Abstract
The JAK2V617F mutation has been detected in most cases of Ph-negative myeloproliferative neoplasms (MPNs). The JAK2V617F protein is a constitutively activated tyrosine kinase that leads to transformation of hematopoietic progenitors. Previous studies have shown that several tyrosine residues within JAK2 are phosphorylated on growth factor or cytokine stimulation. However, the role of these tyrosine residues in signaling and transformation mediated by JAK2V617F remains unclear. In this study, we sought to determine the role of tyrosine 201, which is a potential binding site for Src homology 2 domain-containing proteins, in JAK2V617F-induced hematopoietic transformation by introducing a tyrosine-to-phenylalanine point mutation (Y201F) at this site. We observed that the Y201F mutation significantly inhibited cytokine-independent cell growth and induced apoptosis in Ba/F3-EpoR cells expressing JAK2V617F. The Y201F mutation also resulted in significant inhibition of JAK2V617F-mediated transformation of hematopoietic cells. Biochemical analyzes revealed that the Y201F mutation almost completely inhibited constitutive phosphorylation/activation of JAK2V617F. We also show that the Y201 site of JAK2V617F promotes interaction with Stat5 and Shp2, and constitutive activation of downstream signaling pathways. Furthermore, using a BM transduction/transplantation approach, we found that tyrosine 201 plays an important role in the induction of MPNs mediated by JAK2V617F.
Introduction
The somatic JAK2V617F mutation has been found in most patients with polycythemia vera (PV) and 50%-60% of patients with essential thrombocythemia and primary myelofibrosis.1-5 The JAK2V617F mutant is a constitutively active tyrosine kinase that can transform factor-dependent hematopoietic cell lines to cytokine independence.1,2,6 Expression of JAK2V617F in the BM and spleen of mice results in erythropoietin (Epo)–independent erythroid colonies,6-9 indicating that JAK2V617F mutant can transform erythroid progenitors. Several studies using BM transplantation, transgenic, or knock-in mouse models of Jak2V617F have shown that Jak2V617F is sufficient to induce PV7-16 and may contribute to the pathogenesis of essential thrombocythemia and primary myelofibrosis.8,13,14,17
Expression of JAK2V617F mutant activates multiple downstream signaling pathways, such as Stat5, Stat3, Erk/MAP kinase and PI3 kinase/Akt pathways.1,6,9 Lu et al have suggested that coexpression of a homodimeric type I cytokine receptor, such as Epo receptor (EpoR), is required for JAK2V617F-mediated transformation of hematopoietic cells.18 Subsequent studies by the same group have found that higher level of JAK2V617F expression can circumvent the requirement of a homodimeric type I cytokine receptor for transformation.19
JAK2 belongs to the Janus family of nonreceptor protein tyrosine kinases, which also includes JAK1, JAK3, and TYK2. JAK2 serves as a key mediator of cytokine receptor signaling and plays an important role in hematopoiesis.20-22 All JAK family members contain 7 JAK homology domains (JH1-JH7), which include a catalytically active kinase domain (JH1) and a pseudo-kinase domain (JH2). It has been suggested that the JH2 domain plays a negative autoregulatory role in that deletion of the JH2 domain results in constitutive activation of JAK2 kinase.23 It has recently been shown that the JH2 pseudo-kinase domain negatively regulates cytokine signaling by phosphorylating negative regulatory sites.24 JAK2 also contains a FERM domain in the N-terminal region (JH4-JH7), which is required for association with cytokine receptors.25 Previous studies have suggested a requirement for the intact FERM domain for JAK2V617F-induced transformation.26,27 In addition, JAK2 contains an SH2-like domain, which has been shown to be required for transphosphorylation of JAK2V617F.27 Phosphorylation of tyrosine 1007 in the activation loop of the kinase domain is required for JAK2 kinase activity.28 Several other tyrosine residues, including Tyr221, Tyr570, Tyr317, Tyr637, Tyr813, Tyr868, Tyr966, Tyr972, Tyr913, Tyr372, and Tyr201, in JAK2 are also phosphorylated in response to growth factors and cytokines.29-37 Phosphorylations of these tyrosine residues have been shown to either positively or negatively regulate JAK2 kinase activity. However, the contributions of these tyrosine residues in the constitutive activation and transformation mediated by JAK2V617F remain unknown.
Tyrosine 201 (Y201) of JAK2 has been shown to be phosphorylated in response to angiotensin II signaling.37 Y201 of JAK2 lies within the YXX(L/V/I/M) motif, which is a potential binding site for the SH2 domain-containing proteins. We and other investigators have observed that Stat5 and Shp2 are constitutively phosphorylated in cells expressing JAK2V617F.1,2,6,9 In normal EpoR signaling, Stat5 is recruited to specific tyrosine residues within the cytoplasmic region of EpoR and becomes phosphorylated by JAK2.38 It has been found that Stat5 could be phosphorylated by JAK2V617F in Ba/F3 cells expressing EpoR mutant with all the tyrosines mutated to phenylalanines.19 This raises the possibility that a receptor-independent mechanism for activation of Stat5 by JAK2V617F may also exist. In addition, it has been shown that the N-terminal SH2 domain of Shp2 directly binds to JAK2 through tyrosine 201.37 Therefore, we hypothesized that SH2 domain-containing proteins, such as Stat5 and Shp2, might be recruited to JAK2V617F through tyrosine 201 and become phosphorylated by JAK2V617F.
In this study, we sought to determine the role of tyrosine 201 in constitutive activation of JAK2V617F and hematopoietic transformation mediated by JAK2V617F by introducing a tyrosine-to-phenylalanine point mutation (Y201F) at this site. We observed that Y201F mutation almost completely inhibited the constitutive activation of JAK2V617F, significantly attenuated the binding of Shp2 and Stat5 with JAK2V617F, and inhibited activation of downstream signaling pathways. In addition, we demonstrated that tyrosine 201 is required for efficient transformation of hematopoietic cells and induction of MPNs evoked by JAK2V617F.
Methods
Plasmids and reagents
The cDNA encoding murine JAK2 was obtained from Dr James Ihle (St Jude Children's Hospital, TN). JAK2V617F, JAK2V617F/Y201F, and JAK2/Y201F mutants were generated using the Quick Change site-directed mutagenesis kit (Stratagene) and subcloned into MSCV-IRES-GFP or pCDNA3.1 vector. The monoclonal anti-phosphotyrosine antibody (4G10) was purchased from Millipore. The antibodies directed against phospho-JAK2 (Y1007/Y1008), phospho-Stat5 (Y694), phospho-Shp2 (Y542), phospho-Akt, phospho-Erk1/2, and phospho-p70S6 kinase were purchased from Cell Signaling Technologies, and the antibodies against total JAK2, Stat5, Shp2, Akt, Erk2, and p70S6K were obtained from Santa Cruz Biotechnology.
Cell culture, proliferation, and apoptosis assay
Ba/F3-EpoR cells were maintained in RPMI-1640 medium containing 10% FBS and IL-3 (1 ng/mL). To generate Ba/F3-EpoR cells with stable expression of different JAK2 mutants, Ba/F3-EpoR cells were transduced with MSCV-IRES-GFP retroviruses expressing JAK2WT, JAK2/Y201F, JAK2V617F or JAK2V617F/Y201F, and sorted for GFP-positive cells. For proliferation assays, cells were washed 3 times with RPMI-1640 medium containing 10% FBS and then seeded in a 96-well plate (2.5 × 103 per well) in triplicates. Cell proliferation was measured in the absence or presence of Epo (3 U/mL) at 24 hours, 48 hours, and 72 hours after plating by WST assay using a Quick Cell Proliferation Assay Kit (Biovision). Optical density was measured at 440 nm and 650 nm using a plate reader.
For cell viability measurement, 1.5 × 105 cells were cultured in RPMI 1640 medium with 10% FBS in the absence of cytokine. Viable cell number was assessed by trypan blue exclusion every 24 hours for 5 days.
Apoptosis of cells was determined by annexin V staining. The cells were cultured in RPMI 1640 plus 10% FBS in the absence of cytokine for 48 hours. Cells were harvested and stained with allophycocyanin-conjugated annexin V and propidium iodide according to the manufacturer's instructions (eBioscience) and analyzed by flow cytometry.
Colony-forming assay
Ba/F3-EpoR cells expressing different JAK2 mutants were plated (250 cells/dish) in duplicates in methylcellulose medium (M3231, StemCell Technologies) without any cytokine. Cytokine-independent colonies were counted 5 days after plating. To determine the colony forming unit-erythroid (CFU-E) colonies, spleen cells (1 × 105) from the transplanted mice were plated in methylcellulose medium without any cytokine (Methocult M3234, StemCell Technologies). CFU-E colonies were counted after 2 days in culture after staining with benzidine solution (Sigma-Aldrich).
Immunoblotting and immunoprecipitation
For immunoblot analysis, Ba/F3-EpoR cells expressing different JAK2 mutants were starved for 6 hours in RPMI 1640 medium containing 0.5% BSA at 37°C. Cells were washed with PBS and lysed in a radioimmunoprecipitation assay (RIPA) buffer (50mM Tris-HCl, 150mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2mM Na3VO4, 5mM NaF, 100 μg/mL PMSF, and protease inhibitor cocktail; Sigma-Aldrich). Total proteins were measured using the Bio-Rad Bradford protein assay kit, and equal amounts of proteins were separated by SDS-PAGE. Immunoblotting was performed using phospho-specific JAK2, Stat5, Shp2, p70S6 kinase, Akt, and Erk1/2 antibodies (obtained from Cell Signaling Technology) as indicated. To control for loading, blots were reprobed with antibodies against corresponding total proteins. Blots were developed using enhanced chemiluminescent substrate. The images were obtained using a Bio-Rad ChemiDoc imager, and relative quantification of the phospho-proteins was performed using Image Lab Version 4.0 software (Bio-Rad).
For immunoprecipitation, cells were harvested, washed with cold PBS, and lysed in a buffer containing 1% Triton X-100, 50mM Tris-HCl, 150mM NaCl, 2mM Na3VO4, 5mM NaF, 100 μg/mL PMSF, and protease inhibitor cocktail (Sigma-Aldrich). A total of 1 mg of total protein was incubated with protein A-Sepharose beads and respective antibodies for 4 hours. The bound beads were washed 5 times in lysis buffer, boiled 2 times in sample buffer, and separated by SDS-PAGE, followed by immunoblotting with anti-phosphotyrosine, anti-JAK2, anti-Stat5, or anti-Shp2 antibody.
Retroviral transduction and transplantation
High-titer retroviral stocks of MSCV-IRES-GFP vector, MSCV-JAK2WT-IRES-GFP, MSCV-JAK2V617F-IRES-GFP, and MSCV-JAK2V617F/Y201F-IRES-GFP were prepared by transient transfection of 293T cells as described previously.39 Retroviral titers were determined in Ba/F3 cells by analyzing GFP-positive cells using flow cytometry 2 days after transduction. BM cells from 5-fluorouracil–primed Balb/c mouse were transduced with equal titer retroviruses expressing vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F by 2 rounds of spin infection.39 Transduced BM cells (106) were injected into tail veins of lethally irradiated (2 × 550 cGy) Balb/c recipient mice. Mice were maintained on acidified water. All animal studies were approved by the Committee for the Humane Use of Animals of State University of New York Upstate Medical University.
Flow cytometry
Single-cell suspensions were prepared from the spleen, and red cells were lysed with red cell lysis solution. Cells were washed and resuspended in PBS plus 2% FBS, and stained for 20 minutes on ice with directly conjugated (either PE or allophycocyanin) monoclonal antibodies against erythroid (Ter119, CD71) and myeloid (Mac-1, Gr-1) cell surface markers. Flow cytometry was performed with an LSRII (BD Biosciences) and analyzed by using FlowJo Version 8.8.6 software (TreeStar).
Southern blot analysis
Genomic DNA was extracted from the BM of transplanted mice. A total of 12 μg of genomic DNA was digested with BglII and was subjected to agarose gel electrophoresis and transferred to Hybond N membrane (GE Healthcare) according to standard protocol. The DNA was hybridized with a GFP probe (isolated from MSCV-IRES-GFP vector by NcoI and SalI digestion) labeled with radioactive [32P] dCTP, as previously described.12
Blood and tissue analysis
Peripheral blood counts were determined using Hemavet 950FS (Drew Scientific). For histopathologic analysis, mouse tissue specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin.
Statistical analysis
Results are expressed as mean ± SEM, and data were analyzed by ANOVA or Student t test using GraphPad Version 5 software. P < .05 was considered to be statistically significant.
Results
JAK2V617F requires Y201 site for cytokine-independent growth and survival of Ba/F3-EpoR cells
It has been shown that MPN-associated JAK2V617F mutant can transform factor-dependent hematopoietic cell lines to cytokine independence.1,2 Several tyrosine residues, including Y201 in JAK2, are phosphorylated in response to growth factors and cytokines.29-37 However, the role of Y201 in constitutive activation of JAK2V617F and cellular transformation mediated by JAK2V617F remains unknown.
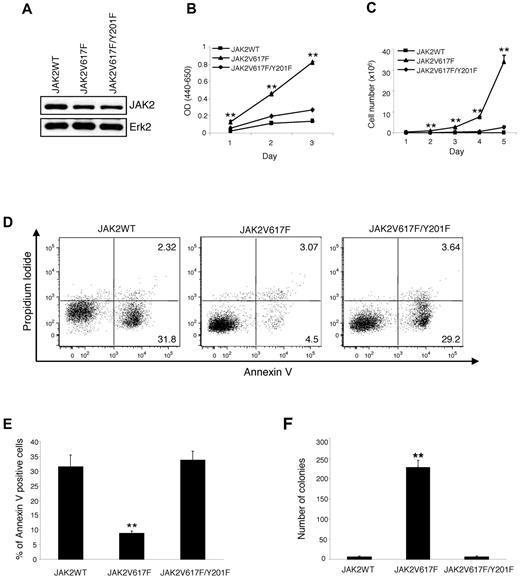
To examine the role of Y201 in hematopoietic transformation mediated by JAK2V617F, we established Ba/F3-EpoR cells stably expressing wild-type JAK2 (JAK2WT), JAK2V617F, or JAK2V617F/Y201F. For this purpose, Ba/F3-EpoR cells were infected with MSCV-IRES-GFP–based retroviruses expressing JAK2WT, JAK2V617F, or JAK2V617F/Y201F and sorted for GFP. We used pools of sorted cells instead of individual clone to avoid clonal variation in JAK2 protein expression. As shown in Figure 1A, total JAK2 protein level was comparable between cells expressing JAK2V617F and JAK2V617F/Y201F mutants. However, JAK2WT protein expression was slightly higher than the mutant JAK2 proteins (Figure 1A). Next, we assessed the effects of wild-type and mutant JAK2 expression on proliferation of hematopoietic Ba/F3-EpoR cells in the absence of cytokine. As expected, Ba/F3-EpoR cells expressing JAK2WT failed to proliferate in the absence of cytokine (Figure 1B-C). Whereas expression of JAK2V617F resulted in cytokine-independent proliferation of Ba/F3-EpoR cells, the JAK2V617F/Y201F mutant was defective in inducing cytokine-independent proliferation of Ba/F3-EpoR cells (Figure 1B-C). Y201F mutation in JAK2, however, did not alter the rate of cell growth compared with JAK2WT-expressing cells in the presence of Epo (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These results suggest that Y201 is required by JAK2V617F for cytokine-independent proliferation of hematopoietic cells.
JAK2V617F requires tyrosine 201 for cytokine-independent proliferation and transformation of Ba/F3-EpoR cells. Ba/F3-EpoR cells stably expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were used. (A) JAK2 protein expression was assessed in these cell lines by Western blot. (B) Cytokine-independent cell proliferation was determined over 3 days using the WST assay. (C) Total numbers of viable cells were counted over 5 days in the absence of cytokine by trypan blue exclusion. In both cases, JAK2V617F-induced cytokine-independent cell proliferation was significantly inhibited by the Y201F mutation. Results shown are representative of 3 independent experiments. Data are mean ± SEM. (D) Flow cytometric analysis shows a marked increase in apoptosis (annexin V+) in Ba/F3-EpoR cells expressing JAK2V617F/Y201F compared with those expressing JAK2V617F 2 days after cytokine withdrawal. Representative dot plots from 3 independent experiments are shown. (E) Percentages of annexin V+ cells from 3 independent experiments are shown in bar graphs as mean ± SEM. Noticeably, the population undergoing apoptosis (annexin V+) in Ba/F3-EpoR cells expressing JAK2V617F/Y201F was significantly higher than those expressing JAK2V617F and identical to those expressing JAK2WT. (F) The Y201F mutation remarkably inhibited the transformation induced by JAK2V617F. Ba/F3-EpoR cells expressing different JAK2 mutants (2.5 × 102 cells/dish) were plated in duplicate in methylcellulose medium without any cytokine. Colonies were counted after 5 days. Data are mean ± SEM from 3 independent experiments. **P < .005 (1-way ANOVA).
JAK2V617F requires tyrosine 201 for cytokine-independent proliferation and transformation of Ba/F3-EpoR cells. Ba/F3-EpoR cells stably expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were used. (A) JAK2 protein expression was assessed in these cell lines by Western blot. (B) Cytokine-independent cell proliferation was determined over 3 days using the WST assay. (C) Total numbers of viable cells were counted over 5 days in the absence of cytokine by trypan blue exclusion. In both cases, JAK2V617F-induced cytokine-independent cell proliferation was significantly inhibited by the Y201F mutation. Results shown are representative of 3 independent experiments. Data are mean ± SEM. (D) Flow cytometric analysis shows a marked increase in apoptosis (annexin V+) in Ba/F3-EpoR cells expressing JAK2V617F/Y201F compared with those expressing JAK2V617F 2 days after cytokine withdrawal. Representative dot plots from 3 independent experiments are shown. (E) Percentages of annexin V+ cells from 3 independent experiments are shown in bar graphs as mean ± SEM. Noticeably, the population undergoing apoptosis (annexin V+) in Ba/F3-EpoR cells expressing JAK2V617F/Y201F was significantly higher than those expressing JAK2V617F and identical to those expressing JAK2WT. (F) The Y201F mutation remarkably inhibited the transformation induced by JAK2V617F. Ba/F3-EpoR cells expressing different JAK2 mutants (2.5 × 102 cells/dish) were plated in duplicate in methylcellulose medium without any cytokine. Colonies were counted after 5 days. Data are mean ± SEM from 3 independent experiments. **P < .005 (1-way ANOVA).
Next, we examined the effects of Y201F mutation on growth factor-independent survival/apoptosis of Ba/F3-EpoR cells as measured by annexin V and propidium iodide staining. We observed significant apoptosis in Ba/F3-EpoR cells expressing JAK2WT within 48 hours of cytokine withdrawal (Figure 1D-E). Expression of JAK2V617F resulted in increased survival and decreased apoptosis in Ba/F3-EpoR cells in the absence of cytokine (Figure 1D-E). In contrast, Ba/F3-EpoR cells expressing JAK2V617F/Y201F exhibited marked apoptosis in the absence of cytokine (Figure 1D-E), indicating that Y201F mutation in JAK2V617F rendered Ba/F3-EpoR cells to be cytokine-dependent.
We also determined the effects of Y201F on JAK2V617F-evoked transformation of hematopoietic cells using a colony-forming assay. As expected, Ba/F3-EpoR cells expressing JAK2WT failed to give rise to cytokine-independent colonies (Figure 1F). Expression of JAK2V617F resulted in a large number of cytokine-independent colonies, whereas JAK2V617F/Y201F mutant was defective in inducing cytokine-independent colonies (Figure 1F). Together, these results strongly suggest an important role for Y201 in hematopoietic transformation mediated by JAK2V617F.
Constitutive activation of JAK2V617F and downstream signaling pathways are inhibited by the Y201F mutation
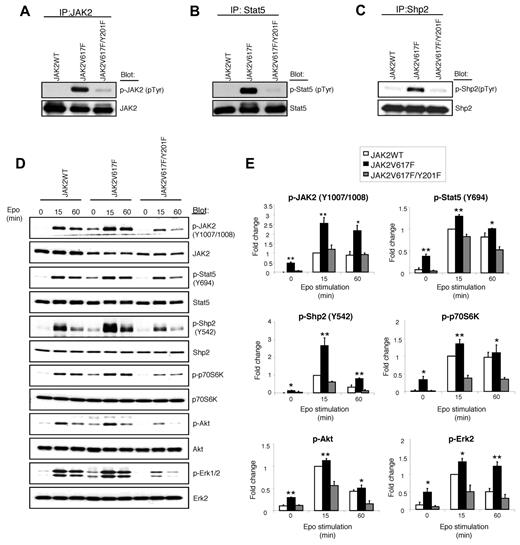
In normal hematopoietic cells, JAK2 becomes phosphorylated on ligand binding to the cytokine receptors. JAK2V617F mutant protein and several downstream signaling molecules, including Stat5 and Shp2, are constitutively phosphorylated in cells expressing JAK2V617F.1,6,9 Therefore, we determined the effects of Y201F mutation on tyrosyl phosphorylation of JAK2, Stat5, and Shp2. As expected, JAK2V617F, but not JAK2WT, was phosphorylated in Ba/F3-EpoR cells in the absence of cytokine (Figure 2A). Y201F mutation, however, blocked the constitutive phosphorylation of JAK2V617F (Figure 2A). Y201F mutation also markedly inhibited the constitutive phosphorylation of Stat5 and Shp2 mediated by JAK2V617F (Figure 2B-C).
Tyrosine 201 is required for constitutive activation of JAK2V617F and its downstream signaling. (A-C) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were deprived of cytokine and serum for 6 hours. Tyrosyl phosphorylation of JAK2 (A), Stat5 (B), and Shp2 (C) was detected by immunoprecipitation with specific antibodies against JAK2, Stat5, and Shp2, followed by immunoblotting with phosphotyrosine antibody (4G10). Membranes were reprobed with total antibodies. Note that constitutive tyrosine phosphorylation of JAK2, Stat5, and Shp2 induced by JAK2V617F was significantly inhibited by the Y201F mutation. (D) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were cytokine and serum-starved followed by stimulation with Epo (3 U/mL) for the indicated times. Cell lysates were prepared and directly immunoblotted with phospho-specific antibodies or total antibodies as indicated. (E) Histograms demonstrate the fold changes in phosphorylation of JAK2, Stat5, Shp2, p70S6K, Akt, and Erk2 compared with the phosphorylation levels in cells expressing JAK2WT. All the data are normalized for the JAK2WT value at 15 minutes, which is set to 1. Data from 3 independent experiments are shown as mean ± SEM. *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA). Notably, Y201F mutation markedly inhibited the activation of JAK2V617F and downstream signaling pathways, including Stat5, Shp2, p70S6 kinase, Akt, and Erk.
Tyrosine 201 is required for constitutive activation of JAK2V617F and its downstream signaling. (A-C) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were deprived of cytokine and serum for 6 hours. Tyrosyl phosphorylation of JAK2 (A), Stat5 (B), and Shp2 (C) was detected by immunoprecipitation with specific antibodies against JAK2, Stat5, and Shp2, followed by immunoblotting with phosphotyrosine antibody (4G10). Membranes were reprobed with total antibodies. Note that constitutive tyrosine phosphorylation of JAK2, Stat5, and Shp2 induced by JAK2V617F was significantly inhibited by the Y201F mutation. (D) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, and JAK2V617F/Y201F were cytokine and serum-starved followed by stimulation with Epo (3 U/mL) for the indicated times. Cell lysates were prepared and directly immunoblotted with phospho-specific antibodies or total antibodies as indicated. (E) Histograms demonstrate the fold changes in phosphorylation of JAK2, Stat5, Shp2, p70S6K, Akt, and Erk2 compared with the phosphorylation levels in cells expressing JAK2WT. All the data are normalized for the JAK2WT value at 15 minutes, which is set to 1. Data from 3 independent experiments are shown as mean ± SEM. *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA). Notably, Y201F mutation markedly inhibited the activation of JAK2V617F and downstream signaling pathways, including Stat5, Shp2, p70S6 kinase, Akt, and Erk.
We also assessed the effects of Y201F on phosphorylation of JAK2, Stat5, and Shp2 by using phospho-specific antibodies. Similar to our observation on total tyrosyl phosphorylation of JAK2 (in Figure 2A), we observed no phosphorylation of JAK2WT at Y1007/Y1008 sites in the absence of Epo (Figure 2D-E). However, Epo stimulation resulted in phosphorylation of JAK2WT at these sites. As expected, JAK2V617F was constitutively phosphorylated at Y1007/Y1008, and Epo stimulation further enhanced phosphorylation at these sites. Strikingly, Y201F mutation significantly inhibited phosphorylation of JAK2V617F at Y1007/Y1008 sites (Figure 2D-E). Constitutive phosphorylation of Stat5 (at Y694) and Shp2 (at Y542) induced by JAK2V617F was also significantly inhibited by the Y201F mutation (Figure 2D-E). Together, these results clearly indicate that Y201 is required for constitutive activation of JAK2V617F.
Next, we assessed the effects of Y201F mutation on downstream signaling mediated by JAK2V617F. We and other investigators have shown that Akt, Erk, and p70S6 kinase pathways are activated in response to JAK2V617F.1,6,9 Whereas JAK2V617F expression in Ba/F3-EpoR cells resulted in constitutive activation of Akt, p70S6 kinase, and Erk1/2, Y201F mutation markedly inhibited the constitutive activation of Akt, p70S6 kinase, and Erk1/2 induced by JAK2V617F (Figure 2D-E). However, Y201F mutation in the context of JAK2WT did not show any significant defect in signaling in response to Epo (supplemental Figure 2). These results suggest that Y201 is more important for oncogenic JAK2V617F-induced signaling than normal JAK2WT-mediated signaling.
The Y201F mutation prevents the interaction of JAK2V617F with Stat5 and Shp2
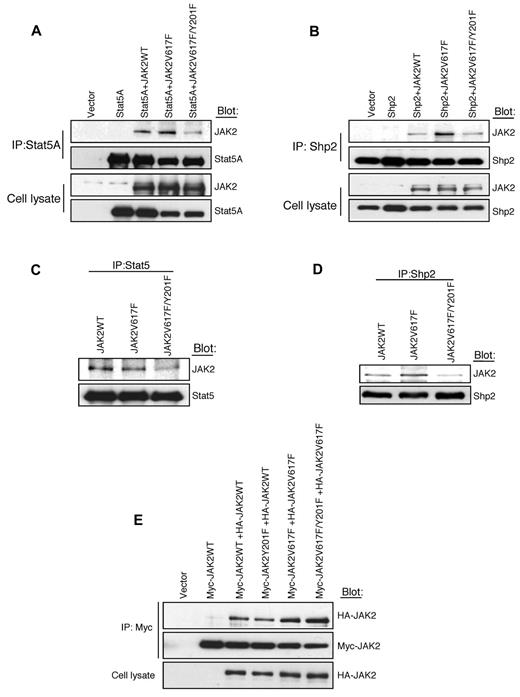
Because Y201 is a potential binding site for SH2 domain-containing protein(s)37 and Y201F mutation inhibits JAK2V617F-induced phosphorylation of Stat5 and Shp2 (Figure 2B-E), we sought to determine whether JAK2V617F directly interacts with Stat5 and/or Shp2 through the Y201 site. To address this question, we cotransfected Stat5A or Shp2 along with JAK2WT, JAK2V617F, or JAK2V617F/Y201F mutant into 293T cells and performed coimmunoprecipitation experiments. As shown in Figure 3A, Stat5A directly interacted with JAK2WT or JAK2V617F, but the interaction between JAK2V617F and Stat5A was markedly reduced by the Y201F mutation. Similarly, Shp2 interacted with JAK2WT or JAK2V617F in 293T cells (Figure 3B). The interaction of Shp2 with JAK2V617F was more pronounced than with JAK2WT. However, Y201F mutation significantly inhibited the interaction between Shp2 and JAK2V617F (Figure 3B).
Tyrosine 201 promotes interaction of Stat5 and Shp2 with JAK2V617F. (A-B) JAK2WT, JAK2V617F, or JAK2V617F/Y201F was coexpressed with Stat5A (A) or Shp2 (B) into 293T cells. Stat5A or Shp2 was immunoprecipitated from cell lysates using antibodies against Stat5A (A) or Shp2 (B). Coprecipitated JAK2, Stat5A, and Shp2 were determined by immunoblotting using specific antibodies as indicated. (C-D) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, or JAK2V617F/Y201F were cytokine and serum deprived before harvesting. Cell lysates were immunoprecipitated by an anti-Stat5 (C) or anti-Shp2 antibody (D) and subjected to immunoblotting with the indicated antibodies. Note that the Y201F mutation significantly inhibited the interaction between JAK2V617F and Stat5 as well as the interaction between JAK2V617F and Shp2. (E) Y201 is not required for dimerization of JAK2WT or JAK2V617F. The 293T cells were cotransfected with Myc- and HA-tagged JAK2WT or different JAK2 mutants as indicated. Myc-tagged JAK2WT or mutants were efficiently coimmunoprecipitated with HA-tagged JAK2WT or JAK2 mutants. Notably, Y201F mutation did not affect the self-association or dimerization of JAK2WT or JAK2V617F.
Tyrosine 201 promotes interaction of Stat5 and Shp2 with JAK2V617F. (A-B) JAK2WT, JAK2V617F, or JAK2V617F/Y201F was coexpressed with Stat5A (A) or Shp2 (B) into 293T cells. Stat5A or Shp2 was immunoprecipitated from cell lysates using antibodies against Stat5A (A) or Shp2 (B). Coprecipitated JAK2, Stat5A, and Shp2 were determined by immunoblotting using specific antibodies as indicated. (C-D) Ba/F3-EpoR cells expressing JAK2WT, JAK2V617F, or JAK2V617F/Y201F were cytokine and serum deprived before harvesting. Cell lysates were immunoprecipitated by an anti-Stat5 (C) or anti-Shp2 antibody (D) and subjected to immunoblotting with the indicated antibodies. Note that the Y201F mutation significantly inhibited the interaction between JAK2V617F and Stat5 as well as the interaction between JAK2V617F and Shp2. (E) Y201 is not required for dimerization of JAK2WT or JAK2V617F. The 293T cells were cotransfected with Myc- and HA-tagged JAK2WT or different JAK2 mutants as indicated. Myc-tagged JAK2WT or mutants were efficiently coimmunoprecipitated with HA-tagged JAK2WT or JAK2 mutants. Notably, Y201F mutation did not affect the self-association or dimerization of JAK2WT or JAK2V617F.
We also determined the endogenous interaction of JAK2WT or JAK2V617F with Stat5 or Shp2 in Ba/F3-EpoR cells. Stat5 was coimmunoprecipitated with JAK2WT or JAK2V617F in Ba/F3-EpoR cells (Figure 3C). The interaction between JAK2V617F and Stat5 was inhibited by the Y201F mutation (Figure 3C). Similarly, the interaction between Shp2 and JAK2V617F was also inhibited by the Y201F mutation (Figure 3D). Thus, Y201 site promotes interaction of JAK2V617F with Stat5 and Shp2. However, Y201F mutation did not completely block the interaction of JAK2V617F with Stat5 and Shp2.
To determine the role of Y201 in self-association or dimerization of JAK2, we cotransfected HA- and Myc-tagged JAK2 constructs in 293T cells and performed coimmunoprecipitation experiments. We found that HA-tagged JAK2WT or JAK2V617F efficiently coimmunoprecipitated with Myc-tagged JAK2WT or JAK2V617F respectively (Figure 3E). Y201F mutation did not have any effect on interaction between Myc- and HA-tagged JAK2WT or JAK2V617F (Figure 3E), suggesting that Y201 site is not required for self-association or dimerization of JAK2WT or JAK2V617F mutant.
Y201 is required by JAK2V617F for induction of myeloproliferative disease in mice
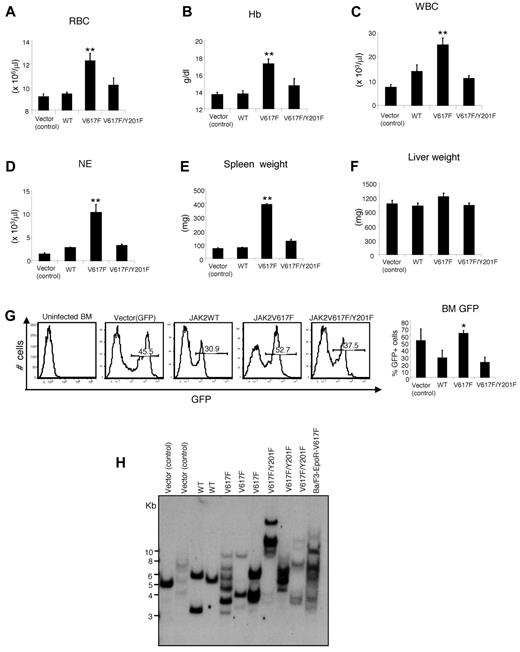
Retroviral expression of JAK2V617F in BM cells results in a PV-like MPN in mice.7,10-12 To examine the in vivo role of Y201 in JAK2V617F-induced transformation and MPNs, we performed retroviral BM transduction and transplantation assays. High-titer retroviruses expressing GFP vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F mutants were produced. Viral titers for vector (control), JAK2WT, JAK2V617F, and JAK2V617F/Y201F expressing retroviruses were assessed in Ba/F3 cells by flow cytometry using GFP expression (supplemental Figure 3). Comparable titer retroviruses were used for the BM transplantation assays. Expression of JAK2V617F in the BM of the transplanted mice resulted in marked increases in red blood cell, hemoglobin, white blood cell, and neutrophil counts in the peripheral blood of transplanted animals compared with mice expressing GFP vector or JAK2WT (Figure 4A-D), as observed previously.7,10-12 In contrast, JAK2V617F/Y201F mutant expression markedly reduced the peripheral blood parameters, and those were more comparable with that observed in mice expressing JAK2WT (Figure 4A-D). JAK2V617F expression also resulted in splenomegaly in the transplanted animals (Figure 4E). Spleen size in mice expressing JAK2V617F/Y201F was much smaller than that observed in JAK2V617F-transplanted mice and was more comparable with that seen in mice expressing GFP vector or JAK2WT (Figure 4E). However, we did not observe any significant difference in the liver size/weight among GFP vector, JAK2WT, JAK2V617F and JAK2V617F/Y201F-transplanted animals (Figure 4F).
Tyrosine 201 is required for efficient induction of MPNs by JAK2V617F. BM from 6-week female Balb/c mice was infected with retroviruses expressing vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F and then transplanted into lethally irradiated Balb/c recipient mice. Peripheral blood red blood cell (A), hemoglobin (B), white blood cell (C), and neutrophil (D) counts in mice receiving JAK2V617F/Y201F-transduced BM (n = 10) were significantly reduced compared with mice receiving JAK2V617F-transduced BM (n = 10) and were comparable with those receiving vector control- or JAK2WT-transduced BM (n = 5) 8 weeks after transplantation. (E) Spleen weight. Mice receiving JAK2V617F-transduced BM developed profound splenomegaly compared with the control (vector or JAK2WT) mice. The Y201F mutation significantly inhibited the JAK2V617F-evoked increase in spleen weight (n = 5 for mice receiving vector control- or JAK2WT-transduced BM; n = 6 for mice receiving JAK2V617F- or JAK2V617F/Y201F-transduced BM). (F) Liver weight. No significant difference was observed in the liver size among all 4 groups of mice (n = 5). (G) Flow cytometric analysis of GFP expression in the BM 8 weeks after transplantation. Histogram represents the percentage of GFP+ population in the BM of the transplanted mice (n = 5). *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA). Data are mean ± SEM. (H) Southern blot analysis with a radioactive GFP probe demonstrating oligoclonal integration of proviral clones in the BM of the transplanted animals expressing vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F. DNA from Ba/F3-EpoR-JAK2V617F cells is included at right. DNA size markers are shown in kilobases at left.
Tyrosine 201 is required for efficient induction of MPNs by JAK2V617F. BM from 6-week female Balb/c mice was infected with retroviruses expressing vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F and then transplanted into lethally irradiated Balb/c recipient mice. Peripheral blood red blood cell (A), hemoglobin (B), white blood cell (C), and neutrophil (D) counts in mice receiving JAK2V617F/Y201F-transduced BM (n = 10) were significantly reduced compared with mice receiving JAK2V617F-transduced BM (n = 10) and were comparable with those receiving vector control- or JAK2WT-transduced BM (n = 5) 8 weeks after transplantation. (E) Spleen weight. Mice receiving JAK2V617F-transduced BM developed profound splenomegaly compared with the control (vector or JAK2WT) mice. The Y201F mutation significantly inhibited the JAK2V617F-evoked increase in spleen weight (n = 5 for mice receiving vector control- or JAK2WT-transduced BM; n = 6 for mice receiving JAK2V617F- or JAK2V617F/Y201F-transduced BM). (F) Liver weight. No significant difference was observed in the liver size among all 4 groups of mice (n = 5). (G) Flow cytometric analysis of GFP expression in the BM 8 weeks after transplantation. Histogram represents the percentage of GFP+ population in the BM of the transplanted mice (n = 5). *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA). Data are mean ± SEM. (H) Southern blot analysis with a radioactive GFP probe demonstrating oligoclonal integration of proviral clones in the BM of the transplanted animals expressing vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F. DNA from Ba/F3-EpoR-JAK2V617F cells is included at right. DNA size markers are shown in kilobases at left.
Next, we assessed the engraftment of hematopoietic cells in the BM of the transplanted animals receiving vector, JAK2WT-, JAK2V617F-, or JAK2V617F/Y201F-transduced BM using GFP expression. As shown in Figure 4G, a significant proportion of cells (∼ 30%) in the BM of transplanted mice receiving JAK2WT- or JAK2V617F/Y201F-transduced BM cells were GFP+, suggesting that JAK2WT- or JAK2V617F/Y201F-transduced BM cells were efficiently engrafted. JAK2V617F expression, however, caused increased percentage of GFP+ cells in the BM of JAK2V617F transplanted animals (Figure 4G), possibly because of its role in expansion of hematopoietic progenitors/precursors, as previously described.9,40 Southern blot analysis demonstrated oligoclonal retroviral integration in the recipients of vector, JAK2WT-, JAK2V617F-, and JAK2V617F/Y201F-transduced BM (Figure 4H). The presence of multiple proviral clones in the recipients of JAK2V617F/Y201F-transduced BM similar to that seen in recipients of JAK2V617F-transduced BM (Figure 4H) further confirms a similar level of oligoclonality/polyclonality in these groups of mice.
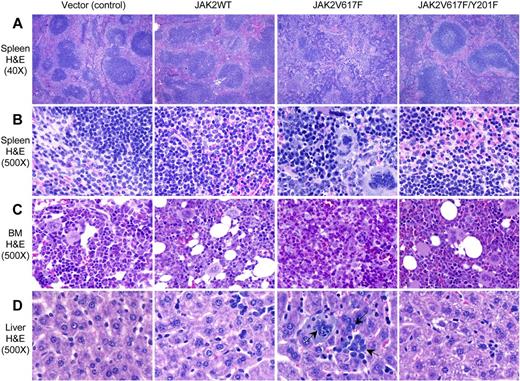
Histopathologic analyses also revealed that Y201F significantly attenuated the development of PV-like MPNs in mice expressing JAK2V617F. Spleens of vector (control) and JAK2WT-expressing mice showed normal architecture and minimal extramedullary hematopoiesis (Figure 5A-B). Spleens of JAK2V617F-expressing mice showed marked distortion of the architecture with attenuated white pulp and expanded red pulp containing abundant extramedullary hematopoiesis with infiltration of immature erythroid and granulocytic precursors and clusters of megakaryocytes (Figure 5A-B). In contrast, spleen sections from transplanted animals expressing JAK2V617F/Y201F double-mutant showed near-normal architecture with only mild extramedullary hematopoiesis (Figure 5A-B). BM sections of JAK2V617F-expressing mice showed hypercellularity with trilineage hyperplasia, whereas JAK2V617F/Y201F-expressing BM exhibited less cellularity and more closely resembled the BM of the vector (control) or JAK2WT-expressing mice (Figure 5C). Liver sections of JAK2WT-expressing mice exhibited minimal extramedullary hematopoiesis, consisting of a few granulocyte precursors (Figure 5D). Livers from JAK2V617F-expressing mice showed scattered but readily visible islands of dysplastic megakaryocytes, granulocytes, and erythroid precursors whereas JAK2V617F/Y201F mutant livers appeared normal and identical to those of control (vector or JAK2WT) transplanted animals (Figure 5D).
Histopathologic characterization of the transplanted animals. (A-B) Hematoxylin and eosin stains of the spleens (original magnification ×40 and 500) obtained from vector (control) and JAK2WT expressing mice show normal architecture and minimal extramedullary hematopoiesis. Spleens of JAK2V617F-expressing mice show marked distortion of the splenic architecture with an attenuation of the white pulp and expansion of the red pulp containing abundant extramedullary hematopoiesis, including megakaryocytes and erythroid and granulocyte precursors. JAK2V617F/Y201F-expressing mice show minimal alteration of the architecture with only mild extramedullary hematopoiesis. (C) BM sections (hematoxylin and eosin stain; original magnification ×500) from JAK2V617F-expressing mice show hypercellularity with increased and dysplastic megakaryocytes and increase in granulopoiesis. JAK2V617F/Y201F mouse BM was essentially normal and resembles that of control animals. (D) Liver sections (hematoxylin and eosin stain; original magnification ×500) from JAK2V617F-expressing mice show extramedullary hematopoiesis, whereas livers of JAK2V617F/Y201F mice appear identical to those of control animals. Arrows indicate megakaryocytes and granulocytes in the liver of JAK2V617F mice.
Histopathologic characterization of the transplanted animals. (A-B) Hematoxylin and eosin stains of the spleens (original magnification ×40 and 500) obtained from vector (control) and JAK2WT expressing mice show normal architecture and minimal extramedullary hematopoiesis. Spleens of JAK2V617F-expressing mice show marked distortion of the splenic architecture with an attenuation of the white pulp and expansion of the red pulp containing abundant extramedullary hematopoiesis, including megakaryocytes and erythroid and granulocyte precursors. JAK2V617F/Y201F-expressing mice show minimal alteration of the architecture with only mild extramedullary hematopoiesis. (C) BM sections (hematoxylin and eosin stain; original magnification ×500) from JAK2V617F-expressing mice show hypercellularity with increased and dysplastic megakaryocytes and increase in granulopoiesis. JAK2V617F/Y201F mouse BM was essentially normal and resembles that of control animals. (D) Liver sections (hematoxylin and eosin stain; original magnification ×500) from JAK2V617F-expressing mice show extramedullary hematopoiesis, whereas livers of JAK2V617F/Y201F mice appear identical to those of control animals. Arrows indicate megakaryocytes and granulocytes in the liver of JAK2V617F mice.
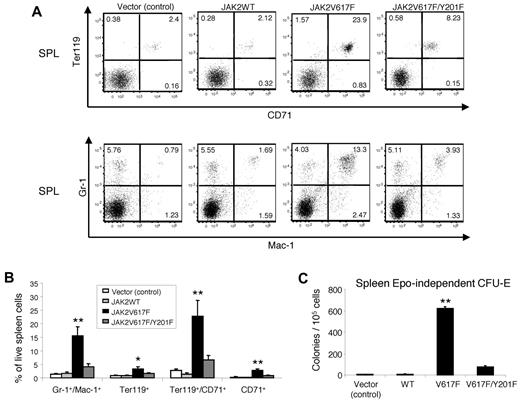
Flow cytometric analysis showed marked increase in erythroid (Ter119+ CD71+) and myeloid (Gr-1+Mac-1+) precursors in the spleens of JAK2V617F-expressing mice compared with vector (control) or JAK2WT-expressing mice (Figure 6A-B). JAK2V617F/Y201F double-mutant expression, however, significantly reduced the expansion of erythroid and myeloid precursors in the spleens of the transplanted animals (Figure 6A-B).
Effects of the Y201F mutation on hematopoietic progenitors expressing JAK2V617F. (A) Flow cytometric analysis demonstrates significant decrease in Ter119+CD71+ erythroid precursors and Gr-1+Mac-1+ myeloid population in the spleens of mice transplanted with JAK2V617F/Y201F-transduced BM compared with mice transplanted with JAK2V617F-transduced BM. Representative dot plots are presented. (B) Histogram shows the percentage of Gr-1+Mac-1+, Ter119+, Ter119+CD71+, and CD71+ populations in the spleens (n = 4 for mice expressing vector control or JAK2WT; n = 6 for mice expressing JAK2V617F; n = 7 for mice expressing JAK2V617F/Y201F). Noticeably, the Y201F mutation markedly inhibited the JAK2V617F-evoked increase in Gr-1+Mac-1+, Ter119+, Ter119+CD71+, and CD71+ populations, and there were no significant differences in those populations among mice transplanted with vector control, JAK2WT-, or JAK2V617F/Y201F-transduced BM. (C) Analysis of Epo-independent CFU-E colony formation in the spleen. A total of 1 × 105 spleen cells were seeded in methylcellulose medium without any cytokine (Methocult M3234). CFU-E colonies were scored after 2 days. Data are mean ± SEM from 3 independent experiments. *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA).
Effects of the Y201F mutation on hematopoietic progenitors expressing JAK2V617F. (A) Flow cytometric analysis demonstrates significant decrease in Ter119+CD71+ erythroid precursors and Gr-1+Mac-1+ myeloid population in the spleens of mice transplanted with JAK2V617F/Y201F-transduced BM compared with mice transplanted with JAK2V617F-transduced BM. Representative dot plots are presented. (B) Histogram shows the percentage of Gr-1+Mac-1+, Ter119+, Ter119+CD71+, and CD71+ populations in the spleens (n = 4 for mice expressing vector control or JAK2WT; n = 6 for mice expressing JAK2V617F; n = 7 for mice expressing JAK2V617F/Y201F). Noticeably, the Y201F mutation markedly inhibited the JAK2V617F-evoked increase in Gr-1+Mac-1+, Ter119+, Ter119+CD71+, and CD71+ populations, and there were no significant differences in those populations among mice transplanted with vector control, JAK2WT-, or JAK2V617F/Y201F-transduced BM. (C) Analysis of Epo-independent CFU-E colony formation in the spleen. A total of 1 × 105 spleen cells were seeded in methylcellulose medium without any cytokine (Methocult M3234). CFU-E colonies were scored after 2 days. Data are mean ± SEM from 3 independent experiments. *P < .05 (1-way ANOVA). **P < .005 (1-way ANOVA).
Expression of JAK2V617F results in Epo-independent endogenous erythroid colonies in the spleens of humans, a hallmark feature of PV.41 So, we asked whether Y201 plays any role in JAK2V617F-evoked transformation of erythroid progenitors. Whereas JAK2V617F expression resulted in a large number of Epo-independent CFU-E colonies in the spleens, JAK2V617F/Y201F double-mutant expression markedly inhibited Epo-independent CFU-E colony formation in the spleens of the transplanted animals (Figure 6C). Together, these results suggest that tyrosine 201 is required by JAK2V617F for efficient induction of MPN-like disease in mice.
Discussion
The JAK2V617F mutant is a constitutively active tyrosine kinase that induces hematopoietic transformation and plays an important role in the pathogenesis of MPNs, but the mechanisms of constitutive activation of JAK2V617F and the requirements for transformation induced by JAK2V617F are not clearly understood. Several tyrosine residues within JAK2 are phosphorylated in response to various growth factors or cytokines; and although some of those tyrosines have been shown to regulate JAK2 activity,29-37 the contributions of these tyrosines in constitutive activation of JAK2V617F and downstream signaling are unknown. Here we show that the tyrosine 201, which is a potential binding site for SH2 domain-containing proteins, is required for constitutive activation of JAK2V617F and efficient induction of hematopoietic transformation and MPN-like disease in mice.
Previous studies have suggested a requirement for homodimeric type I cytokine receptors, such as EpoR, in JAK2V617F-induced transformation of hematopoietic Ba/F3 cells.18 It has been found that higher expression level of JAK2V617F can bypass the requirement of homodimeric type I cytokine receptor for transformation.19 We also observed that JAK2V617F expressed in parental Ba/F3 cells is activated in the absence of cytokine, but is not sufficient for cytokine-independent cell growth (H. Zou, D.Y., and G.M., unpublished observation, April 2009). To determine the role of Y201 in JAK2V617F-mediated transformation under physiologic condition with cytokine receptor (EpoR) expression, we used Ba/F3 cells expressing EpoR. Consistent with previous results, expression of JAK2V617F was sufficient to transform Ba/F3-EpoR cells to growth factor independence.18,26 JAK2V617F/Y201F double-mutant expressing cells were unable to survive and proliferate in the absence of cytokine (Figure 1), indicating that the Y201F mutation abrogated the transforming potential of JAK2V617F.
Stat5 and Shp2 are SH2 domain-containing proteins that are phosphorylated by JAK2 in response to cytokine or growth factor stimulation.42,43 Constitutive phosphorylation of Stat5 and Shp2 was observed in cells expressing JAK2V617F.1,6,9 We found the JAK2V617F/Y201F mutant to be defective in constitutive phosphorylation of JAK2 and its downstream targets Stat5 and Shp2. We and other investigators have shown that Akt, p70S6 kinase, and Erk signaling pathways are constitutively activated in cells expressing JAK2V617F.1,6,9 We observed that the JAK2V617F/Y201F mutant was defective in inducing constitutive activation of Akt, p70S6 kinase, and Erk pathways. Interestingly, Y201F mutation in JAK2WT did not result in any significant defects in activation of downstream signaling in response to Epo (supplemental Figure 2). Thus, the requirements for signaling by JAK2WT and JAK2V617F could be different. This may have important implication in designing therapies for JAK2V617F-positive MPNs (discussed later in “Discussion”).
We also have provided evidence on how the Y201 site of JAK2V617F might contribute to signaling. We found that the Y201 site promotes binding of JAK2V617F with Stat5 and Shp2. Y201F mutation markedly inhibited the interactions between JAK2V617F and Stat5, and between JAK2V617F and Shp2 in both 293T and Ba/F3-EpoR cells (Figure 3A-D). According to the conventional model, Stat5 and Shp2 are recruited to the phosphorylated tyrosine residues in the cytoplasmic portion of the EpoR on ligand-induced activation of JAK2.38,44 It has been demonstrated that higher expression levels of JAK2V617F in parental Ba/F3 cells (without EpoR) cause constitutive activation of JAK2V617F and transformation of Ba/F3 cells.19 We also observed that overexpression of JAK2V617F in parental Ba/F3 or 293T cells results in constitutive activation of JAK2V617F and phosphorylation of Stat5 and Shp2 (data not shown). It has also been found that EpoR mutant with all the intracellular tyrosines mutated to phenylalanines could lead to activation/phosphorylation of JAK2V617F and Stat5, although the phosphorylation level of Stat5 could be lower than in cells expressing wild-type EpoR.19 Thus, both EpoR-dependent and -independent mechanisms exist for recruitment and activation of Stat5 (and Shp2). Our results suggest that Y201 of JAK2V617F may recruit Stat5 and Shp2, thereby facilitating their phosphorylations by JAK2V617F. Phosphorylation of Stat5 may facilitate translocation of Stat5 to the nucleus, and regulation of transcription of genes required for cell survival/proliferation.42 Recently, we also have shown that Stat5 deficiency significantly inhibits JAK2V617F-evoked activation of p70S6 kinase, suggesting that p70S6 kinase is a downstream signaling component of the JAK2V617F/Stat5 pathway.40 On the other hand, Shp2 has been shown to regulate activation of both Erk and Akt pathways.43,45 Thus, Y201 may regulate signaling pathways downstream of JAK2V617F by facilitating the recruitment of Stat5 and Shp2.
Our results demonstrate that the Y201 site is required for cytokine-independent phosphorylation of Y1007/Y1008, the key sites associated with kinase activation of JAK2V617F. However, the Y201 site is not essential for cytokine-induced phosphorylation of JAK2WT at these sites. There are several possible explanations for the greater importance of Y201 for JAK2V617F-mediated signaling compared with cytokine-induced signaling through JAK2WT. First, the Y201 site might be important for proper configuration of the JAK2V617F that is capable of transphosphorylation at the catalytic active sites (Y1007/Y1008). Second, Y201 phosphorylation could be a priming event that allows phosphorylation of other tyrosine residues in JAK2V617F, but not in JAK2WT. Third, ligand-independent activation of JAK2V617F may require JAK2V617F molecules to anchor on the receptors to transphosphorylate each other, and Y201 may facilitate interaction of JAK2V617F with the receptors in the absence of ligands. On the other hand, JAK2WT cannot be activated in the absence of ligands. Ligand binding to the receptors facilitates the conformational change needed for transphosphorylation and activation of JAK2WT, and Y201 in JAK2WT may not be important in this process. Crystal structure of full-length JAK2 has not been solved yet. Available computer-based models of JAK2 structure46 could not explain how the tyrosine residues within the N-terminal region of JAK2WT or JAK2V617F regulate the catalytic activity of JAK2 kinase. High-resolution crystal structure of full-length JAK2 would be required to better understand the role of Y201 in constitutive activation of the JAK2V617F.
To assess the contribution of Y201 in JAK2V617F-evoked transformation of hematopoietic progenitors in vivo, we expressed GFP vector (control), JAK2WT, JAK2V617F, or JAK2V617F/Y201F in the BM cells by retroviral transduction/transplantation. Consistent with previous reports, retroviral expression of JAK2V617F resulted in an MPN-like disorder characterized by increased red blood cells and hemoglobin, leukocytosis, neutrophilia, and splenomegaly (Figure 4A-E). In contrast, blood cell parameters and spleen size were markedly reduced in recipients of BM expressing the JAK2V617F/Y201F mutant compared with recipients of JAK2V617F-transduced BM and were more comparable with those observed in control mice. Flow cytometric and hematopoietic progenitor colony-forming assays also showed marked reduction in myeloid and erythroid progenitors/precursors and decreased transformation of erythroid progenitors in mice expressing JAK2V617F/Y201F compared with JAK2V617F (Figure 6A-C), indicating that JAK2V617F/Y201F significantly attenuated the development of MPN phenotype. These results strongly suggest that Y201 is required by JAK2V617F for efficient hematopoietic transformation and induction of MPNs.
The discovery of the JAK2V617F mutation in a majority of patients with MPNs has led to the development of JAK2 inhibitors. Several JAK2 inhibitors are currently undergoing clinical trials, and ruxolitinib (a JAK1/JAK2 inhibitor) has recently been approved for the treatment of myelofibrosis. However, MPN patients treated with JAK2 inhibitors have exhibited significant hematologic toxicities,47-49 consistent with an important role of JAK2 in normal hematopoiesis.20,21 The current inhibitors of JAK2 cannot distinguish between JAK2WT and JAK2V617F. Because Y201 is more important for oncogenic JAK2V617F-induced signaling than normal JAK2WT-mediated signaling, inhibitors targeting Y201 of JAK2V617F may have less hematopoietic toxicity and might be beneficial for treatment of JAK2V617F-positive MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr James Ihle (St Jude Children's Hospital) for providing the JAK2 cDNA construct.
This work was supported by the Association for International Cancer Research (08-0136) and National Institutes of Health (grant R01 HL095685, G.M.).
National Institutes of Health
Authorship
Contribution: D.Y. performed research, analyzed data, and wrote the paper; R.E.H. performed histopathologic analysis and revised the manuscript; and G.M. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Golam Mohi, Department of Pharmacology, State University of New York Upstate Medical University, 750 E Adams St, Syracuse, NY 13210; e-mail: mohim@upstate.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal