Abstract
Bruton tyrosine kinase (Btk) has a well-defined role in B-cell development, whereas its expression in osteoclasts (OCs) further suggests a role in osteoclastogenesis. Here we investigated effects of PCI-32765, an oral and selective Btk inhibitor, on osteoclastogenesis as well as on multiple myeloma (MM) growth within the BM microenvironment. PCI-32765 blocked RANKL/M-CSF–induced phosphorylation of Btk and downstream PLC-γ2 in OCs, resulting in diminished TRAP5b (ED50 = 17nM) and bone resorption activity. PCI-32765 also inhibited secretion of multiple cytokines and chemokines from OC and BM stromal cell cultures from both normal donors (ED50 = 0.5nM) and MM patients. It decreased SDF-1–induced migration of MM cells, and down-regulated MIP1-α/CCL3 in MM cells. It also blocked MM cell growth and survival triggered by IL-6 or coculture with BM stromal cells or OCs in vitro. Importantly, PCI-32765 treatment significantly inhibits in vivo MM cell growth (P < .03) and MM cell–induced osteolysis of implanted human bone chips in SCID mice. Moreover, PCI-32765 prevents in vitro colony formation by stem-like cells from MM patients. Together, these results delineate functional sequelae of Btk activation mediating osteolysis and growth of MM cells, supporting evaluation of PCI-32765 as a novel therapeutic in MM.
Introduction
Multiple myeloma (MM) is a clonal malignancy of plasma cells accumulating in the BM. Myeloma cells have a high capacity to induce osteolytic bone lesions in patients, especially in the advanced stages.1 One key clinical feature of this cancer is the hyperactive bone resorption and minimal bone regeneration because of overactive osteoclasts (OCs) and inactive osteoblasts (OBs) via unbalanced regulation of cytokines and chemokines in the BM microenvironment.2 MM cells are highly dependent on the BM microenvironment for growth and survival through interactions particularly with BM stromal cells (BMSCs), OCs, and OBs, all of which secrete important MM growth factors and cytokines. Understanding and defining these BM factors are critical to provide the rationale to functionally target these factors and/or kinases as novel biologically based therapeutics for MM.
Bruton tyrosine kinase (Btk), a nonreceptor tyrosine kinase resembling the src family, plays a key role in the development and function of normal B cells through activation of the B-cell antigen receptor signaling pathway on binding to antigens.3 Btk is regulated by membrane recruitment via its pleckstrin homology domain, tyrosine residue 551 (Y551) in the activation loop, and Y223 auto-phosphorylation site in the SH3 domain.4 It further phosphorylates PLC-γ, leading to activation of MAPK, NFκB, and AKT signaling pathways. Mutations in the gene encoding Btk causes a B-cell defect, which manifests in boys during early childhood as X-linked agammaglobulinemia,5 a primary immunodeficiency originally described by Bruton in 1952. The Btk mutations in X-linked agammaglobulinemia disrupt Btk function and prevent B-cell maturation and secretion of immunoglobulins. Btk also contributes to the initiation and maintenance of B cell malignancies and autoimmune diseases.6 It is involved in myeloid cell function via immune complex stimulation of Fcγ receptor (FcγR) signaling.7 Most recently, Btk inhibition in macrophages was shown to abolish FcγRIII-induced TNF-α, IL-1β, and IL-6 production as well as block B-cell receptor-dependent B-cell proliferation via NF-κB activation, providing convincing evidence for Btk as a promising new therapeutic target in rheumatoid arthritis and B-cell lymphoma.8-14 Encouragingly, the irreversible Btk inhibitor PCI-32765 (IC50 = 0.5nM) has demonstrated clinical activity against a variety of B-cell malignancies in ongoing phase 1 or 2 trials, including mantle cell lymphoma, chronic lymphocytic leukemia, follicular lymphoma, and diffuse large B-cell lymphoma, with excellent tolerability.
A novel role for Btk was identified in OC differentiation. Btk is selectively expressed in OCs originating from BM-derived monocyte/macrophage precursor cells, but not OBs derived from mesenchymal lineage.15 In a genome-wide screening of mRNA for nonreceptor tyrosine kinases expressed during OC and OB differentiation in mice, high expression of Lyn and Syk, which are upstream of Btk, as well as Src, were identified in OCs. In addition, Btk regulates OC maturation by modulating the activity of NFATc1, the major OC transcriptional factor activated after RANKL stimulation.16 These recent findings prompted us to hypothesize a potential role of Btk in mediating osteolytic bone disease in MM.
Although Btk is expressed in all hematopoietic lineages except T and NK cells, it has not been studied in plasma cell cancers, including MM and Waldenström macroglobulinemia (WM). However, gene expression profiling showed robust Btk expression in malignant plasma cells from the majority of MM patients (> 85%) as well as lymphoplasmacytic cells from WM patients. We therefore here aimed to identify molecular mechanisms regulating OC function via Btk activation during MM-induced bone disease as well as to investigate the biologic significance of Btk in MM cells. We examined the potential therapeutic impact of PCI-32765 on OCs, OBs, and BMSCs in the BM microenvironment, as well as on MM cell lines and patient MM cells in vitro and in vivo. Our results demonstrate that Btk activation in the BM milieu promotes MM cell growth, survival, and interaction with other BM stromal components, in addition to triggering MM-induced bone lysis. Our studies therefore provide the framework for clinical development of PCI-32765 as a novel therapeutic strategy in MM.
Methods
Cell lines and patient sample processing
All CD138-expressing MM cell lines were grown in RPMI 1640 (Invitrogen) with 10% FBS (Hyclone), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). They were kindly provided by sources previously described,17,18 ATCC, or the German Collection of Microorganisms and Cell Cultures. Patient MM samples were obtained after informed consent was provided, in accordance with the Declaration of Helsinki and under the auspices of a Dana-Farber Cancer Institute Institutional Review Board–approved protocol. Primary CD138+ MM cells from patients were purified using positive selection with CD138-microbeads (Miltenyi Biotec). Residual CD138− BM mononuclear cells were cultured in RPMI 1640/10% FCS for 3 to 6 weeks to generate BMSCs.17,19,20
In vitro OC culture and determination of OC formation
CD14+ OC precursor cells were selected by monocyte-cell enrichment RS cocktail (StemCell Technologies) from peripheral blood mononuclear cells from normal donors or MM patients after informed consent.17,20 They were seeded in 96-well plates at 5 × 104 cells per well on plastic or 1.5 × 105 cells per well on dentine discs with DMEM/10% FCS supplemented with RANKL (50 ng/mL; PeproTech) and M-CSF (25 ng/mL; PeproTech), in the presence of serial dilutions of PCI-32765 (0-10μM; Pharmacyclics). OCs were identified by staining for tartrate-resistant acid phosphatase activity (TRAP), according to the manufacturers' instructions (Sigma-Aldrich). TRAP+ cells (> 3 nuclei and > 50 μm per cell) were considered to be mature OCs. In some experiments, they were also measured in a microplate reader (SpectraMax M2; Molecular Devices).
For immunofluorescence analysis, cells were fixed with 1% paraformaldehyde, permeabilized with 0.05% Triton, and stained with Alexa 568-nm Phalloidin (Invitrogen) for F-actin and 4,6-diamidino-2-phenylindole (Invitrogen) to detect nuclei. Confocal images were acquired using a LSM510 Zeiss confocal microscope and then analyzed by the LSM 5 Image browser software to delineate the OC margin and determine the area of spread. Cultures seeded on dentine discs were immersed in 1M NH4OH overnight to remove cells, washed in distilled water, dried, and stained with 0.1M Toluidine blue. Bright-field images of dried dentine discs were acquired using a Leica microscope attached to a Leica camera, using a 10× lens and processed with Photoshop to obtain a binary image. Bone pits were represented by black pixels. The percentage of black pixels occupied by resorption pits per field was determined using UTHSCSA Image Tool Version 3.0 software.
Cytotoxicity assays
The growth inhibitory and antisurvival effects of PCI-32765 (Pharmacyclics) on MM and WM cells, alone or in cocultures, were assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Sigma-Aldrich) dye absorbance, [3H]thymidine incorporation, and CellTiter-Glo Luminescent Cell Viability Assay (Promega).
Multiple cytokine/chemokine secretion assay
Cell culture supernatants collected from PCI-32765-treated OC cultures from normal donors or MM patients were subjected to ELISA specific for TRAP5b (Quidel), activin A (eBioscience), APRIL (eBioscience), BAFF (eBioscience), and a multiplexed immunoassay (Affymetrix) for other cytokines/chemokines.
Immunoblotting and immunoprecipitation analysis
Total cell lysates were subjected to 4% to 15% SDS-PAGE and transferred onto polyvinylidene fluoride membranes,18 followed by probing with specific Abs (Cell Signaling Technology). To show Btk phosphorylation after SDF-1 stimulation, cell lysates from MM cells were first immunoprecipitated with an anti-Btk Ab (Santa Cruz Biotechnology), and then immunoblotted with anti-pBtk Ab (Epitomics).
Cell adhesion and migration assays
Cell adhesion assays were done by adding MM cell lines or patient MM cells to BMSC-coated 6-well plates in the presence or absence of PCI-32765 (100nM) overnight and then examined with a Leica DM LB research microscope using Leica IM50 Image Manager (Leica Microsystems) with processing using Adobe Photoshop Version 7.0 software (Adobe Systems). Migration assays were performed in triplicate with transwell insert chambers (8 μm insert, Costar; Fisher Scientific) coated with 1 μg/mL sVCAM.21,22 The lower compartment contained SDF-1 (200 ng/mL; R&D Systems), and MM cells were added to the upper chamber in the presence of PCI-32765 and allowed to migrate for 4 hours at 37°C. The migration of control untreated cells on VCAM-1–coated transwells in the presence of SDF-1 was normalized to 100%.
Multiplex gene expression analysis
To study effects of PCI-32765 on gene expression in OC lineage cells and INA-6 MM cells, QuantiGene Plex Version 2.0 Assay was used according to manufacturer's protocol (Panomics, Affymetrix). In each experiment, 32 to 35 target genes were selected. PCI-32765–inhibited genes were further confirmed by real-time quantitative RT-PCR using specific primers from Applied Biosystems and the ABI Prism 7000 Sequence Detection System, with analysis using the ABI Prism 7000 SDS Version 1.0 software. Specific primers were used for MIP1-α (Hs00234142_m1), IL-8 (Hs00174103_m1), RANTES (Hs00174575_m1), TRAF2 (Hs00184192_m1), LAT (Hs01065378_g1), MYD88 (Hs01573837_g1), MIP1-β (Hs00237011_m1), BAFF (Hs00606874_g1), CXCR4 (Hs00607978_s1), TGF-β1 (Hs00998133_m1), PLC-γ2 (Hs00182192_m1), and NFATc1 (Hs00542678_m1). Gene expression was normalized using 18S (Hs99999901_s1) and HPRT (Hs99999909_m1) internal controls, which was compared with control vehicle to show relative percentage inhibition by PCI-32765.
SCID-hu model of human MM
All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee (Veterans Administration Boston Healthcare System). Human fetal bone grafts were implanted subcutaneously into CB-17 severe combined immunodeficient mice (SCID-hu mice; Taconic Farms), which were housed in our Animal Research Facility.23 Four weeks after bone implantation, 3 × 106 INA6 MM cells were injected directly into the human bone implant. Mouse sera were serially monitored for shuIL-6R by ELISA (R&D Systems) as a surrogate marker for tumor growth. Mice (n = 6 for each group) were treated with oral PCI-32765 (12 mg/kg) or control vehicle for 4 weeks after MM cell injection.
Histologic and immunohistochemistry analysis
Four weeks after treatment with PCI-32765, implanted human bones were retrieved, fixed in freshly prepared 4% paraformaldehyde overnight, washed with 70% ethanol, and analyzed for bone histology.24 Fixed specimens were demineralized with 14% EDTA in PBS. The specimens were processed and embedded in paraffin, and 5-μm sections were prepared for histologic analysis. Immunohistochemical staining was performed for CD138, alkaline phosphatase (ALP), and TRAP.
Micro-CT analysis
High-resolution CT scanning was performed using a GE RS150 small animal micro-CT scanner on implanted human bones containingINA-6 MM cells, which were retrieved from mice treated with either PCI-32765 or vehicle control for approximately 2 to 4 weeks. The acquisition parameters were: 70 kVp, 25 mA, 20-ms exposure time, 500 views more than 200 degrees. Three-dimensional isosurface renderings were generated from the CT scans using Amira 5.2 Visage Imaging processing software.12
Methylcellulose colony formation assays
CD138−CD34− BM mononuclear cells were treated, washed free of drug, and plated at a density of 2 × 105 cells/mL in 1.2% methylcellulose (R&D Systems), 30% FBS, 1% BSA, 10−4M 2-mercaptoethanol, 2mM l-glutamine, and 10% lymphocyte conditioned media as a source of growth factors, as previously described.25,26 Samples were plated in triplicate onto 35-mm2 tissue culture dishes. MM colonies consisting of more than 40 cells were scored at 14 to 21 days with an inverted microscope.
Statistical analysis
In vitro experiments were performed in triplicate and repeated at least 2 times; a representative experiment (mean ± SE) was selected for the figures, except when otherwise specified. Statistical significance of differences observed in PCI-32765 treated compared with control cultures was determined using 1-way ANOVA with Bonferroni posthoc comparison (for more than 3 groups) or a 2-tailed unpaired Student t test (for 2 groups) with all the analyses using Prism Version 4.0 (GraphPad Software). The minimal level of significance was P less than .05.
Lentiviral Btk shRNA transduction
Btk shRNA vs GFP control lentiviral particles (RNAi Consortium of Dana-Farber Cancer Institute18 ) were transduced into Btk-expressing MM cell lines, followed by adhesion assay 3 days after transduction. Trypan blue exclusion, Caspase-Glo 3/7 (Promega), and [3H]thymidine uptake were done 4 days after infection to assess impact on MM cell growth and survival.
Results
PCI-32765 blocks Btk-mediating osteoclastogenic signaling pathway and bone resorption activity
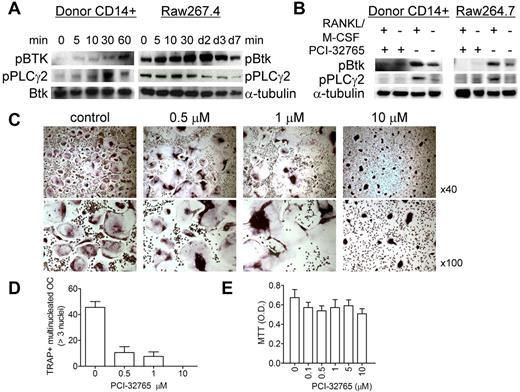
We first confirmed by immunoblotting that activation of Btk mediates osteoclastogenesis induced by M-CSF and RANKL in CD14+ OC precursor cells. Phosphorylation of Btk and its downstream PLC-γ2 was induced by M-CSF/RANKL in CD14+ monocytes from human donors and mouse RAW 264.7 cells (Figure 1A). Conversely, therapeutic Btk inhibitor PCI-32765 completely blocked baseline and induced phosphorylation of Btk and PLC-γ2 (Figure 1B). At the end of 2-week culture of M-CSF/RANKL–stimulated normal donor monocytes (donor number > 4) in plastic tissue culture plates, we observed a significant reduction in multinucleated mature OC numbers (TRAP+, > 3 nuclei and > 50 μm per OC cell, P < .01), as well as irregular TRAP staining pattern of multinucleated OCs influenced by PCI-32765, in a dose-dependent fashion (Figure 1C-E; supplemental Figures 1 and 2B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
PCI-32765 blocked Btk-mediating osteoclastogenic signaling pathway and impacted osteoclastogenesis. (A) CD14+ OC precursor cells (OCPs) from normal human donor or mouse raw267.4 cells were stimulated with RANKL/M-CSF for the indicated time intervals. Cell lysates were subjected to immunoblotting with antiphosphotyrosine antibodies. Anti–α-tubulin and -Btk mAbs served as loading controls. (B) OCPs were pretreated with (+) or without (−) PCI-32765 (100nM) for 2 hours before stimulation with RANKL/M-CSF. (C) TRAP staining was performed at 10 days of OC culture to identify mature OCs (> 3 nuclei, > 50 μm per cell; original magnification ×40 and ×100). (D) TRAP-positive multinucleated OCs were quantified (P < .01) and assayed by MTT (E).
PCI-32765 blocked Btk-mediating osteoclastogenic signaling pathway and impacted osteoclastogenesis. (A) CD14+ OC precursor cells (OCPs) from normal human donor or mouse raw267.4 cells were stimulated with RANKL/M-CSF for the indicated time intervals. Cell lysates were subjected to immunoblotting with antiphosphotyrosine antibodies. Anti–α-tubulin and -Btk mAbs served as loading controls. (B) OCPs were pretreated with (+) or without (−) PCI-32765 (100nM) for 2 hours before stimulation with RANKL/M-CSF. (C) TRAP staining was performed at 10 days of OC culture to identify mature OCs (> 3 nuclei, > 50 μm per cell; original magnification ×40 and ×100). (D) TRAP-positive multinucleated OCs were quantified (P < .01) and assayed by MTT (E).
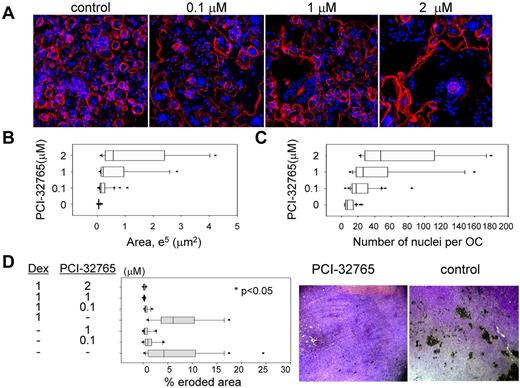
To further assess the effects of PCI-32765 on osteoclastogenesis, OC precursor cells from human donors were cultured on glass cover slips, followed by immunofluorescence staining for actin cytoskeleton and pit formation assay for bone resorption activity. Significantly reduced numbers of mature OCs were observed in PCI-32765 treated versus normal OC cultures (Figure 2A), in accordance with TRAP staining (Figure 1C). PCI-32765–impaired OCs had expanded spreading area per cell associated with increased number of nuclei per OC compared with normal OCs (Figure 2B-C, P < .01). Most importantly, PCI-32765 profoundly diminished bone erosion area formed by OCs cultured on dentine slices in pit formation assays, with or without dexamethasone (Figure 2D). Using Alizarin red quantitation to measure calcium deposition, minimal effects of PCI-32765 on OBs derived from human osteoprogenitor cells were observed; moreover, INA6 MM cell–suppressed OB function was not further impacted, even at higher PCI-32765 concentration (5μM, supplemental Figure 3), indicating that PCI-32765 specifically blocked Btk-mediated OC function, without affecting OBs.
PCI-32765 diminished bone resorption activity. (A) Human OCPs from normal donors were stimulated with RANKL/M-CSF and cultured on glass cover slips for 15 to 17 days, followed by immunofluorescence staining to observe OC morphology using Alexa-568–conjugated phalloidin (red) for actin and 4,6-diamidino-2-phenylindole (blue) for nuclei. (B) Abnormal OCs observed in panel A were further quantitated for extended spreading area per multinucleated OC (> 3 nuclei) and number of nuclei per OC (C). (D) OCPs were cultured on the dentine slice for 2 weeks, in the presence or absence of PCI-32765, alone or with dexamethasone (Dex), and analyzed for pit formation to determine percentage of bone erosion area. Images of representative bone resorption on dentine slices, with or without PCI-32765 treatment, are shown on the right (10× lens).
PCI-32765 diminished bone resorption activity. (A) Human OCPs from normal donors were stimulated with RANKL/M-CSF and cultured on glass cover slips for 15 to 17 days, followed by immunofluorescence staining to observe OC morphology using Alexa-568–conjugated phalloidin (red) for actin and 4,6-diamidino-2-phenylindole (blue) for nuclei. (B) Abnormal OCs observed in panel A were further quantitated for extended spreading area per multinucleated OC (> 3 nuclei) and number of nuclei per OC (C). (D) OCPs were cultured on the dentine slice for 2 weeks, in the presence or absence of PCI-32765, alone or with dexamethasone (Dex), and analyzed for pit formation to determine percentage of bone erosion area. Images of representative bone resorption on dentine slices, with or without PCI-32765 treatment, are shown on the right (10× lens).
Multiple MM- and OC-related cytokine/chemokine secretion is significantly down-regulated by PCI-32765
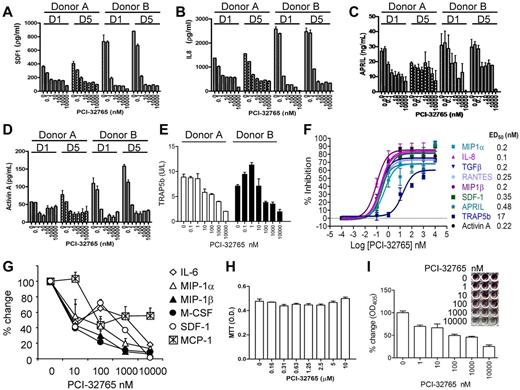
PCI-32765 consistently induced changes of cell supernatants in a dose-dependent manner in representative OC cultures from 2 normal donors, regardless of whether drug was added on day 1 when M-CSF/RANKL stimulation was started or on day 5 after cytokine stimulation (supplemental Figure 2A). Again, multinucleated TRAP+ mature OCs (> 3 nuclei per OC) were decreased in a dose-dependent fashion at the end of 2-week OC cultures (supplemental Figure 2B). Culture supernatants were collected and subject to multiplexed immunoassay for 10 cytokines, as well as specific ELISA for measuring APRIL, BAFF, activin A, and TRAP5b. Diverse MM- and OC-related growth factors, cytokines, and chemokines were potently inhibited by the drug (ED50 of ∼ 0.5nM; Figure 3A-D,F), including IL-8, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, TGF-β1, and SDF-1. PCI-32765 also markedly reduced APRIL (and BAFF, see Figure 6C at mRNA level), which is predominantly secreted by OC in the MM BM microenvironment,27 and activin A, which promotes MM-induced osteolysis and itself a target of other investigational agents.28 MCP-1 was also modestly down-regulated by PCI-32765 (supplemental Figure 2C). Moderate or low levels of sCD40L, IL-1β, and IL-6 were detected and were not significantly altered by PCI-32765. Importantly, TRAP5b, a specific osteoclastic TRAP isoform for bone resorption,29,30 was also down-regulated (ED50 of ∼ 17nM, Figure 3E-F), confirming TRAP IHC staining (supplemental Figure 2B).
PCI-32765 potently down-regulates cytokines/chemokine in supernatants from human OC and BMSC cultures from normal donors and MM patients. Supernatants collected at day 14 of human OC cultures treated with PCI-32765 starting on day 1 and day 5 were subjected to ELISA and Multiplex Luminex Assay (A-E). Many MM- and OC-related cytokines and chemokines were decreased (ED50 = 0.1-0.48nM). TRAP5b, a mature OC-specific marker, was also inhibited (ED50 = 17nM; F). (G) Survival of CD138-negative BMSCs from MM patients with active disease was measured by MTT assay, and supernatants assayed for secretion of cytokine/chemokines (H). (I) TRAP staining was performed at day 21 culture, visualized, and further measured using a spectrophotometer.
PCI-32765 potently down-regulates cytokines/chemokine in supernatants from human OC and BMSC cultures from normal donors and MM patients. Supernatants collected at day 14 of human OC cultures treated with PCI-32765 starting on day 1 and day 5 were subjected to ELISA and Multiplex Luminex Assay (A-E). Many MM- and OC-related cytokines and chemokines were decreased (ED50 = 0.1-0.48nM). TRAP5b, a mature OC-specific marker, was also inhibited (ED50 = 17nM; F). (G) Survival of CD138-negative BMSCs from MM patients with active disease was measured by MTT assay, and supernatants assayed for secretion of cytokine/chemokines (H). (I) TRAP staining was performed at day 21 culture, visualized, and further measured using a spectrophotometer.
Because the aforementioned cytokines/chemokines are also produced from BMSCs, we next examined whether PCI-32765 diminished secretion of these factors from BM stromal cell cultures derived from CD138− cells of MM patients with active disease. PCI-32765 decreased secretion of IL-6, MIP-1α, MIP1-β, SDF-1, M-CSF, and MCP-1, to a lesser extent, without affecting viability of BMSCs (Figure 3G-H). Furthermore, PCI-32765 decreased TRAP staining in a dose-dependent manner (Figure 3I). Therefore, PCI-32765 significantly inhibited cytokine/chemokine secretion and osteoclastogenesis from both MM patients and human normal donors, further validating the role of Btk in OC formation and function.
PCI-32765 is cytotoxic against Btk-expressing plasma tumor cells and abolishes MM cell growth and survival triggered by IL-6 and by coculture with OCs or BMSCs
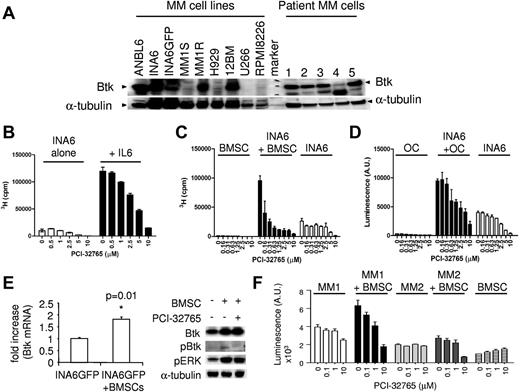
Because Btk is widely expressed at the mRNA level in patient MM cells (supplemental Figure 4A), we next confirmed its protein expression by immunoblotting. All CD138+ patient cells from 5 persons expressed Btk protein, whereas MM cell lines expressed Btk more heterogeneously (Figure 4A), correlating with results from microarray gene expression profiling. Dexamethasone-resistant MM1R cells express much higher Btk level than its parental dexamethasone-sensitive MM1S cells, consistent with a previous report.31 Importantly, PCI-32765 inhibited MM cell proliferation induced by IL-6 in the IL-6–dependent ANBL6 and INA6 MM cells (Figure 4B; supplemental Figure 4B). It also induced cytotoxicity against INA6 cells cocultured with patient BMSCs or OCs (Figure 4C-D). Further, Btk was up-regulated at both mRNA and protein levels in PCI-32765–responsive INA6GFP cells on binding to patient BMSCs (P = .01; Figure 4E; supplemental Figure 4C), supporting its biologic role in promoting MM cell growth, survival, and drug resistance in the BM microenvironment. Importantly, PCI-32765 induced cytotoxicity against CD138+ MM patient cells was enhanced in the coculture with BMSCs, whereas no toxicity was observed in BMSCs alone (Figure 4F). PCI-32765 also significantly blocked cell proliferation and viability, as well as induced apoptosis, in WM cells (supplemental Figure 4E-G). Thus, PCI-32765 induced direct cytotoxicity against Btk-expressing MM and WM tumor cells. Further, it remains active against MM cells stimulated with IL-6, BMSCs, or OCs.
PCI-32765 induces cytotoxicity against Btk-expressing MM cells stimulated by IL-6 or coculture with BMSCs or OCs. (A) Immunoblotting analysis showed Btk expression in CD138+ MM cell lines and patient MM cells (n = 5). (B) INA6 cells, with or without IL-6 (B) or BMSCs (C) or OC (D), prepared from MM patients were cultured with PCI-32765. (E) INA6-GFP cells were cocultured with (+) or without (−) BMSCs overnight, sorted by FACS, and subjected to mRNA and protein extraction for Btk levels by quantitative RT-PCR and immunoblotting. (F) PCI-32765 was added for 3 days to CD138+ cells from 2 representative MM patients, alone or with BMSCs, followed by a luminescent cell viability assay.
PCI-32765 induces cytotoxicity against Btk-expressing MM cells stimulated by IL-6 or coculture with BMSCs or OCs. (A) Immunoblotting analysis showed Btk expression in CD138+ MM cell lines and patient MM cells (n = 5). (B) INA6 cells, with or without IL-6 (B) or BMSCs (C) or OC (D), prepared from MM patients were cultured with PCI-32765. (E) INA6-GFP cells were cocultured with (+) or without (−) BMSCs overnight, sorted by FACS, and subjected to mRNA and protein extraction for Btk levels by quantitative RT-PCR and immunoblotting. (F) PCI-32765 was added for 3 days to CD138+ cells from 2 representative MM patients, alone or with BMSCs, followed by a luminescent cell viability assay.
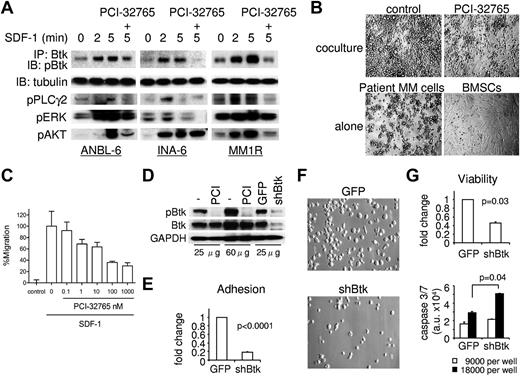
SDF-1–induced MM cell adhesion and migration is inhibited by PCI-32765 via blockade of Btk signaling cascade
Given that SDF-1 was greatly reduced in OC lineage cells and BMSCs by PCI-32765 treatment (Figure 3) and SDF-1 is known to trigger MM cell migration and homing,22,32 we next determined whether SDF-1 induced Btk activation in MM cells and, conversely, whether PCI-32765 blocked SDF-1–triggered adhesion and migration of MM cells via inhibition of Btk signaling cascade. By coimmunoprecipitation (IP) of cell lysates with anti-Btk antibody followed by immunoblotting with anti-pBtk antibody, we showed that SDF-1 induced phosphorylation of Btk in 4 MM cell lines in a time-dependent manner, whereas PCI-32765 specifically blocked Btk/PLC-γ signaling (Figure 5A; supplemental Figure 5A). Without IP, baseline Btk phosphorylation was detectable in cell lysates of ANBL6 and MM1R MM cells, which express high levels of Btk (Figure 6A). With IP, Btk phosphorylation was easily identified after SDF-1 stimulation in MM1S cells, which express low levels of Btk (supplemental Figure 5A). Thus, the intensity of Btk phosphorylation was correlated with Btk protein level. Importantly, PCI-32765 decreased SDF-1–induced cell adhesion to BMSCs and migration via VCAM in Btk-expressing MM cell lines and patient MM cells (Figure 5B-C; supplemental Figure 5B-C). Therefore, SDF-1 activates Btk in MM cells; conversely, SDF-1–induced MM adhesion and migration via Btk signaling cascade was abrogated by PCI-32765.
SDF-1–induced adhesion and migration in MM cells were inhibited by PCI-32765, and Btk directly regulates MM cell survival. (A) MM cells were preincubated with PCI-32765 (100nM, +) or control media; the drug was then washed out before stimulation with SDF-1 for 2 and 5 minutes. Lysates were immunoprecipitated (IP) with anti-Btk, and the IPs probed with anti-pBtk. Total cell lysates were further probed with indicated phopho-specific Abs and anti–α-tubulin as a loading control. (B) PCI-32765 was added to patient MM cells in overnight coculture with BMSCs. Images were taken using a Leica DFC300FX and Leica IM50 Image Manager (original magnification ×100). (C) CD138+ patient MM cells were treated with PCI-32765 and allowed to migrate in the absence (−) or presence of SDF-1 (200 ng/mL) in transwells coated with 1 μg/mL sVCAM-1. Bars represent the mean ± SD of triplicates. (D) MM1R cells were transduced with control GFP or shBtk lentiviruses; cell lysates were then prepared for immunoblotting. MM1R cells pretreated with (PCI) or without (−) PCI-32765 served as controls. (E) Three days after lentivirus infection, MM1R cells were labeled with calcein-AM and adherence to BMSCs was assayed in 2 hours. Four days after lentiviral transduction, cell images (F, original magnification ×400) were taken, and cell viability as well as caspase 3/caspase 7 activity (G) were determined.
SDF-1–induced adhesion and migration in MM cells were inhibited by PCI-32765, and Btk directly regulates MM cell survival. (A) MM cells were preincubated with PCI-32765 (100nM, +) or control media; the drug was then washed out before stimulation with SDF-1 for 2 and 5 minutes. Lysates were immunoprecipitated (IP) with anti-Btk, and the IPs probed with anti-pBtk. Total cell lysates were further probed with indicated phopho-specific Abs and anti–α-tubulin as a loading control. (B) PCI-32765 was added to patient MM cells in overnight coculture with BMSCs. Images were taken using a Leica DFC300FX and Leica IM50 Image Manager (original magnification ×100). (C) CD138+ patient MM cells were treated with PCI-32765 and allowed to migrate in the absence (−) or presence of SDF-1 (200 ng/mL) in transwells coated with 1 μg/mL sVCAM-1. Bars represent the mean ± SD of triplicates. (D) MM1R cells were transduced with control GFP or shBtk lentiviruses; cell lysates were then prepared for immunoblotting. MM1R cells pretreated with (PCI) or without (−) PCI-32765 served as controls. (E) Three days after lentivirus infection, MM1R cells were labeled with calcein-AM and adherence to BMSCs was assayed in 2 hours. Four days after lentiviral transduction, cell images (F, original magnification ×400) were taken, and cell viability as well as caspase 3/caspase 7 activity (G) were determined.
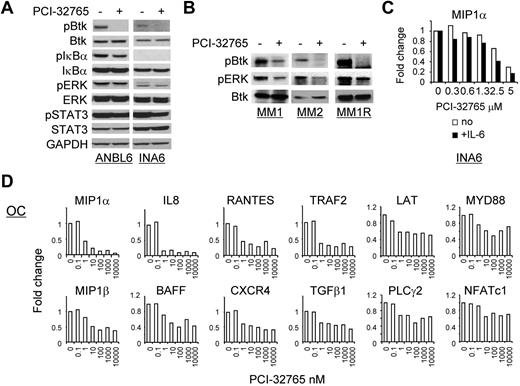
PCI-32765 significantly altered gene expression in MM cells and OC lineage cells stimulated with M-CSF/RANKL. (A) MM (ANBL6, INA-6) were treated with PCI-32765 (1μM) for an hour, and cell lysates were subjected to immunoblotting using specific Abs to determine the effects of the drug on Btk signaling cascade. (B) Patient MM cells (MM1, MM2) and MM1R cells were treated with PCI-32765. (C) INA6 cells, with or without IL-6, were treated with PCI-32765 overnight followed by real-time quantitative RT-PCR for MIP-1α/CCL3, which was normalized to HPRT as an internal control. Fold changes triggered by the drug were further normalized relative to the medium control. (D) Human donor OCPs stimulated with M-CSF/RANKL were treated with PCI-32765 for 7 days, and mRNA changes of these target genes were assayed by real-time quantitative RT-PCR using specific primers.
PCI-32765 significantly altered gene expression in MM cells and OC lineage cells stimulated with M-CSF/RANKL. (A) MM (ANBL6, INA-6) were treated with PCI-32765 (1μM) for an hour, and cell lysates were subjected to immunoblotting using specific Abs to determine the effects of the drug on Btk signaling cascade. (B) Patient MM cells (MM1, MM2) and MM1R cells were treated with PCI-32765. (C) INA6 cells, with or without IL-6, were treated with PCI-32765 overnight followed by real-time quantitative RT-PCR for MIP-1α/CCL3, which was normalized to HPRT as an internal control. Fold changes triggered by the drug were further normalized relative to the medium control. (D) Human donor OCPs stimulated with M-CSF/RANKL were treated with PCI-32765 for 7 days, and mRNA changes of these target genes were assayed by real-time quantitative RT-PCR using specific primers.
Btk knockdown significantly blocks MM cell adherence to BMSC and MM cell viability
To directly define the function of Btk in MM cells, its expression was knocked down with Btk shRNA (shBtk) lentivirus infection in Btk-expressing MM cells. Lentiviral shBtk down-regulated pBtk and Btk, as confirmed by immunoblotting in infected MM1R (Figure 5D), INA6, and ANBL6 MM cells (supplemental Figure 5D). Three days after lentiviral infection, MM1RshBtk transfectants showed significantly reduced adherence to BMSCs relative to control GFP cells (P < .0001; Figure 5E). Control MM1RGFP exhibited typical adherent morphology with protrusions, whereas MM1RshBtk transfectants did not (Figure 5F; supplemental Figure 5E). Furthermore, cell viability by trypan blue exclusion was decreased (P = .03), whereas caspase 3/caspase 7 activation (P = .04) was increased, in MM1RshBtk versus MM1RGFP transfectants (Figure 5G; supplemental Figure 5F). Btk knockdown also significantly reduced cell proliferation in ANBL6 cells (supplemental Figure 5G). Thus, Btk regulates MM cell growth and survival as well as adherence to BMSCs.
PCI-32765 affects gene expression in MM cells and OC lineage cells
We further studied whether PCI-32765 blocked Btk downstream signaling cascade in MM and WM cells. As expected, PCI-32765 inhibited baseline Btk phosphorylation in Btk-expressing MM cells (Figure 6A; supplemental Figure 6A) and WM cells (supplemental Figure 6B), shown by direct immunoblotting analysis. PCI-32765 further reduced baseline NFκB activation because pIκBα was decreased. Importantly, phosphorylated Btk was detected in CD138+ patient MM cells (n = 2); conversely, PCI-32765 blocked baseline Btk activity and pERK (Figure 6B). We next defined Btk downstream target genes in INA6 MM cells as well as OC lineage cells by multiple real-time quantitative RT-PCR analysis. In INA6 MM cells, MIP1-α was consistently reduced in a dose-dependent fashion, either in the presence or absence of IL-6 stimulation (Figure 6C). Decreased MIP-1α was also observed in PCI-32765–treated WM cells (supplemental Figure 6C). In addition, IL-6R/gp80 was decreased by PCI-32765 in INA6 cells (supplemental Figure 6D).
In OC lineage cells from 2 representative normal donors, M-CSF/RANKL up-regulated the majority of 32 selected genes, including Btk, Syk, RANK, NFATc1, TRAF2, NFκB, TGF-β1, BAFF, APRIL, MIP1-α, MIP-1β, and IL-8. Induction of Btk mRNA after M-CSF/RANKL activation is consistent with elevated Btk protein expresion as OCs mature in mice.16 Only 5 genes (PLC-γ1, BMX, BLK, IL-6, and RANKL) were either expressed at low levels or not induced after M-CSF/RANKL stimulation for either 1 or 7 days, confirming the purity of OC lineage cells (Table 1). Additional genes were induced after 7-day stimulation by M-CSF/RANKL. Importantly, PCI-32765 consistently down-regulated 12 genes (MIP1-α, MIP-1β, IL-8, BAFF, RANTES, TRAF2, CXCR4, TGF-β1, LAT, PLC-γ2, MYD88, and NFATc1) in OC lineage cells, which was further validated in an additional donor (Figure 6C). Thus, PCI-32765 potently blocked gene expression in OC lineage cells, consistent with inhibition of protein secretion. Furthermore, PCI-32765 reduced MIP1-α expression in MM cells, which could negatively feedback on OC activation in the BM microenvironment.
PCI-32765 affects gene expression in OC lineage cells activated with M-CSF/RANKL
| . | Donor 1 . | Donor 2 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Fold increase by M-CSF and RANKL . | % inhibition by PCI-32765 (10nM) . | Fold increase by M-CSF and RANKL . | % inhibition by PCI-32765 (10nM) . | |||||
| Day 1 . | Day 7 . | Day 1 . | Day 7 . | Day 1 . | Day 7 . | Day 1 . | Day 7 . | |
| Btk | 6.44 ± 0.83 | 14.18 ± 1.36 | 0 | 23.14 | 7.50 ± 0.62 | 11.51 ± 0.92 | 0 | 0 |
| Syk | 3.16 ± 0.79 | 16.38 ± 0.78 | 0 | 29.30 | 3.68 ± 0.14 | 6.99 ± 0.86 | 0 | 0 |
| RANK | 8.71 ± 2.63 | 13.05 ± 0.50 | 0 | 0 | 6.25 ± 0.12 | 6.89 ± 0.18 | 0 | 0 |
| TRAF2 | 11.43 ± 1.35 | 15.61 ± 0.71 | 0 | 75.05 | 11.35 ± 0.15 | 3.72 ± 0.20 | 0 | 16.13 |
| MAPK8 | 4.73 ± 0.91 | 4.6 ± 0.14 | 0 | 39.08 | 4.75 ± 0.26 | 3.56 ± 1.23 | 0 | 0 |
| BAFF | 2.25 ± 0.6 | 14.56 ± 0.36 | 0 | 54.32 | 3.5 ± 0.10 | 8.35 ± 0.72 | 0 | 20.74 |
| LAT | 3.5 ± 0.74 | 24.74 ± 0.39 | 0 | 59.78 | 3.77 ± 0.56 | 8.39 ± 0.49 | 0 | 18.51 |
| NFATc1 | 16.18 ± 2.10 | 29.37 ± 1.34 | 0 | 27.00 | 23.66 ± 1.19 | 15.23 ± 0.52 | 29.14 | 0 |
| I-κB | 4.23 ± 0.43 | 6.16 ± 0.31 | 0 | 36.49 | 4.87 ± 0.14 | 3.64 ± 0.72 | 0 | 0 |
| APRIL | 1.62 ± 0.34 | 4.21 ± 0.04 | 0 | 0 | 2.52 ± 0.14 | 4.32 ± 0.62 | 0 | 0 |
| RANTES | 1.29 ± 0.10 | 7.44 ± 0.15 | 0 | 70.98 | 0.97 ± 0.01 | 2.61 ± 0.23 | 0 | 0 |
| NF-κB1 | 5.97 ± 0.99 | 8.41 ± 0.08 | 0 | 0 | 7.32 ± 0.75 | 6.03 ± 0.40 | 0 | 0 |
| IL-8 | 20.91 ± 4.29 | 13.65 ± 0.45 | 0 | 87.00 | 13.64 ± 0.81 | 2.02 ± 0.17 | 32.12 | 0 |
| MAPK14 | 2.08 ± 0.03 | 3.22 ± 0.26 | 0 | 0 | 2.49 ± 0.09 | 2.08 ± 0.31 | 0 | 0 |
| IL-6* | 1.08 ± 0.05* | 0.96 ± 0.04* | 0 | 0 | 1.19 ± 0.08* | 1.05 ± 0.05* | 0 | 0 |
| MIP1-β | 15.65 ± 2.74 | 5.37 ± 0.88 | 59.91 | 87.00 | 7.52 ± 0.62 | 4.48 ± 0.35 | 80.92 | 47.65 |
| MIP1-α | 12.10 ± 2.64 | 29.03 ± 0.77 | 75.25 | 84.04 | 10.35 ± 1.09 | 7.83 ± 1.41 | 76.61 | 54.02 |
| STAT3 | 4.25 ± 0.60 | 14.53 ± 1.83 | 0 | 30.17 | 4.86 ± 0.07 | 6.41 ± 0.35 | 0 | 0 |
| CHUK | 5.60 ± 0.48 | 5.43 ± 0.24 | 0 | 35.60 | 7.25 ± 0.17 | 4.31 ± 0.08 | 0 | 0 |
| CXCR4 | 0.79 ± 0.16 | 17.27 ± 0.02 | 0 | 57.20 | 0.80 ± 0.04 | 6.66 ± 1.43 | 0 | 13.95 |
| TGF-β1 | 4.82 ± 0.65 | 24 ± 0.67 | 0 | 52.22 | 6.41 ± 0.28 | 12.30 ± 0.45 | 0 | 13.15 |
| MAPK1 | 2.99 ± 0.87 | 12.43 ± 0.17 | 0 | 25.60 | 2.30 ± 0.06 | 8.02 ± 1.27 | 0 | 0 |
| BLNK | 2.52 ± 0.02 | 4.38 ± 0.02 | 0 | 38.81 | 3.37 ± 0.43 | 2.56 ± 0.13 | 0 | 0 |
| MYD88 | 3.12 ± 0.37 | 7.82 ± 1.41 | 0 | 50.87 | 4.13 ± 0.00 | 5.07 ± 0.16 | 0 | 13.15 |
| AKT1 | 3.28 ± 0.37 | 10.43 ± 0.74 | 0 | 28.47 | 3.78 ± 0.09 | 7.43 ± 0.29 | 0 | 0 |
| TRAF6 | 3.56 ± 0.65 | 6.06 ± 1.26 | 0 | 27.92 | 4.40 ± 0.55 | 3.89 ± 1.04 | 0 | 0 |
| PLC-β2 | 4.07 ± 0.24 | NA | 0 | NA | 5.55 ± 0.28 | NA | 0 | NA |
| PLC-γ2 | NA | 12.71 ± 0.75 | NA | 46.84 | NA | 3.77 ± 0.05 | NA | 0 |
| BLK* | 1.02 ± 0.01* | 1.00 ± 0.08* | ND | ND | 0.97 ± 0.05* | 1.10 ± 0.2* | ND | ND |
| PLC-γ1* | 1.31 ± 0.06* | 1.48 ± 0.17* | ND | ND | 1.49 ± 0.18* | 2.11 ± 0.63* | ND | ND |
| BMX* | 1.00 ± 0.06* | 0.81 ± 0.01* | ND | ND | 0.81 ± 0.01* | 1.10 ± 0.03* | ND | ND |
| RANKL* | 0.99 ± 0.04* | 0.94 ± 0.05* | ND | ND | 1.04 ± 0.09* | 0.93 ± 0.06* | ND | ND |
| . | Donor 1 . | Donor 2 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Fold increase by M-CSF and RANKL . | % inhibition by PCI-32765 (10nM) . | Fold increase by M-CSF and RANKL . | % inhibition by PCI-32765 (10nM) . | |||||
| Day 1 . | Day 7 . | Day 1 . | Day 7 . | Day 1 . | Day 7 . | Day 1 . | Day 7 . | |
| Btk | 6.44 ± 0.83 | 14.18 ± 1.36 | 0 | 23.14 | 7.50 ± 0.62 | 11.51 ± 0.92 | 0 | 0 |
| Syk | 3.16 ± 0.79 | 16.38 ± 0.78 | 0 | 29.30 | 3.68 ± 0.14 | 6.99 ± 0.86 | 0 | 0 |
| RANK | 8.71 ± 2.63 | 13.05 ± 0.50 | 0 | 0 | 6.25 ± 0.12 | 6.89 ± 0.18 | 0 | 0 |
| TRAF2 | 11.43 ± 1.35 | 15.61 ± 0.71 | 0 | 75.05 | 11.35 ± 0.15 | 3.72 ± 0.20 | 0 | 16.13 |
| MAPK8 | 4.73 ± 0.91 | 4.6 ± 0.14 | 0 | 39.08 | 4.75 ± 0.26 | 3.56 ± 1.23 | 0 | 0 |
| BAFF | 2.25 ± 0.6 | 14.56 ± 0.36 | 0 | 54.32 | 3.5 ± 0.10 | 8.35 ± 0.72 | 0 | 20.74 |
| LAT | 3.5 ± 0.74 | 24.74 ± 0.39 | 0 | 59.78 | 3.77 ± 0.56 | 8.39 ± 0.49 | 0 | 18.51 |
| NFATc1 | 16.18 ± 2.10 | 29.37 ± 1.34 | 0 | 27.00 | 23.66 ± 1.19 | 15.23 ± 0.52 | 29.14 | 0 |
| I-κB | 4.23 ± 0.43 | 6.16 ± 0.31 | 0 | 36.49 | 4.87 ± 0.14 | 3.64 ± 0.72 | 0 | 0 |
| APRIL | 1.62 ± 0.34 | 4.21 ± 0.04 | 0 | 0 | 2.52 ± 0.14 | 4.32 ± 0.62 | 0 | 0 |
| RANTES | 1.29 ± 0.10 | 7.44 ± 0.15 | 0 | 70.98 | 0.97 ± 0.01 | 2.61 ± 0.23 | 0 | 0 |
| NF-κB1 | 5.97 ± 0.99 | 8.41 ± 0.08 | 0 | 0 | 7.32 ± 0.75 | 6.03 ± 0.40 | 0 | 0 |
| IL-8 | 20.91 ± 4.29 | 13.65 ± 0.45 | 0 | 87.00 | 13.64 ± 0.81 | 2.02 ± 0.17 | 32.12 | 0 |
| MAPK14 | 2.08 ± 0.03 | 3.22 ± 0.26 | 0 | 0 | 2.49 ± 0.09 | 2.08 ± 0.31 | 0 | 0 |
| IL-6* | 1.08 ± 0.05* | 0.96 ± 0.04* | 0 | 0 | 1.19 ± 0.08* | 1.05 ± 0.05* | 0 | 0 |
| MIP1-β | 15.65 ± 2.74 | 5.37 ± 0.88 | 59.91 | 87.00 | 7.52 ± 0.62 | 4.48 ± 0.35 | 80.92 | 47.65 |
| MIP1-α | 12.10 ± 2.64 | 29.03 ± 0.77 | 75.25 | 84.04 | 10.35 ± 1.09 | 7.83 ± 1.41 | 76.61 | 54.02 |
| STAT3 | 4.25 ± 0.60 | 14.53 ± 1.83 | 0 | 30.17 | 4.86 ± 0.07 | 6.41 ± 0.35 | 0 | 0 |
| CHUK | 5.60 ± 0.48 | 5.43 ± 0.24 | 0 | 35.60 | 7.25 ± 0.17 | 4.31 ± 0.08 | 0 | 0 |
| CXCR4 | 0.79 ± 0.16 | 17.27 ± 0.02 | 0 | 57.20 | 0.80 ± 0.04 | 6.66 ± 1.43 | 0 | 13.95 |
| TGF-β1 | 4.82 ± 0.65 | 24 ± 0.67 | 0 | 52.22 | 6.41 ± 0.28 | 12.30 ± 0.45 | 0 | 13.15 |
| MAPK1 | 2.99 ± 0.87 | 12.43 ± 0.17 | 0 | 25.60 | 2.30 ± 0.06 | 8.02 ± 1.27 | 0 | 0 |
| BLNK | 2.52 ± 0.02 | 4.38 ± 0.02 | 0 | 38.81 | 3.37 ± 0.43 | 2.56 ± 0.13 | 0 | 0 |
| MYD88 | 3.12 ± 0.37 | 7.82 ± 1.41 | 0 | 50.87 | 4.13 ± 0.00 | 5.07 ± 0.16 | 0 | 13.15 |
| AKT1 | 3.28 ± 0.37 | 10.43 ± 0.74 | 0 | 28.47 | 3.78 ± 0.09 | 7.43 ± 0.29 | 0 | 0 |
| TRAF6 | 3.56 ± 0.65 | 6.06 ± 1.26 | 0 | 27.92 | 4.40 ± 0.55 | 3.89 ± 1.04 | 0 | 0 |
| PLC-β2 | 4.07 ± 0.24 | NA | 0 | NA | 5.55 ± 0.28 | NA | 0 | NA |
| PLC-γ2 | NA | 12.71 ± 0.75 | NA | 46.84 | NA | 3.77 ± 0.05 | NA | 0 |
| BLK* | 1.02 ± 0.01* | 1.00 ± 0.08* | ND | ND | 0.97 ± 0.05* | 1.10 ± 0.2* | ND | ND |
| PLC-γ1* | 1.31 ± 0.06* | 1.48 ± 0.17* | ND | ND | 1.49 ± 0.18* | 2.11 ± 0.63* | ND | ND |
| BMX* | 1.00 ± 0.06* | 0.81 ± 0.01* | ND | ND | 0.81 ± 0.01* | 1.10 ± 0.03* | ND | ND |
| RANKL* | 0.99 ± 0.04* | 0.94 ± 0.05* | ND | ND | 1.04 ± 0.09* | 0.93 ± 0.06* | ND | ND |
CD14+ OC precursor cells from 2 normal donors were plated (6 × 104 cells per well) in 96-well plates and stimulated with M-CSF/RANKL for 1 and 7 days in the presence or absence of PCI-32765 (10nM). Cells were harvested and subject to QuantiGene Expression Assay to determine gene expression at each condition. Expression levels were normalized with the internal control HPRT. % inhibition on gene expression by PCI-32765 was determined as follows: % inhibition = 100 × [1 − (expression with PCI-32765 and cytokine stimulation)/(expression with cytokine stimulation)].
0 indicates no inhibition; NA, not available; and ND, not determined due to insignificant expression level.
Gene expression is neglected because of low level.
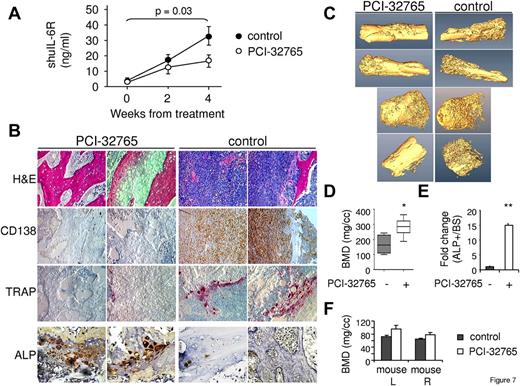
PCI-32765 has anti-MM activity in vivo and decreases MM-induced bone lysis in the SCID-hu model of human MM
To investigate whether Btk inhibition by PCI-32765 could suppress MM cell growth and overcome MM-induced osteolysis in vivo, we used a mouse model of MM bone disease, the SCID-hu model.23,33 MM cell growth was quantitated by measuring soluble IL6R (sIL6R) secreted by INA-6 MM cells in murine blood, and mice were treated with PCI-32765 after first detection of tumor growth. Continuous PCI-32765 (12 mg/kg) treatment significantly inhibited MM cell growth after 4 weeks (P = .03), indicating in vivo antitumor activity of PCI-32765. Histologic analysis and immunohistochemistry for CD138 and TRAP staining confirmed decreased numbers of MM cells and reduced bone resorption activity in the human bones retrieved from PCI-32765–treated mice compared with the control group (Figure 7B). Furthermore, ALP expression, an enzyme marker of OBs and osteogenesis, was significantly more prominent in implanted human bone tissues from PCI-32765 versus control mice (P < .01; Figure 7B,E; supplemental Figure 7), indicating increased bone formation activity in PCI-32765–treated mice. These results confirmed that PCI-32765 blocked MM cell growth in vivo, associated with decreased MM-induced bone lysis. High-resolution micro-CT scan performed on the human bone chips retrieved from these mice further demonstrated that MM-induced bone lysis was significantly ameliorated after PCI-32765 treatment (Figure 7C-D). No adverse effect of PCI-32765 was observed in normal mouse bones (Figure 7E). Indeed, PCI-32765 slightly increased BMD in normal mouse extremities.
PCI-32765 inhibits MM cell growth and MM-induced bone lysis in a murine model of human MM. (A) SCID-hu mice were injected with INA-6 MM cells into the implanted human bone and continuously treated with PCI-32765 (12 mg/kg, n = 6) or vehicle control (n = 5) beginning after first detection of tumor by monitoring shuIL-6R in mouse serum samples weekly. (B) Bone chips were retrieved from SCID-hu mice, decalcified, and sectioned. Tissue slides were stained with H&E and immunohistochemically analyzed for CD138 (MM), TRAP (OC), and ALP (OB). Original magnification ×200, except for ALP (original magnification ×400). (C) Representative cross-sectional images by 3-dimensional reconstruction of the harvested human bones obtained after performing high-resolution micro-CT scan are shown and quantified (D). *P < .04. (E) Osteogenic activity per bone surface (ALP+/BS), indicating bone formation activity. **P < .01. The PCI-32765-treated group displayed significantly reduced osteolysis induced by MM cells and enhanced osteogenic activity, compared with vehicle control group. Effects of PCI-32765 were also quantitated in the left (mouse L) and right (mouse R) normal mouse extremities (F).
PCI-32765 inhibits MM cell growth and MM-induced bone lysis in a murine model of human MM. (A) SCID-hu mice were injected with INA-6 MM cells into the implanted human bone and continuously treated with PCI-32765 (12 mg/kg, n = 6) or vehicle control (n = 5) beginning after first detection of tumor by monitoring shuIL-6R in mouse serum samples weekly. (B) Bone chips were retrieved from SCID-hu mice, decalcified, and sectioned. Tissue slides were stained with H&E and immunohistochemically analyzed for CD138 (MM), TRAP (OC), and ALP (OB). Original magnification ×200, except for ALP (original magnification ×400). (C) Representative cross-sectional images by 3-dimensional reconstruction of the harvested human bones obtained after performing high-resolution micro-CT scan are shown and quantified (D). *P < .04. (E) Osteogenic activity per bone surface (ALP+/BS), indicating bone formation activity. **P < .01. The PCI-32765-treated group displayed significantly reduced osteolysis induced by MM cells and enhanced osteogenic activity, compared with vehicle control group. Effects of PCI-32765 were also quantitated in the left (mouse L) and right (mouse R) normal mouse extremities (F).
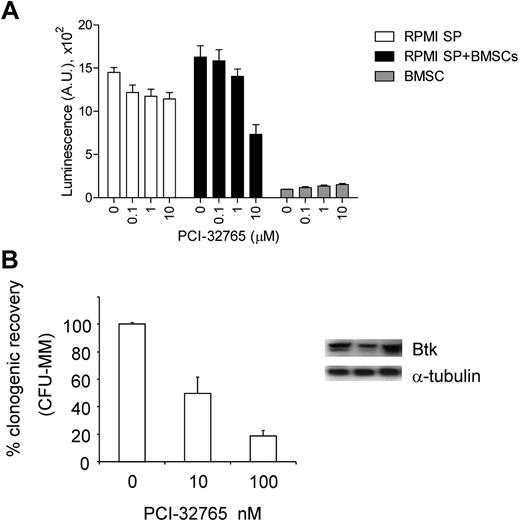
PCI-32765 inhibits clonogenic myeloma stem-like cells from MM patients
We finally assessed Btk expression in clonogenic tumor cells from MM patients because this stem-like cell subpopulation is hypothesized to be more resistant to chemotherapies, contribute to tumor relapse, and be responsible for disease maintenance. Myeloma stem-like cells purified from RPMI8226 (RPMI SP) and BMMCs of MM patients (n = 5) were monitored by luminescence cell viability assay and colony formation assay in methylcellulose, respectively, in the presence or absence of PCI-32765. PCI-32765 inhibited cell viability of RPMI SP cells cocultured with BMSCs in a dose-dependent manner, whereas BMSCs were spared (Figure 8A). Further, immunoblotting analysis demonstrated Btk expression in MM stem-like cells and PCI-32765 suppressed their potential to form colonies (Figure 8B).
PCI-32765 inhibited MM stem-like cells. (A) Stem-like PRMI8226 MM cells (RPMI SP) sorted by flow cytometry were incubated with PCI-32765 in the presence or absence of BMSCs for 3 days, followed by a luminescent cell viability assay. (B) Clonogenic stem-like cells, purified from MM patient (n = 5) BMMCs by immunomagnetic depletion of CD138+ plasma cells and CD34+ normal hematopoietic progenitors, were subjected to colony formation assays in methylcellulose in the presence of PCI-32765. Btk expression in such clonogenic populations from 3 MM patients was shown by immunoblotting.
PCI-32765 inhibited MM stem-like cells. (A) Stem-like PRMI8226 MM cells (RPMI SP) sorted by flow cytometry were incubated with PCI-32765 in the presence or absence of BMSCs for 3 days, followed by a luminescent cell viability assay. (B) Clonogenic stem-like cells, purified from MM patient (n = 5) BMMCs by immunomagnetic depletion of CD138+ plasma cells and CD34+ normal hematopoietic progenitors, were subjected to colony formation assays in methylcellulose in the presence of PCI-32765. Btk expression in such clonogenic populations from 3 MM patients was shown by immunoblotting.
Discussion
Because MM expands in the BM and generates devastating bone lesions, it is critical to better understand how MM cells interact with the BM microenvironment and to further elucidate molecular mechanisms controlling osteolysis. Our current study is the first report to show that Btk plays a crucial role in myeloma-associated osteolysis via regulating a broad panel of cytokines and chemokines both at transcriptional and protein levels in OC lineage cells and BMSCs, which are in close contact with MM cells within the BM microenvironment. These Btk-targeted cytokines and chemokines include MIP1-α,34,35 MIP1-β,34,36 SDF-1,37,38 TGF-β1,39 activin A,28 APRIL,27,40,41 BAFF,19,24,27,42 and IL-8,43,44 which have been shown to contribute to MM-related bone lesions and disease progression. Significantly, PCI-32765 blocked secretion of these factors from OC and BM stromal cultures derived from MM patients. In addition, M-CSF, an early OC lineage cell growth and survival factor during OC differentiation, was potently and consistently inhibited by PCI-32765 in cultures of BM stromal cells from MM patients. Importantly, PCI-32765 further impaired osteoclastogenesis and suppressed bone resorption activity.
Btk protein is expressed in MM and WM tumor cells, with baseline activation correlating with levels of protein expression. Btk signaling cascade is further induced in MM cells by SDF-1, which regulates adhesion, migration, and homing of MM cells to the BM microenvironment. Conversely, PCI-32765 blocked these effects. In addition, MIP-1α secretion from MM cells was inhibited by PCI-32765, confirming direct Btk-mediated regulation of MIP1-α in MM cells. Importantly, PCI-32765 blocked MM bone destruction, as well as MM cell growth and survival, in a SCID-hu model of human MM. Moreover, PCI-32765 suppressed the clonogenicity of MM stem-like cells from MM patients, indicating the therapeutic potential to target Btk in this chemo-resistant subpopulation to achieve long-term remission. Therefore, PCI-32765 targets both MM cells and the BM microenvironment, providing the framework for targeting Btk to improve patient outcome in MM and WM.
We have identified a significant array of MM- and OC-related cytokines/chemokines regulated by Btk activation in human OC and BM stromal cell cultures. In the BM stromal cell cultures after 2 to 4 weeks without further cytokine addition, cells remained primarily fibroblast-like without Btk expression. Thus, no cytotoxicity was observed by PCI-32765 treatment against these cells, even though cytokine/chemokine secretion was significantly reduced. Furthermore, PCI-32765 did not adversely affect function of dendritic cells derived from monocytes stimulated with IL-4 and GM-CSF, suggesting minimal toxicity against these monocyte-derived antigen-presenting cells (supplemental Figure 8). Finally and importantly, PCI-32765 mediated activity against OCs while sparing OBs and bone formation.
A significant number of genes critical to OC function and interaction with MM cells were also inhibited by PCI-32765, which correlated with decreased protein secretion. Btk mRNA was markedly increased during osteoclastogenesis, further confirming its role in OC differentiation. MIP1-α, IL-8, MIP1-β, TGF-β1, RANTES, BAFF, and APRIL were consistently down-regulated by PCI-32765, with ED50 of approximately 0.5nM, at both mRNA and protein levels. PCI-32765–inhibited NFATc1 transcription is also associated with decreased TRAP5b protein secretion (ED50 of ∼ 20nM), further confirming the effects of PCI-32765 on OC differentiation and function. Purified human osteoclastic TRAP is specifically the 5b isoform. Therefore, in addition to TRAP IHC staining, we also performed TRAP5b ELISA to verify osteolytic function and number of OC. PCI-32765 significantly reduced Btk-regulated cytokines and chemokines from differentiated OC precursors, indicating that PCI-32765 blocked not only OC differentiation but also secretion of important cytokines from differentiated OC lineage cells. These results further suggest that TRAP5b might serve as a useful biomarker in future clinical trials to evaluate effect of PCI-32765 on MM-related bone disease. We observed that PCI-32765 induced abnormal giant OCs with inactive bone erosion activity. Such giant OCs resemble those reported after long-term oral bisphosphonate therapy,45,46 representing detached OCs with protracted apoptosis. In our study, PCI-32765 did not significantly inhibited survival of OC in vitro, assayed by MTT in short-term cultures; after longer incubation, it may induce significant apoptosis in OC. In addition, the observed abnormal morphology of PCI-32765–affected OCs may be associated with deregulated Btk-mediated interaction with cytoskeletal proteins47 because a 10-amino acid sequence at the N-terminus of Btk contains an F-actin binding site.4 Our results indicate that PCI-32765 caused uncontrolled fusion of immature OC leading to aberrant organization of the actin cytoskeleton critical for bone resorption. Finally, bone erosion activity on dentine slices in PCI-32765–treated OC culture was severely decreased compared with control group, confirming that PCI-32765 induced dysfunctional OCs. These results are in accordance with impaired lacunar in vitro resorption by OCs from human Btk-deficient patients because of deregulation of actin cytoskeletal function.48 However, these XLA patients do not exhibit increased bone density or alterations in serum markers of bone turnover because of the compensatory mechanisms that up-regulate levels of inflammatory cytokines IL-6, IL-1β, and TNF-α.48 In contrast, PCI-32765 profoundly blocks multiple cytokines important for OC function and osteoclastogenic activity in vivo. Indeed, in the SCID-hu model of human MM where MM-induced osteolysis on human bone is recapitulated in SCID mice, PCI-32765 demonstrated significant anti-MM activity and ameliorated lytic bone disease.
This study is the first to identify baseline Btk activation in MM and WM cells. Significant constitutive levels of phosphorylated Btk were seen in MM cells (ANBL6, 12BM, and MM1R) and WM cells with high Btk expression. Moreover, we identify SDF-1 as a Btk-regulated BM factor, which in turn can activate Btk signaling, which regulates MM cell adhesion and migration. In addition, Btk can associate with gp130 to activate IL-6 signaling pathway in the murine pro-B cell line, even in the absence of ligand.49 It is similarly possible that baseline Btk activation was associated with interaction with gp130 in MM and WM cells; moreover, IL-6 associated with gp130 could also stimulate Btk phosphorylation in MM and WM cells. This may explain why IL-6– or stromal cell–dependent MM cells were more sensitive to PCI-32765 than growth factor-independent MM cells in our current study.
In conclusion, we have shown that Btk plays a pathogenic role in MM-related osteolytic bone disease, as well as growth and survival of MM and WM in the BM microenvironment. Many of the Btk-regulated cytokines and chemokines identified here have already been shown to be attractive therapeutic targets for treating MM patients. Furthermore, Btk controls MM cancer stem cells, indicating that targeting Btk might reduce chemoresistance and risk of recurrence. Our results therefore provide the preclinical basis for clinical evaluation of PCI-32765 to simultaneously decrease bone complications and improve patient outcome in MM and related diseases.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michelle Chen, Daniel Tannenbaum, and Komal Sane for excellent technical support for cell culture and MM patient sample maintenance; Samantha Pozzi for her input on IHC studies; Mei Zhang for IHC analysis at Pathology Department in Brigham and Women's Hospital, Boston, MA; and Padmaja Magadala at Pharmacyclics for performing the migration assays.
This work was supported by the National Institutes of Health (grants RO-1 50947, PO1-78378, and DF/HCC SPORE in Multiple Myeloma P50CA100707), the Lebow Fund to Cure Myeloma (K.C.A.), and DF/HCC Myeloma SPORE Career Development Award (Y.-T.T.).
National Institutes of Health
Authorship
Contribution: Y.-T.T., B.Y.C., J.J.B., and K.C.A. conceptualized research and formed the hypothesis; Y.-T.T., B.Y.C., S.-Y.K., M.F., G.Y., and Y.C. designed and performed experiments and analyzed data; Y.C. validated immunofluorescence and bone resorption assay; Y.H., J.L., J.-J.Z., A.C., M.C., M.A.S., M.Y.Z., and C.A. performed the in vitro research and collected data; M.F. and N.C.M. designed, performed, and analyzed animal work; B.Y.C., Y.C., D.R.C., J.-J.Z., J.J.B., and L.E. provided reagents, analytic tools, micro-CT scan analysis, and input to studies; Q.W. and W.M. designed and performed clonogenic myeloma cell work; G.Y. and S.P.T. performed and analyzed data for WM work; P.R., N.C.M., and K.C.A. provided MM patient samples; Y.-T.T. wrote the manuscript; and Y.-T.T., B.Y.C., Y.C., L.E., J.J.B., and K.C.A. critically evaluated and edited the manuscript.
Conflict-of-interest disclosure: B.Y.C., J.J.B., and L.E. are employees of Pharmacyclics, Inc, whose product was used in this research. S.P.T. received research funding for a clinical protocol from Pharmacyclics. P.R. serves on advisory boards to Millennium, Celgene, Novartis, Johnson & Johnson, and Bristol-Myers Squibb. N.C.M. serves on advisory boards to Millennium, Celgene, and Novartis. K.C.A. serves on advisory boards to Millennium, Onyx, and MannKind. The remaining authors declare no competing financial interests.
Correspondence: Yu-Tzu Tai, Department of Medical Oncology, Dana-Farber Cancer Institute, M551, 450 Brookline Ave, Boston, MA 02215; e-mail: yu-tzu_tai@dfci.harvard.edu.