot mutation on the L265P locus was also found to be mutated, although at a lower frequency (<10%), in mucosa-associated lymphoid tissue lymphoma and CLL, principally in mutated immunoglobulin heavy-chain variable-region gene in this latter group.9,14,24-26 We have confirmed the low incidence rate of the MYD88 L265P mutation in several other histologies of B-cell types, although our studied populations were limited in size in certain subgroups.
It can be difficult to distinguish WM from MZL in some cases, and this could be partially the result of the existence of a large overlapping of common immunophenotypic and genomic aberrations across the 2 entities, although clearly different small B-cell lymphomas.27,28 We therefore carefully studied the patients in the MYDwild group to identify any specific profile of WM with MYDwild. Few clinical and biological data distinguished MYDwild from MYDmut subgroups in our series: gender and the presence of a splenomegaly and of a trisomy 3. We have not observed any particular immunologic characteristics according to MYD mutation status, but a higher frequency of CD5 expression.
Interestingly, we found that CD27 (expressed in nearly half the patients in our WM series) was mainly observed in WM with MYD88wild. CD27 is induced on B lymphocytes after antigenic stimulation and interacts with CD70 to differentiate mature B cells into plasma cells.19 CD27 is also considered a marker of memory B cells from which WM is thought to derive,19,20 and it was found to be heterogeneously expressed in WM.19,29 Further studies are needed to confirm the high frequency of CD27 in WM with MYD88wild in a larger WM cohort and to elucidate the potential role of membrane CD27 in MYDwild WM pathogenesis. The MYDmut WM cells could arise from a “memory-like,” somatically mutated precursor that had lost memory markers, such as CD27, due to shedding from the cell surface in relation to the BM microenvironment stimuli.11,29
The role of the TLR pathway in promoting tumor survival and growth was established in several models, although not in WM.30 MYD88 is an adaptator protein through which most TLRs (except for TLR3), and IL-1 and IL-18 receptors, activate several signaling pathways.1 The high frequency of MYD88 mutation in WM highlights the TLR pathway as a new potential pathogenic pathway in WM, previously not identified using gene expression profiling.8,20 The L265P mutation occurred in the MYD88 Toll/IL-1 receptor domain, and the MYD88 L265P protein was shown to form a stable complex containing phosphorylated IRAK1/4 and to promote NF-κB and JAK-STAT3 signaling in ABC-DLBCL, conferring a selective advantage in cell survival.4 Ngo et al4 found that inhibition of MYD88 signaling decreased NF-κB activity and survival of ABC cell lines expressing MYD88 L265P. Our preliminary observations showed that blocking of MYD88 signaling using an inhibitor of MYD88 homodimerization induced cytotoxicity and apoptosis, and reduced cell proliferation, through decreased IRAK4 and STAT3 phosphorylation. These data also suggested that direct targeting of MYD88 signaling may provide a novel approach for the treatment of MW in the future, although future studies will need to confirm this hypothesis.
The MYD88 gene may be altered via several mechanisms in WM, beyond mutation of the L265P locus. We found that other processes might alter MYD88 in the MYDwild subgroup, as the most frequent genetic aberration observed in the MYDwild subgroup was gain of chromosome 3, suggesting a potential gene dosage effect, and not solely a specific gene disruption mechanism in the development of WM. However, gain of copy number of the MYD88 gene may also constitute an alternative mechanism of MYD88 alteration in WM. This preliminary observation needs to be confirmed in a larger cohort with high-throughput genomic analysis. Ngo et al4 reported an association between gain or amplification of the MYD88 locus and L265P mutation in ABC-DLBCL, indicating selection by the cancer cells for this mutant allele. Interestingly, we have described a novel alteration of MYD88 that combined UPD and mutation of MYD88 in 1 patient. Of interest, UPD suggested a potentiating mechanism of gene deregulation by duplication of an activating mutation in the MYD88 gene.31,32 This combined mechanism was rare in our series, as suggested in a previous whole exome–sequencing study in WM.9 Even if different oncogenic pathways are involved in these genetic alterations (either UPD or copy number variation) at the MYD88 locus, gain function mutation may be considered as a major genetic aberration in MW. The therapeutic perspective accompanying the discovery of the MYD88 mutation and of its importance in WM remains even in patients with homozygous MYD88 mutation, as exemplified by the similar cell death–based effect observed on the MYD88 mutated cell lines, either heterozygous or homozygous for the MYD88 mutation, by the MYD88 inhibitor.
MYD88 L265P mutation appears to be the most frequent mutation described to date in WM.9 MYD88 L265P mutation may be considered as the first genetic hit in WM that promotes NF-κB and JAK-STAT3 signaling and subsequently initiates alteration of major pathways, such as apoptotic pathways. Subsequently, WM cells may acquire additional genetic hits over time, mediated through LOH, gene amplification, or epigenetic changes that may potentially contribute to further deregulation of the WM clone and promote tumor progression. In our series, additional genetic alterations targeting NF-κB genes such as deletion of TNFAIP3, a negative regulator of NF-κB pathway, were observed in addition to MYD88 L265P mutation. Further studies will have to concentrate on whether other alterations, part of a complex genetic event including MYD88 mutation, back up MYD88 in driving normal B cells into WM tumoral cells and favor thereafter progression of WM tumoral B cells.
In conclusion, we have confirmed the high incidence of somatically acquired MYD88 L265P mutation in WM. Our study also confirmed that the MYD88 gene might undergo genetic alterations through other mechanisms, such as UPD, as a new pathogenic mechanism likely underlying WM pathogenesis, or gain of chromosome 3. The discovery of MYD L265P mutation might be an important breakthrough in the understanding of the pathogenesis of WM. Future studies will determine whether MYD L265P mutation might help with the diagnosis of WM. From a therapeutic standpoint, the signaling complex coordinated by MYD88 L265P might represent a new enticing target in WM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Anne Sophie Blanchis, Valérie Grandieres, Claudine Delsault, and Axelle Séghir for their technical assistance.
This work was supported by the Comité du Pas de Calais de la Ligue contre le Cancer.
Authorship
Contribution: S.P. and X.L. designed the overall study, performed research, collected data, performed data analysis and interpretation, and prepared and wrote the manuscript; C.R. performed flow cytometry, data analysis and interpretation, and manuscript preparation; E.B. and C.H. performed sampling preparation and survival experiments; A. Decambron performed flow cytometry; A. Daudignon, C.R.-L., O.N., V.S., and O.T. performed conventional cytogenetic and FISH analysis; X.L., C.H., and S.T. provided patient sampling and clinical data; A.R. and N.G. performed molecular study; S.P., C.P., B.Q., P.D., C.R., and X.L. performed final review of manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xavier Leleu, Service des Maladies du Sang, Hôpital Huriez, Centre Hospitalier Régional Universitaire, Lille, France; e-mail: xavier.leleu@chru-lille.fr.