Abstract
Clonal cytogenetic abnormalities are a major risk factor for relapse after hematopoietic cell transplantation (HCT) for myelodysplastic syndrome (MDS). We determined the impact of the recently established 5-group cytogenetic classification of MDS on outcome after HCT. Results were compared with the impact of the International Prognostic Scoring System (IPSS) 3 cytogenetic risk groups, and the additional effect of a monosomal karyotype was assessed. The study included data on 1007 patients, 1-75 years old (median 45 years), transplanted from related (n = 547) or unrelated (n = 460) donors. Various conditioning regimens were used, and marrow, peripheral blood, or cord blood served as stem cell source. Both IPSS and 5-group cytogenetic risk classifications were significantly associated with post-HCT relapse and mortality, but the 5-group classification discriminated more clearly among the lowest- and highest-risk patients. A monosomal karyotype tended to further increase the rates of relapse and mortality, even after considering the IPSS or 5-group classifications. In addition, the pathologic disease category correlated with both relapse and mortality. Mortality was also impacted by patient age, donor type, conditioning regimen, platelet count, and etiology of MDS. Although mortality declined significantly in recent years, novel strategies are needed to overcome the barrier of high-risk cytogenetics.
Introduction
The prognosis of patients with myelodysplastic syndromes (MDSs) is dependent on disease and patient characteristics at the time of presentation.1 MDS tends to progress more rapidly in older than in younger patients, in those with greater comorbidities as determined by the ACE-27 instrument,1 and in patients with higher scores based on the International Prognostic Scoring Stem (IPSS)2 or the World Health Organization (WHO)–based prognostic scoring system.3,4 These factors also impact survival with various treatment modalities, including hematopoietic cell transplantation (HCT),5-7 currently the only therapy with proven curative potential.
A major cause of failure after HCT for MDS is disease relapse, profoundly affected by the clonal karyotype and the proportion of marrow myeloblasts at the time of HCT. Many recent studies have applied the 3 cytogenetic risk categories defined by the IPSS (good, intermediate, and poor2 ; Table 1) to the analysis of HCT results,6,7 although it has been suggested that categorization of cytogenetics into 2 risk groups (IPSS good/intermediate vs poor) is sufficient.8 However, a recent joint report analyzing the impact of cytogenetics on survival in nearly 3000 nontransplanted patients with MDS indicated that a distinction of 5 cytogenetic risk groups (very good, good, intermediate, poor, and very poor; Table 1) provided for more accurate prognostication than the 3 IPSS categories, identifying patients with median life expectancies that ranged from 61 months (very good group) to 6 months (very poor group).9
| Classification/prognostic group . | Abnormalities . | ||
|---|---|---|---|
| Single . | Double . | Complex . | |
| IPSS | |||
| Good | Normal; −Y; | — | — |
| del(5q); del(20q) | |||
| Intermediate | Other | Any | — |
| Poor | 7* | — | ≥ 3† |
| 5-group | |||
| Very good | −Y; del(11q) | — | — |
| Good | Normal; del(5q); del (20q); del(12p) | Including del(5q) | — |
| Intermediate | del(7q); +8; i(17q); +19; any other | Any other | — |
| Poor | −7; Inv(3)/t(3q)/del(3q) | Including −7/ del(7q) | 3† |
| Very poor | — | — | > 3† |
| Classification/prognostic group . | Abnormalities . | ||
|---|---|---|---|
| Single . | Double . | Complex . | |
| IPSS | |||
| Good | Normal; −Y; | — | — |
| del(5q); del(20q) | |||
| Intermediate | Other | Any | — |
| Poor | 7* | — | ≥ 3† |
| 5-group | |||
| Very good | −Y; del(11q) | — | — |
| Good | Normal; del(5q); del (20q); del(12p) | Including del(5q) | — |
| Intermediate | del(7q); +8; i(17q); +19; any other | Any other | — |
| Poor | −7; Inv(3)/t(3q)/del(3q) | Including −7/ del(7q) | 3† |
| Very poor | — | — | > 3† |
— indicates not applicable.
Any chromosome 7 abnormality.
Number of clonal abnormalities.
In the present analysis, we determined the impact of this 5-group classification along with that of a monosomal karyotype on transplant outcome in a large cohort of patients with MDS who had undergone HCT at our center.
Methods
Patients
Through June 2010, 1007 patients with MDS (including patients whose disease had transformed to acute myeloid leukemia [tAML]) underwent HCT at the Fred Hutchinson Cancer Research Center; 3 patients were transplanted before 1980, and 89% of HCT were carried out since 1990. Patient and disease characteristics are summarized in Table 2. All protocols were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Patient and disease characteristics
| Variable . | No. (%) of patients . |
|---|---|
| No. of patients | 1007 |
| Patient age, y, range (median) | 1-75 (45) |
| Sex (male/female) | 588 (58)/419 (42) |
| De novo MDS | 771 (77) |
| Secondary MDS | 236 (23) |
| Antecedent disorders* | 228 (23) |
| Prior therapy† | 374 (37) |
| FAB/WHO classification | |
| RA‡ | 377 (37.4) |
| RCMD‡ | 82 (8.1) |
| MDS-U | 25 (2.5) |
| RARS | 15 (1.5) |
| RAEB§ | 304 (30.2) |
| RAEB-1 | 46 (4.5) |
| RAEB-2 | 42 (4.2) |
| RAEB-T | 91 (9.1) |
| tAML | 113 (11.2) |
| Cytogenetics | |
| IPSS | |
| Good | 434 (43.1) |
| Intermediate | 182 (18.1) |
| Poor | 304 (30.2) |
| 5-group | |
| Very good | 13 (1.3) |
| MK+/MK−/unknown | 0/13/0 |
| Good | 440 (43.7) |
| MK+/MK−/unknown | 0/440/0 |
| Intermediate | 175 (17.4) |
| MK+/MK−/unknown | 7/167/1 |
| Poor | 148 (14.7) |
| MK+/MK−/unknown | 60/88/0 |
| Very poor | 97 (9.6) |
| MK+/MK−/unknown | 89/8/0 |
| Data incomplete | 134 (13.3) |
| Variable . | No. (%) of patients . |
|---|---|
| No. of patients | 1007 |
| Patient age, y, range (median) | 1-75 (45) |
| Sex (male/female) | 588 (58)/419 (42) |
| De novo MDS | 771 (77) |
| Secondary MDS | 236 (23) |
| Antecedent disorders* | 228 (23) |
| Prior therapy† | 374 (37) |
| FAB/WHO classification | |
| RA‡ | 377 (37.4) |
| RCMD‡ | 82 (8.1) |
| MDS-U | 25 (2.5) |
| RARS | 15 (1.5) |
| RAEB§ | 304 (30.2) |
| RAEB-1 | 46 (4.5) |
| RAEB-2 | 42 (4.2) |
| RAEB-T | 91 (9.1) |
| tAML | 113 (11.2) |
| Cytogenetics | |
| IPSS | |
| Good | 434 (43.1) |
| Intermediate | 182 (18.1) |
| Poor | 304 (30.2) |
| 5-group | |
| Very good | 13 (1.3) |
| MK+/MK−/unknown | 0/13/0 |
| Good | 440 (43.7) |
| MK+/MK−/unknown | 0/440/0 |
| Intermediate | 175 (17.4) |
| MK+/MK−/unknown | 7/167/1 |
| Poor | 148 (14.7) |
| MK+/MK−/unknown | 60/88/0 |
| Very poor | 97 (9.6) |
| MK+/MK−/unknown | 89/8/0 |
| Data incomplete | 134 (13.3) |
MDS-U indicates myelodysplastic syndrome, unclassified.
Among these, 54 had aplastic anemia and 8 had Fanconi anemia or other constitutional marrow failure states; 20 had Crohn disease, juvenile rheumatoid arthritis, or other autoimmune disorders; 9 had myeloproliferative neoplasms that evolved to MDS, 56 had Hodgkin or non-Hodgkin lymphoma, and 5 had multiple myeloma. Seventeen had previously been treated for other lymphoid or myeloid leukemias and 57 for solid tumors; 4 had received solid organ transplants, 2 had been accidentally exposed to radiation, and in 4 patients the etiology could not be ascertained.
Unknown in 34.
WHO categories defined only in more recent patients; RA includes patients with RCMD transplanted in earlier years.
In 139 patients with RAEB (categorized by FAB), a breakdown into RAEB-1 and RAEB-2 was not possible.
Cytogenetic findings were categorized according to the IPSS (information complete in 918 patients) as well as the 5-group classification recently presented by Schanz et al10 (information complete in 871 patients). In addition, we categorized patients by monosomal and nonmonosomal karyotypes, as originally defined for patients with AML. Thus, patients who had 2 (or more) autosomal monosomies or one monosomy in combination with other structural abnormalities were considered as having a monosomal karyotype; marker chromosomes were not considered in this assessment11 (information complete in 905 patients). Cytogenetic classifications were applied both to patients with de novo and with secondary MDS. The reason for “incomplete data” was 2-fold: (1) in some instances, cultures to induce cell division for banding analysis were unsuccessful; and (2) others were categorized as intermediate or poor according to the IPSS without providing the specific clonal abnormalities.
Comorbidity scores as determined by the HCT comorbidity index (HCT-CI)12 were available in 440 patients (44%), representing the most recently transplanted subcohort.
Donors and sources of stem cells
Donors were related in 547 patients (54%), including 461 HLA-identical siblings (46%), 9 syngeneic donors (1%), and 77 (8%) HLA nonidentical family members, and unrelated in 460 patients (46%), including 21 patients (2%) who received cord blood as a source of stem cells. The level of HLA typing varied over the reporting period, ranging from serologic typing and mixed leukocyte cultures in the earlier transplant recipients to high resolution typing (DNA sequencing) in the more recently transplanted patients. Among the 439 patients with unrelated donors (noncord blood), 266 (61%) were determined (prospectively or retrospectively) to be matched by high-resolution typing at HLA-A, -B, -C, -DRB1, and -DQB1.13 There were 147 (33%) who were mismatched for at least one allele, and for 26 patients (6%) high resolution typing was not performed.
The source of hematopoietic stem cells was bone marrow in 502 patients (50%), G-CSF mobilized peripheral blood progenitor cells (PBPCs) in 480 patients (48%), a combination of marrow and PBPCs in 4 patients (< 0.5%) and cord blood in 21 patients (2%).
Complete information on CMV immune status in both patient and donor was available for 916 transplants (91%), whereas the information was incomplete for 91 (9%; Table 3).
Donor and transplant characteristics
| Variable . | No. of patients (%) . |
|---|---|
| Donors, related | 547 (54) |
| HLA-matched sibling* | 470 (47) |
| HLA-mismatched/nonsibling† | 77 (8) |
| Unrelated‡ | 460 (46) |
| HLA-matched§ | 266 (26) |
| HLA mismatched‖ | 158 (16) |
| Source of stem cells | |
| Marrow | 502 (50) |
| PBPC¶ | 484 (48) |
| Cord blood | 21 (2) |
| Donor/patient CMV status** | |
| D+/P+ | 280 (28) |
| D+/P− | 112 (11) |
| D−/P− | 272 (27) |
| D−/P+ | 252 (25) |
| Conditioning regimens | |
| BU + CY (± ATG) | 413 (41) |
| FLU + TBI (2–4.5 Gy) | 101 (10) |
| BU + TBI (12 Gy) | 72 (7) |
| FLU + BU | 47 (5) |
| BU + CY + TBI (12 Gy) | 44 (4) |
| 131I-CD45 + FLU + TBI (2 Gy) | 23 (2) |
| FLU + treosulfan | 22 (2) |
| CY + TBI (≥ 12 Gy) ± ATG | 216 (21) |
| Other | 69 (7) |
| GVHD prophylaxis†† | |
| CSP + MTX | 659 (65) |
| Tacrolimus + MTX | 136 (14) |
| MMF + CSP/tacrolimus | 101 (10) |
| CSP ± steroids | 58 (6) |
| Other | 44 (4) |
| None‡‡ | 9 (1) |
| Variable . | No. of patients (%) . |
|---|---|
| Donors, related | 547 (54) |
| HLA-matched sibling* | 470 (47) |
| HLA-mismatched/nonsibling† | 77 (8) |
| Unrelated‡ | 460 (46) |
| HLA-matched§ | 266 (26) |
| HLA mismatched‖ | 158 (16) |
| Source of stem cells | |
| Marrow | 502 (50) |
| PBPC¶ | 484 (48) |
| Cord blood | 21 (2) |
| Donor/patient CMV status** | |
| D+/P+ | 280 (28) |
| D+/P− | 112 (11) |
| D−/P− | 272 (27) |
| D−/P+ | 252 (25) |
| Conditioning regimens | |
| BU + CY (± ATG) | 413 (41) |
| FLU + TBI (2–4.5 Gy) | 101 (10) |
| BU + TBI (12 Gy) | 72 (7) |
| FLU + BU | 47 (5) |
| BU + CY + TBI (12 Gy) | 44 (4) |
| 131I-CD45 + FLU + TBI (2 Gy) | 23 (2) |
| FLU + treosulfan | 22 (2) |
| CY + TBI (≥ 12 Gy) ± ATG | 216 (21) |
| Other | 69 (7) |
| GVHD prophylaxis†† | |
| CSP + MTX | 659 (65) |
| Tacrolimus + MTX | 136 (14) |
| MMF + CSP/tacrolimus | 101 (10) |
| CSP ± steroids | 58 (6) |
| Other | 44 (4) |
| None‡‡ | 9 (1) |
ATG indicates antithymocyte globulin; CSP, cyclosporine; MTX, methotrexate; and MMF, mycophenolate mofetil.
Including 9 syngeneic twins.
HLA mismatches typically involved one class I or class II antigen.
In 36 patients, high-resolution typing was not available.
HLA-matched at the allele level by high-resolution typing.
HLA-mismatched for one antigen, 1 or 2 alleles, or 1 antigen plus 1 allele.
Including 4 patients who receive PBPC + marrow.
Information incomplete in 91 cases.
In addition, 15 patients received ATG and 28 sirolimus in combination with various regimens.
Patients were transplanted from syngeneic twin donors.
Transplant procedure
Patients were prepared for HCT with various conditioning regimens (Table 3), the spectrum reflecting the evolution of transplant regimens over the duration of the study. The analysis of the impact of regimens was based on treatment components and dose intensities, rather than using classification into 2 or 3 subgroups as proposed elsewhere.14
Statistical analysis
Stepwise Cox regression models were fit to assess the association of various factors with the time-to-event outcomes relapse, overall mortality, and the earliest of relapse or mortality (failure of relapse-free survival) with entry and exit P values of .05. For variables with more than 2 categories, a global test was conducted using the score test. Kaplan-Meier estimates were used to determine the probability of overall and relapse-free survival for listed categories, and cumulative incidence estimates were used to summarize the probability of relapse and nonrelapse mortality (NRM), where NRM was regarded as a competing risk for relapse, and relapse as a competing risk for NRM.15 The analysis included patients transplanted as of December 31, 2010.
Results
Changes in cohort composition over time
Patient age increased from a median of 31.7 years before 1990 to a median of 54.3 years in the interval 2006-2010. Concurrently, the proportion of unrelated donor HCT increased from 6% to 57%, and the proportion of patients who received PBPCs rather than marrow increased from 0% before 1990 to 84% from 2006-2010.
The proportion of patients with refractory anemia (RA) decreased from 35% before 1990 to 14% in the period from 2006-2010 with a concurrent increase in more advanced cases of MDS.
Cytogenetic risk groups
The 5-group cytogenetic risks “good” and “intermediate” largely coincided with the IPSS cytogenetic risk groups “good” (99%) and “intermediate” (79%), respectively (Table 1). The IPSS group, “poor,“ was split into the categories “intermediate” (12%), “poor” (47%), and “very poor” (32%) in the novel 5-group classification (in the remaining 9% of patients classified as IPSS risk “poor,” details were insufficient to subcategorize them in the 5-group classification).
Within the 5 risk groups, secondary MDS accounted for 0 of 13, 69 of 440 (16%), 46 of 175 (26%), 46 of 148 (31%), and 28 of 97 (29%) in the very good, good, intermediate, poor, and very poor groups, respectively. Cytogenetic information for 5-group classification was incomplete in 134 patients, 47 (35%) of whom had secondary MDS (Table 4).
IPSS categories and distribution by 5-group risk classification
| IPSS category . | 5-group classification . | |||||
|---|---|---|---|---|---|---|
| Very good . | Good . | Intermediate . | Poor . | Very poor . | Data incomplete . | |
| Good | 3 | 431 | 0 | 0 | 0 | 0 |
| Intermediate | 10 | 9 | 139 | 5 | 0 | 19 |
| Poor | 0 | 0 | 36 | 143 | 97 | 28 |
| Data incomplete | 0 | 0 | 0 | 0 | 0 | 87 |
| IPSS category . | 5-group classification . | |||||
|---|---|---|---|---|---|---|
| Very good . | Good . | Intermediate . | Poor . | Very poor . | Data incomplete . | |
| Good | 3 | 431 | 0 | 0 | 0 | 0 |
| Intermediate | 10 | 9 | 139 | 5 | 0 | 19 |
| Poor | 0 | 0 | 36 | 143 | 97 | 28 |
| Data incomplete | 0 | 0 | 0 | 0 | 0 | 87 |
Considering only patients with complete cytogenetic information, there were 167 patients with monosomal karyotypes, 59 (30%) of 200 with secondary MDS, and 108 (15%) of 705 with de novo MDS (P < .0001). All but 4 monosomal karyotypes were within the IPSS poor-risk cytogenetic group, and all but 7 within the poor-risk and very poor-risk groups in the 5-group classification. The proportion of patients with monosomal karyotype was stable across time, ranging from 19% before 1990 to 20% after 2006.
Disease stage and pre-HCT therapy
There were 371 patients with RA (by French-American-British [FAB] criteria), 15 with RA and ring sideroblasts (RARS), 82 with refractory cytopenia with multilineage dysplasia (RCMD; including 9 with RS), 302 with RA with excess blasts (RAEB, including 46 who were classified by WHO as RAEB-1 and 41 classified as RAEB-2), 89 with RAEB in transformation (RAEB-T), and 107 in whom the disease had transformed to tAML. Thus, by WHO criteria, 196 patients had AML. In 41 patients, MDS could not be further classified.
For 973 patients, the extent of prior therapy (other than transfusion support, hematopoietic growth factors, or antibiotics) could be ascertained. Among these, 599 had received no therapy, 329 had been given induction-type chemotherapy, 41 hypomethylating drugs, and 4 both. In 34 patients, prior therapy could not be verified.
Secondary MDS
MDS was considered to be secondary in 236 patients, the majority of whom had antecedent hematologic disorders or lymphoid neoplasms. Details are provided in Table 2. Among 236 patients with secondary MDS, 65 (28%) advanced to tAML, similar to 214 (28%) of 771 patients with de novo MDS.
Transplant outcome
Overall, 650 patients had died by the time of last contact. The follow-up among the 357 surviving patients was 0.5 to 27.5 years (median, 7.1 years). There were 254 patients in whom the disease progressed or relapsed, and 222 (87%) of these had died at the time of last contact; 428 patients died from nonrelapse causes. The 5-year estimates of relapse, NRM, overall survival, and relapse-free survival were 25%, 40%, 38%, and 35%, respectively. Grades 2-4 acute GVHD occurred in 663 of 964 evaluable patients (69%), and 248 patients (26%) had grades 3-4 disease. Chronic GVHD occurred in 406 patients (40%).
Univariate analysis
Relapse.
As summarized in Table 5, disease stage/risk category (by FAB or WHO), marrow myeloblast count, cytogenetic risk group, patient age, year of HCT, presence of monosomal karyotype, conditioning, etiology, and pre-HCT treatment were each associated with the rate of relapse. The cumulative incidence of relapse by karyotype as determined by IPSS and 5-group criteria is shown in Figure 1A and B. The relapse rate was nonsignificantly increased in patients with secondary MDS compared with patients with de novo MDS (hazard rate [HR] = 1.27). However, as shown in Table 5, the relapse rate was dependent on the antecedent disorders and was significantly lower than with de novo MDS only in patients with prior aplastic anemia (HR = 0.26). When year of HCT was modeled as a continuous linear variable, there was an increase in relapse as the transplant year increased (P = .03). However, the relapse rate from 1996 onward was quite stable (compared with 1996-2000, the HRs for the years 2001-2005 and 2006-2010 were 1.03 and 1.04, respectively).
Univariate regression model for relapse
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| Marrow myeloblasts* | 1.02 | 1.01-1.03 | < .0001 | ||
| Hemoglobin* | 0.96 | 0.89-1.04 | .31 | ||
| Platelet count* | 0.98 | 0.95-1.03 | .50 | ||
| Patient age* | 1.10 | 1.02-1.19 | .01 | ||
| Disease category | MDS | 1 | |||
| tAML | 2.51 | 1.96-3.22 | < .0001 | ||
| IPSS cytogenetic risk | Good | 1 | < .0001 | ||
| Intermediate | 1.10 | 0.75-1.62 | .62 | ||
| Poor | 2.76 | 2.08-3.66 | < .0001 | ||
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 0.82 | 0.20-3.34 | .78 | ||
| Intermediate | 1.23 | 0.84-1.80 | .28 | ||
| Poor | 1.74 | 1.21-2.51 | .003 | ||
| Very poor | 6.68 | 4.67-9.56 | < .0001 | ||
| Data incomplete | 1.72 | 1.15-2.55 | .008 | ||
| Donor type | Matched sibling | 1 | .15 | ||
| Nonsibling relative | 0.69 | 0.39-1.22 | .20 | ||
| URD matched | 0.76 | 0.56-1.02 | .07 | ||
| URD mismatched | 0.83 | 0.58-1.21 | .34 | ||
| URD unknown | 0.46 | 0.19-1.11 | .09 | ||
| Year of HCT* | 1.02 | 1.00-1.04 | .03 | ||
| Monosomal karyotype | No | 1 | |||
| Yes | 3.70 | 2.78-4.92 | < .0001 | ||
| Secondary MDS | No | 1 | |||
| Yes | 1.27 | 0.95-1.68 | .10 | ||
| Conditioning regimen† | BU + CY | 1 | .001 | ||
| FLU + LD TBI | 2.38 | 1.64-3.45 | < .0001 | ||
| FLU + BU | 2.03 | 1.21-3.41 | .008 | ||
| 131I + FLU + TBI | 2.34 | 1.18-4.64 | .02 | ||
| FLU + TREO | 0.39 | 0.10-1.59 | .19 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.56 | 1.12-2.18 | .009 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | < .0001 | ||
| RAEB. | 2.09 | 1.56-2.79 | < .0001 | ||
| RAEB-T | 2.18 | 1.41-3.37 | .0005 | ||
| tAML | 2.70 | 1.82-3.99 | < .0001 | ||
| Etiology‡ | de novo | 1 | .002 | ||
| Aplastic anemia | 0.26 | 0.10-0.70 | .008 | ||
| Lymphoma | 1.70 | 1.09-2.67 | .02 | ||
| Solid tumor | 1.92 | 1.22-3.01 | .005 | ||
| Other leukemias | 2.57 | 1.31-5.01 | .006 | ||
| Treatment pre-HCT | None | 1 | < .0001 | ||
| IC | 2.09 | 1.61-2.71 | < .0001 | ||
| IC + hypomethylation | 0.00 | 0.00-**** | .97 | ||
| Hypomethylation | 2.53 | 1.51-4.23 | .0004 |
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| Marrow myeloblasts* | 1.02 | 1.01-1.03 | < .0001 | ||
| Hemoglobin* | 0.96 | 0.89-1.04 | .31 | ||
| Platelet count* | 0.98 | 0.95-1.03 | .50 | ||
| Patient age* | 1.10 | 1.02-1.19 | .01 | ||
| Disease category | MDS | 1 | |||
| tAML | 2.51 | 1.96-3.22 | < .0001 | ||
| IPSS cytogenetic risk | Good | 1 | < .0001 | ||
| Intermediate | 1.10 | 0.75-1.62 | .62 | ||
| Poor | 2.76 | 2.08-3.66 | < .0001 | ||
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 0.82 | 0.20-3.34 | .78 | ||
| Intermediate | 1.23 | 0.84-1.80 | .28 | ||
| Poor | 1.74 | 1.21-2.51 | .003 | ||
| Very poor | 6.68 | 4.67-9.56 | < .0001 | ||
| Data incomplete | 1.72 | 1.15-2.55 | .008 | ||
| Donor type | Matched sibling | 1 | .15 | ||
| Nonsibling relative | 0.69 | 0.39-1.22 | .20 | ||
| URD matched | 0.76 | 0.56-1.02 | .07 | ||
| URD mismatched | 0.83 | 0.58-1.21 | .34 | ||
| URD unknown | 0.46 | 0.19-1.11 | .09 | ||
| Year of HCT* | 1.02 | 1.00-1.04 | .03 | ||
| Monosomal karyotype | No | 1 | |||
| Yes | 3.70 | 2.78-4.92 | < .0001 | ||
| Secondary MDS | No | 1 | |||
| Yes | 1.27 | 0.95-1.68 | .10 | ||
| Conditioning regimen† | BU + CY | 1 | .001 | ||
| FLU + LD TBI | 2.38 | 1.64-3.45 | < .0001 | ||
| FLU + BU | 2.03 | 1.21-3.41 | .008 | ||
| 131I + FLU + TBI | 2.34 | 1.18-4.64 | .02 | ||
| FLU + TREO | 0.39 | 0.10-1.59 | .19 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.56 | 1.12-2.18 | .009 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | < .0001 | ||
| RAEB. | 2.09 | 1.56-2.79 | < .0001 | ||
| RAEB-T | 2.18 | 1.41-3.37 | .0005 | ||
| tAML | 2.70 | 1.82-3.99 | < .0001 | ||
| Etiology‡ | de novo | 1 | .002 | ||
| Aplastic anemia | 0.26 | 0.10-0.70 | .008 | ||
| Lymphoma | 1.70 | 1.09-2.67 | .02 | ||
| Solid tumor | 1.92 | 1.22-3.01 | .005 | ||
| Other leukemias | 2.57 | 1.31-5.01 | .006 | ||
| Treatment pre-HCT | None | 1 | < .0001 | ||
| IC | 2.09 | 1.61-2.71 | < .0001 | ||
| IC + hypomethylation | 0.00 | 0.00-**** | .97 | ||
| Hypomethylation | 2.53 | 1.51-4.23 | .0004 |
CMV status and source of stem cells had no significant impact on outcome.
URD indicates unrelated donor; and ATG, antithymocyte globulin.
Modeled as continuous linear variables; HR for platelet count represents increase in hazard associated with increase in platelets by 50 000; HR for age represents increase in hazard associated with increase in age by 10 years; HR for year of HCT represents increase in hazard associated with increase in year of HCT by one year; HR for hemoglobin represents increase in hazard associated with increase in hemoglobin by 1 g/dL; HR for blasts represents increase in hazard associated with increase in blasts by 1%.
Other regimens used (CY + TMI; FLU + TBI + CY; CY + BU; BU + TBI; BU + CY + TBI; other regimens) did not result in a significant differences.
Other antecedent conditions (myeloproliferative neoplasms; constitutional marrow failure syndromes; multiple myeloma; autoimmune disoders; prior solid organ transplantation or accidental radiation exposure) had no significant impact.
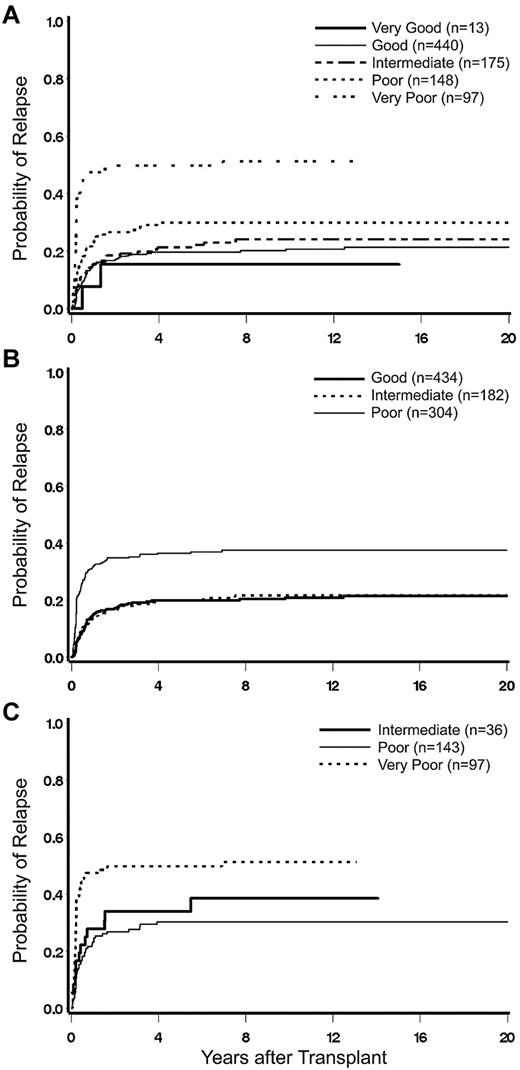
Impact of karyotype on posttransplant relapse. (A) Cumulative incidence (CI) of relapse by 5-group cytogenetic classification. (B) CI of relapse by IPSS cytogenetic classification. (C) CI of relapse among patients in the IPSS group “poor,” reclassified by 5-group criteria.
Impact of karyotype on posttransplant relapse. (A) Cumulative incidence (CI) of relapse by 5-group cytogenetic classification. (B) CI of relapse by IPSS cytogenetic classification. (C) CI of relapse among patients in the IPSS group “poor,” reclassified by 5-group criteria.
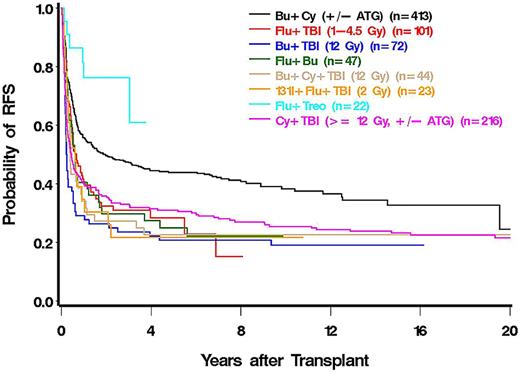
There was a trend to lower rates of relapse with HLA-matched unrelated compared with HLA-matched sibling donors (HR = 0.76). As summarized in Table 5, the relapse rate varied dependent on the type of conditioning regimen; it was highest among patients conditioned with fludarabine (FLU) plus low-dose (2-4.5 Gy) total body irradiation (TBI; HR = 2.38) and in patients conditioned with 131I-CD45 radioimmunotherapy (HR = 2.34) in a phase 1 or 2 study enrolling patients with refractory disease, not eligible for other protocols. The lowest rate of relapse was observed in a cohort of patients conditioned with a treosulfan plus FLU combination.
The incidence of post-HCT relapse was higher among patients given induction chemotherapy (HR = 2.09) or hypomethylating agents (HR = 2.53) before HCT than in those who were untreated.
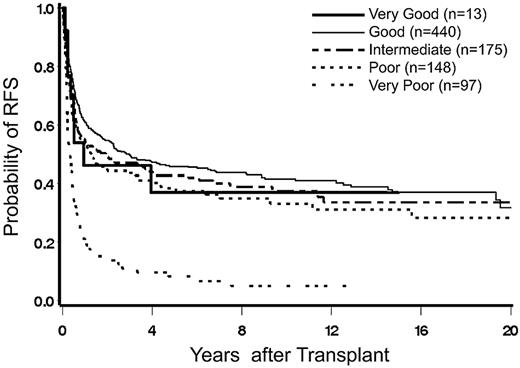
Mortality.
Overall mortality was impacted by the same factors that were associated with relapse, and, in addition, by platelet count, type of donor, and the presence of secondary MDS (Table 6). The probability of survival by karyotype is shown in Figure 2. The rate of mortality increased as patient age increased (P = .005, modeling age as a continuous linear variable). If patient age was categorized as 0-50, 50-60, 60-65, 65-70, and > 70 years, the rate of mortality increased with each category (relative to ≤ 50 years, HR = of 1.09, 1.14, 1.24, and 1.62, respectively).
Univariate regression model for overall mortality
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| Marrow myeloblasts* | 1.02 | 1.01-1.02 | < .0001 | ||
| Hemoglobin* | 0.95 | 0.90-1.01 | .08 | ||
| Platelet count* | 0.93 | 0.90-0.97 | .0005 | ||
| Patient age* | 1.07 | 1.02-1.12 | .005 | ||
| Disease category | MDS | 1 | |||
| tAML | 1.57 | 1.34-1.86 | < .0001 | ||
| IPSS cytogenetic risk | Good | 1 | < .0001 | ||
| Intermediate | 1.16 | 0.93-1.45 | .19 | ||
| Poor | 1.77 | 1.47-2.12 | < .0001 | ||
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 1.15 | 0.57-2.32 | .71 | ||
| Intermediate | 1.13 | 0.90-1.43 | .28 | ||
| Poor | 1.29 | 1.01-1.63 | .04 | ||
| Very poor | 3.08 | 2.40-3.94 | < .0001 | ||
| Data incomplete | 1.88 | 1.50-2.36 | < .0001 | ||
| Donor type | Matched sibling | 1 | .01 | ||
| Nonsibling relative | 1.40 | 1.06-1.85 | .02 | ||
| URD matched | 0.88 | 0.72-1.07 | .19 | ||
| URD mismatched | 1.22 | 0.98-1.52 | .07 | ||
| URD unknown§ | 0.89 | 0.57-1.40 | .61 | ||
| Year of HCT* | 0.97 | 0.96-0.99 | < .0001 | ||
| Monosomal karyotype | No | 1 | |||
| Yes | 2.44 | 2.01-2.96 | < .0001 | ||
| Secondary MDS | No | 1 | |||
| Yes | 1.33 | 1.11-1.58 | .002 | ||
| Conditioning regimen† | BU + CY (± ATG) | 1 | < .0001 | ||
| FLU + LD TBI | 1.49 | 1.14-1.96 | .004 | ||
| BU + TBI (12 Gy) | 2.21 | 1.65-2.95 | < .0001 | ||
| FLU + BU | 1.46 | 1.01-2.10 | .04 | ||
| BU + CY + TBI (12 Gy) | 1.86 | 1.30-2.67 | .0007 | ||
| 131I + FLU + TBI (2 Gy) | 1.86 | 1.15-3.00 | .01 | ||
| FLU + TREO | 0.43 | 0.18-1.04 | .06 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.66 | 1.35-2.03 | < .0001 | ||
| Other | 1.69 | 1.11-2.57 | .01 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | < .0001 | ||
| RAEB | 1.63 | 1.36-1.96 | < .0001 | ||
| RAEB-T | 2.02 | 1.56-2.63 | < .0001 | ||
| tAML | 2.37 | 1.86-3.02 | < .0001 | ||
| Etiology‡ | de novo | 1 | < .0001 | ||
| MPN | 2.36 | 1.17-4.75 | .03 | ||
| Lymphoma | 1.50 | 1.11-2.02 | .008 | ||
| Multiple myeloma | 3.12 | 1.29-7.55 | .01 | ||
| Autoimmune disorders | 1.76 | 1.08-2.85 | .02 | ||
| Organ transplant | 2.64 | 0.85-8.22 | .09 | ||
| Accidental radiation exposure | 11.4 | 2.83-46.1 | .0006 | ||
| Treatment pre-HCT | No | 1 | .01 | ||
| IC | 1.27 | 1.08-1.50 | .004 | ||
| IC + hypomethylation | 0.51 | 0.07-3.63 | .50 | ||
| Hypomethylation | 0.84 | 0.53-1.34 | .47 |
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| Marrow myeloblasts* | 1.02 | 1.01-1.02 | < .0001 | ||
| Hemoglobin* | 0.95 | 0.90-1.01 | .08 | ||
| Platelet count* | 0.93 | 0.90-0.97 | .0005 | ||
| Patient age* | 1.07 | 1.02-1.12 | .005 | ||
| Disease category | MDS | 1 | |||
| tAML | 1.57 | 1.34-1.86 | < .0001 | ||
| IPSS cytogenetic risk | Good | 1 | < .0001 | ||
| Intermediate | 1.16 | 0.93-1.45 | .19 | ||
| Poor | 1.77 | 1.47-2.12 | < .0001 | ||
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 1.15 | 0.57-2.32 | .71 | ||
| Intermediate | 1.13 | 0.90-1.43 | .28 | ||
| Poor | 1.29 | 1.01-1.63 | .04 | ||
| Very poor | 3.08 | 2.40-3.94 | < .0001 | ||
| Data incomplete | 1.88 | 1.50-2.36 | < .0001 | ||
| Donor type | Matched sibling | 1 | .01 | ||
| Nonsibling relative | 1.40 | 1.06-1.85 | .02 | ||
| URD matched | 0.88 | 0.72-1.07 | .19 | ||
| URD mismatched | 1.22 | 0.98-1.52 | .07 | ||
| URD unknown§ | 0.89 | 0.57-1.40 | .61 | ||
| Year of HCT* | 0.97 | 0.96-0.99 | < .0001 | ||
| Monosomal karyotype | No | 1 | |||
| Yes | 2.44 | 2.01-2.96 | < .0001 | ||
| Secondary MDS | No | 1 | |||
| Yes | 1.33 | 1.11-1.58 | .002 | ||
| Conditioning regimen† | BU + CY (± ATG) | 1 | < .0001 | ||
| FLU + LD TBI | 1.49 | 1.14-1.96 | .004 | ||
| BU + TBI (12 Gy) | 2.21 | 1.65-2.95 | < .0001 | ||
| FLU + BU | 1.46 | 1.01-2.10 | .04 | ||
| BU + CY + TBI (12 Gy) | 1.86 | 1.30-2.67 | .0007 | ||
| 131I + FLU + TBI (2 Gy) | 1.86 | 1.15-3.00 | .01 | ||
| FLU + TREO | 0.43 | 0.18-1.04 | .06 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.66 | 1.35-2.03 | < .0001 | ||
| Other | 1.69 | 1.11-2.57 | .01 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | < .0001 | ||
| RAEB | 1.63 | 1.36-1.96 | < .0001 | ||
| RAEB-T | 2.02 | 1.56-2.63 | < .0001 | ||
| tAML | 2.37 | 1.86-3.02 | < .0001 | ||
| Etiology‡ | de novo | 1 | < .0001 | ||
| MPN | 2.36 | 1.17-4.75 | .03 | ||
| Lymphoma | 1.50 | 1.11-2.02 | .008 | ||
| Multiple myeloma | 3.12 | 1.29-7.55 | .01 | ||
| Autoimmune disorders | 1.76 | 1.08-2.85 | .02 | ||
| Organ transplant | 2.64 | 0.85-8.22 | .09 | ||
| Accidental radiation exposure | 11.4 | 2.83-46.1 | .0006 | ||
| Treatment pre-HCT | No | 1 | .01 | ||
| IC | 1.27 | 1.08-1.50 | .004 | ||
| IC + hypomethylation | 0.51 | 0.07-3.63 | .50 | ||
| Hypomethylation | 0.84 | 0.53-1.34 | .47 |
CMV status and source of stem cells had no significant impact on outcome.
URD indicates unrelated donor; ATG, antithymocyte globulin; LD, low-dose TBI (2-4.5 Gy); TREO, treosulfan; MPN, myeloproliferative neoplasm; and IC, induction chemotherapy.
Modeled as continuous linear variables (as specified in Table 5);
Other regimens used (CY + TMI; FLU + TBI + CY; CY + BU) did not result in a significant difference.
Other antecedent conditions (aplastic anemia; constitutional marrow failure syndromes; solid tumors; other leukemias) had no significant impact.
Allele level typing was not performed.
Overall mortality was higher in patients with secondary compared with patients with de novo MDS (HR = 1.33) and was dependent on the type of antecedent disorder (Table 6). Mortality was also higher in patients who had received induction type chemotherapy before HCT (HR = 1.27) compared with patients without chemotherapy. The pre-HCT use of hypomethylating agents had no significant effect on mortality.
Compared with patients conditioned with busulfan/cyclophosphamide (BU/CY), mortality was increased to various degrees, particularly in patients conditioned with regimens, including high-dose TBI. Mortality was nonsignificantly reduced in patients conditioned with a treosulfan plus FLU regimen (HR = 0.43) compared with BU/CY (Figure 3).
Relapse-free survival.
Because most patients (87%) who experienced post-HCT disease progression or relapse subsequently died, the associations of all variables with relapse or mortality were very similar to the associations with mortality (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Multivariable analysis
Relapse.
In multivariable analysis, the 5-group cytogenetic risk categories, FAB/WHO classification, conditioning regimen, and progression of MDS to tAML were significant risk factors for relapse (Table 7). Patients with very poor cytogenetics had a much higher rate of relapse than patients with good-risk cytogenetics (HR = 5.26). Patients with RAEB-T were significantly more likely to relapse than were RA patients (HR = 2.14). Patients who received FLU plus low-dose TBI had a higher relapse rate than patients conditioned with BU/CY (HR = 2.01). Patients in whom MDS transformed to tAML had a higher rate of relapse compared with those who did not progress (HR = 2.39).
Multivariable regression model for relapse
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 0.75 | 0.18-3.09 | .70 | ||
| Intermediate | 1.19 | 0.81-1.76 | .38 | ||
| Poor | 1.82 | 1.24-2.67 | .002 | ||
| Very poor | 5.26 | 3.59-7.71 | < .0001 | ||
| Data incomplete | 1.60 | 1.03-2.47 | .04 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | .001 | ||
| RAEB | 1.81 | 1.33-2.47 | .0002 | ||
| RAEB-T | 2.14 | 1.35-3.40 | .001 | ||
| tAML | 1.29 | 0.81-2.05 | .28 | ||
| Disease category | MDS | 1 | |||
| tAML | 2.39 | 1.77-3.23 | < .0001 | ||
| Conditioning regimen* | BU + CY | 1 | .02 | ||
| FLU + LD TBI | 2.01 | 1.36-2.96 | .0004 | ||
| CY + TMI | 2.62 | 0.94-7.28 | .06 | ||
| FLU + TREO | 0.34 | 0.08-1.37 | .13 |
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 0.75 | 0.18-3.09 | .70 | ||
| Intermediate | 1.19 | 0.81-1.76 | .38 | ||
| Poor | 1.82 | 1.24-2.67 | .002 | ||
| Very poor | 5.26 | 3.59-7.71 | < .0001 | ||
| Data incomplete | 1.60 | 1.03-2.47 | .04 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | .001 | ||
| RAEB | 1.81 | 1.33-2.47 | .0002 | ||
| RAEB-T | 2.14 | 1.35-3.40 | .001 | ||
| tAML | 1.29 | 0.81-2.05 | .28 | ||
| Disease category | MDS | 1 | |||
| tAML | 2.39 | 1.77-3.23 | < .0001 | ||
| Conditioning regimen* | BU + CY | 1 | .02 | ||
| FLU + LD TBI | 2.01 | 1.36-2.96 | .0004 | ||
| CY + TMI | 2.62 | 0.94-7.28 | .06 | ||
| FLU + TREO | 0.34 | 0.08-1.37 | .13 |
LD indicates low dose; TMI, total marrow irradiation (with lung and liver shielding); and TREO, treosulfan.
Other regimens (CY + TMI; CY + BU; FLU + TBI + CY; FLU + BU; BU + TBI; BU + CY + TBI;131I + FLU + TBI; FLU + LD TBI + CY; CY + BU; CY + TBI [≥ 12 Gy]) had no significant impact.
Mortality.
Mortality was similarly impacted by each of the factors significant for relapse and, in addition, by etiology, platelet count, patient age, type of donor, and year of HCT (Table 8). Patients with very poor cytogenetics were 2.68 times more likely to die than patients with good-risk cytogenetics. Mortality also increased as patient age increased (P < .0001). The rates of mortality for patients with RAEB or RAEB-T were approximately 1.4 times as high as the rate among patients with RA. The HR was similar (1.41) for patients who transformed to tAML (including myeloblast counts ≥ 30%). Among patients with secondary MDS, the rate of mortality was dependent on the type of antecedent disease (Table 8). Patients transplanted from unrelated donors who were HLA mismatched by high-resolution typing had a higher mortality rate than patients transplanted from HLA-matched siblings (HR = 1.58). A delay of HCT resulting from a prolonged donor search might have been a contributing factor.16
Multivariable regression model for overall mortality
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 1.15 | 0.54-2.47 | .72 | ||
| Intermediate | 1.04 | 0.82-1.32 | .75 | ||
| Poor | 1.18 | 0.91-1.52 | .21 | ||
| Very poor | 2.68 | 2.05-3.50 | < .0001 | ||
| Data incomplete | 1.74 | 1.34-2.28 | < .0001 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | .008 | ||
| tAML | 1.44 | 1.04-2.00 | .03 | ||
| RAEB | 1.42 | 1.15-1.74 | .001 | ||
| RAEB-T | 1.45 | 1.08-1.94 | .01 | ||
| Disease category | MDS | 1 | |||
| tAML | 1.41 | 1.13-1.76 | .003 | ||
| Platelet count* | 0.92 | 0.88-0.96 | .0002 | ||
| Patient age* | 1.18 | 1.10-1.26 | < .0001 | ||
| Year of HCT* | 0.96 | 0.94-0.98 | .0002 | ||
| Donor type | Matched sibling | 1 | .003 | ||
| Nonsibling relative | 1.35 | 0.99-1.85 | .06 | ||
| URD matched | 1.19 | 0.95-1.48 | .14 | ||
| URD mismatched | 1.58 | 1.24-2.01 | .0002 | ||
| URD unknown† | 0.98 | 0.60-1.61 | .93 | ||
| Conditioning regimen‡ | BU + CY | 1 | .003 | ||
| FLU + LD TBI | 1.56 | 1.14-2.15 | .006 | ||
| BU + TBI | 1.81 | 1.31-2.51 | .0003 | ||
| BU + CY + TBI | 1.53 | 1.01-2.34 | .05 | ||
| 131I + FLU + TBI | 1.77 | 1.07-2.93 | .03 | ||
| FLU + TREO | 0.55 | 0.22-1.36 | .20 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.29 | 0.97-1.73 | .08 | ||
| Other | 1.90 | 1.09-3.30 | .02 | ||
| Etiology§ | de novo | 1 | < .0001 | ||
| Aplastic anemia | 1.45 | 0.97-2.17 | .07 | ||
| MPN | 2.19 | 1.04-4.60 | .04 | ||
| Multiple myeloma | 9.00 | 3.24-25.0 | < .0001 | ||
| Solid tumor | 1.67 | 1.16-2.40 | .006 | ||
| Autoimmune disorders | 1.60 | 0.97-2.62 | .06 | ||
| Organ transplantation | 23.1 | 6.53-81.8 | < .0001 | ||
| Accidental radiation exposure | 9.66 | 1.29-72.2 | .03 |
| Variable . | Group . | HR . | 95% CI . | P . | Global P . |
|---|---|---|---|---|---|
| 5-group cytogenetic risk | Good | 1 | < .0001 | ||
| Very good | 1.15 | 0.54-2.47 | .72 | ||
| Intermediate | 1.04 | 0.82-1.32 | .75 | ||
| Poor | 1.18 | 0.91-1.52 | .21 | ||
| Very poor | 2.68 | 2.05-3.50 | < .0001 | ||
| Data incomplete | 1.74 | 1.34-2.28 | < .0001 | ||
| FAB/WHO classification | RA/RARS/RCMD | 1 | .008 | ||
| tAML | 1.44 | 1.04-2.00 | .03 | ||
| RAEB | 1.42 | 1.15-1.74 | .001 | ||
| RAEB-T | 1.45 | 1.08-1.94 | .01 | ||
| Disease category | MDS | 1 | |||
| tAML | 1.41 | 1.13-1.76 | .003 | ||
| Platelet count* | 0.92 | 0.88-0.96 | .0002 | ||
| Patient age* | 1.18 | 1.10-1.26 | < .0001 | ||
| Year of HCT* | 0.96 | 0.94-0.98 | .0002 | ||
| Donor type | Matched sibling | 1 | .003 | ||
| Nonsibling relative | 1.35 | 0.99-1.85 | .06 | ||
| URD matched | 1.19 | 0.95-1.48 | .14 | ||
| URD mismatched | 1.58 | 1.24-2.01 | .0002 | ||
| URD unknown† | 0.98 | 0.60-1.61 | .93 | ||
| Conditioning regimen‡ | BU + CY | 1 | .003 | ||
| FLU + LD TBI | 1.56 | 1.14-2.15 | .006 | ||
| BU + TBI | 1.81 | 1.31-2.51 | .0003 | ||
| BU + CY + TBI | 1.53 | 1.01-2.34 | .05 | ||
| 131I + FLU + TBI | 1.77 | 1.07-2.93 | .03 | ||
| FLU + TREO | 0.55 | 0.22-1.36 | .20 | ||
| CY + TBI (≥ 12 Gy) (± ATG) | 1.29 | 0.97-1.73 | .08 | ||
| Other | 1.90 | 1.09-3.30 | .02 | ||
| Etiology§ | de novo | 1 | < .0001 | ||
| Aplastic anemia | 1.45 | 0.97-2.17 | .07 | ||
| MPN | 2.19 | 1.04-4.60 | .04 | ||
| Multiple myeloma | 9.00 | 3.24-25.0 | < .0001 | ||
| Solid tumor | 1.67 | 1.16-2.40 | .006 | ||
| Autoimmune disorders | 1.60 | 0.97-2.62 | .06 | ||
| Organ transplantation | 23.1 | 6.53-81.8 | < .0001 | ||
| Accidental radiation exposure | 9.66 | 1.29-72.2 | .03 |
URD indicates unrelated donor; LD, low dose; TREO, treosufan; ATG, antithymocyte globulin; MPN, myeloproliferative neoplasm; and TMI, total marrow irradiation (with lung and liver shielding).
Modeled as a continuous linear variables (as specified in Table 5).
HLA allele level typing was not carried out.
Other regimens (CY + TMI; CY + BU; FLU + TBI + CY; FLU + BU) had no significant impact.
Other antecedent conditions had no significant impact.
Patients conditioned with FLU plus low-dose TBI (HR = 1.56) or BU/TBI (HR = 1.81) had significantly higher death rates than patients who received BU/CY, and although not statistically significantly different from BU/CY, patients conditioned with treosulfan plus FLU had the lowest rate of death (HR = 0.55, P = .20) of all regimens.
Overall, the rate of death decreased with more recent years of HCT (P = .0002).
Relapse-free survival.
Factors affecting relapse-free survival (supplemental Table 2) were similar to those impacting overall survival (Table 8) and, in addition, cytogenetics, disease stage, platelet count, etiology of the disease, patient age, donor type, conditioning regimen, and year of HCT.
Impact of monosomal karyotype on outcome.
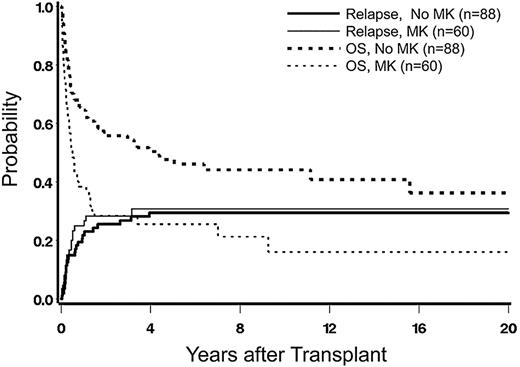
Because there was a strong correlation between 5-group cytogenetics and the presence of monosomal karyotypes (Table 2), we carried out an additional analysis of the impact of monosomal karyotype in patients with poor-risk cytogenetics by the 5-group classification (which contained a mix of patients with and without monosomal karyotype). The incidence of relapse was higher in patients with monosomal karyotype compared with those without (HR = 1.56; confidence interval, 0.85-2.87), although the difference was not statistically significant (P = .15), presumably because of insufficient power (Figure 4). Yet, the rate of overall mortality was significantly higher among patients with monosomal karyotype than in those without (HR = 2.02; confidence interval, 1.34-3.04; P = .0007), an expression of significantly increased NRM, possibly related to a more severe disease pathophysiology.
Impact of monosomal karyotype (MK) on posttransplant relapseand overall survival (OS). Incidence among patients with poor-risk karyotype (by 5-group classification), without or with concurrent MK.
Impact of monosomal karyotype (MK) on posttransplant relapseand overall survival (OS). Incidence among patients with poor-risk karyotype (by 5-group classification), without or with concurrent MK.
Impact of comorbidity.
HCT-CI scores were not included in the overall analysis because they were available for only 441 patients (44%). Among these, increasing scores were associated with an increased rate of mortality (P < .0001) and relapse (P < .0001); however, inclusion into the multivariable model did not qualitatively change the associations with the other variables, probably because comorbidity was associated with disease stage at HCT.17
Comparison of IPSS and 5-group cytogenetic classifications.
There was almost complete agreement for patients categorized as “good” or intermediate” risk between the IPSS and the 5-group cytogenetic classifications. However, patients within the IPSS “poor” risk group were separated by the 5-group classification into 3 subcohorts, “very poor,” “poor,” and “intermediate.“ Among these subcohorts, the rate of relapse was similar for those with intermediate- and poor-risk cytogenetics (HR = 0.86; confidence interval, 0.46-1.61; P = .65) but was significantly higher in patients with very poor cytogenetics (HR = 2.92; confidence interval, 1.58-5.42; P = .0007; Figure 1C).
The risk of mortality relative to patients with intermediate cytogenetics was significantly higher for patients with very poor cytogenetics (HR = 2.59; confidence interval, 1.62-4.15; P < .0001) but did not differ significantly for those with poor cytogenetics (HR = 1.13; confidence interval, 0.71-1.79; P = .62). The 5-group category “very good” was too small to draw firm conclusions.
Discussion
Clonal cytogenetic abnormalities, determined by classic banding technique or by FISH, are strongly associated with the prognosis of myeloid malignancies. The present data in a large cohort of patients with MDS transplanted at a single center underscore the profound effect of cytogenetics on relapse and mortality after HCT, currently the only treatment modality with curative potential. Moreover, results indicate that the newly proposed 5-group cytogenetic risk classification, developed for nontransplanted patients,9 distinguishes patients with different post-HCT prognosis more clearly than do the 3 risk groups of the original IPSS classification,2 particularly among patients with the lowest and highest risk of post-HCT relapse. Although retrospective data in a rather heterogeneous group of patients must be interpreted with caution, it is of note that the small cohort of patients in the very good risk group had a 5-year probability of relapse of 8% with 48% survival, compared with 50% and 8%, respectively, in the very poor risk group. By IPSS criteria, the relapse incidence was similar for the cytogenetic groups good and intermediate, and significantly lower than for the poor group, supporting the proposal by others that a separation into 2 risk categories by IPSS criteria was sufficient to distinguish patients with different post-HCT prognosis.18 However, patients with IPSS poor-risk cytogenetics, by 5-group criteria, was composed of 3 subcohorts, intermediate, poor, and very poor, with relapse incidence rates of 30% and 50% for the poor and very poor subcohorts, respectively. If, in addition, the presence of a monosomal karyotype is considered, this combination may select a subcohort of patients for whom HCT efforts with currently used modalities prove to be futile. Those results confirm reports by others on the negative impact of a monosomal karyotype19-21 in patients with AML; only one previous report22 failed to show a significant contribution of monosomy to prognosis beyond that of a complex karyotype. It is of note, however, that the present analysis of transplant outcomes indicates a negative impact of monosomal karyotype not only on relapse, but even more so on NRM. Further investigations into this effect of monosomal karyotype are warranted.
In addition to cytogenetics, myeloblast count (categorized according to FAB or WHO), proved significant for outcome, the former reflecting, presumably, the biologic risk profile of the disease, and the latter underscoring primarily the impact of tumor burden. Based on current clinical opinion, pre-HCT debulking therapy should reduce the incidence of relapse after HCT.23-25 The present results, however, revealed a negative impact of pre-HCT therapy on post-HCT outcome, either reflecting disease progression while on therapy26,27 or a lack of response to induction therapy,23,28 although our dataset did not allow to analyze results by depth of response (to either hypomethylating or induction type therapy).25,28 Alternatively, inferior outcome in these patients may have been related to intrinsic characteristics of the disease, such as high-risk cytogenetics, which had led to the decision to administer pre-HCT therapy in the first place; however, there was no statistical evidence of a preferential use of pre-HCT therapy in patients with high-risk cytogenetics (not shown).
Other risk factors for post-HCT outcome included HCT from donors other than HLA-identical siblings, although the relapse rate was lower in patients transplanted from unrelated donors, implying a clinically relevant graft-versus-leukemia effect. Secondary MDS was significant only for mortality, but not for relapse after adjusting for cytogenetics, in agreement with previous studies.29,30 The negative impact of lower platelet counts was thought to be related to the disease stage and underlying disease biology.
As expected, older patient age was associated with a higher probability of death, although it had no significant impact on relapse in multivariable analysis. Several studies reported that transplant outcome in “older” patients was not significantly different from results in younger persons.31,32 However, the definition of “older” (and “younger”) has varied from study to study, the methods used to quantify age (linear vs categorical) have been inconsistent, and a selection bias (for HCT) is highly probable.33 The present analysis included patients up to 75 years of age, many of whom were conditioned with high-intensity regimens as used in younger patients, suggesting that those patients were considered biologically younger than their chronologic age. The inclusion of comorbidities did not further improve the model, but comorbidity scores were available only in the most recently transplanted patients. The rate of mortality was not lower with the use of a low-intensity conditioning regimen (FLU + low-dose TBI). Indeed, there was evidence of higher mortality and relapse rates than observed with more intensive regimens, most likely related to patient selection17 ; however, the effect remained significant in multivariable analysis.
The finding that more recent transplant years were associated with a reduced rate of mortality is consistent with the report from our center in a large cohort of patients with various diagnoses,34 reflecting progressive improvement in supportive care measures.35 These results are encouraging, particularly because the median age of transplanted patients has increased by more than 2 decades from before 1990 to 2005, and the proportion of unrelated donor transplants has risen from 6% to 57%. The concurrent trend of an increase in relapse appears to be related to the declining proportion of patients with low-grade/early MDS (RA) and the increasing numbers of patients with advanced MDS/tAML. In addition, many MDS patients are currently being treated with hypomethylating agents as standard therapy36 and are being referred for HCT with greater delay after diagnosis.27 Prébet et al recently reported that, although the most promising option for patients with disease progression on azacitidine was HCT, the median survival after HCT was only 17 months.26 The present analysis failed to show a significant advantage of any particular regimen over a BU/CY regimen that uses BU targeting.6,37 Nevertheless, there was a trend for a treosulfan plus FLU combination38 to lead to improved survival. The high rate of failure in the small cohort of patients conditioned with radioimmunotherapy very likely was related to the enrollment of high-risk patients in a phase 1 or 2 study.39
Both acute and chronic GVHD occurred with frequencies as reported in previous studies.40 GVHD did not represent a focus of the present analysis, and the interactions of cytogenetics with GVHD, the source of stem cells and relapse, will be the subject of a separate report.
The present analysis was based on clonal chromosomal abnormalities as defined by classic banding technique or FISH. It is likely, however, that differences in outcome within a given cytogenetic risk group as currently defined are related to gene mutations not reflected in cytogenetic findings41 and possibly other factors, such as DNA methylation, histone methylation, or acetylation or elevated ferritin levels.42-45 Further, recent studies show that mutations in genes, such as TP53, ASXL1, EZH2, or RUNX1, or genes involved in transcription, such as SF3B1, significantly affect prognosis of nontransplanted patients with MDS.41,46 Such mutations are also present in approximately half of all patients with a normal conventional karyotype and are correlated with significantly shortened survival in nontransplanted patients.41 As molecular mutation data are being validated, it will be necessary to test their relevance for HCT outcome. It is likely that there is a population of patients who, despite normal cytogenetics, should be considered high risk and, possibly, should be transplanted earlier in the disease course. Conversely, patients with normal cytogenetics may include a subcohort without mutations, which very likely has a better prognosis than the “lumped” data on patients with normal karyotype would suggest.
Thus, this analysis shows that the 5-group cytogenetic risk classification has greater discriminating power for post-HCT relapse and mortality than the IPSS cytogenetic risk classification, by separating subgroups within the IPSS cytogenetic poor-risk category. The analysis further shows that advances in the management of HCT patients in recent years has resulted in considerable improvements in survival, overcoming otherwise negative impact factors, such as older age, more advanced MDS at the time of HCT, and the increasing use of unrelated donors. This finding indicates that considerable progress has been made with the overall approach to HCT. However, future trials must focus on conditioning regimens that lead to reduced NRM in patients with low risk and reduced relapse incidence in patients with high-risk cytogenetics. Such improvement may not be achievable with conventional modalities but will require innovative strategies, involving, possibly, immunotherapeutic approaches, the use of novel compounds directed at mutated gene products, the signaling pathways dependent on those genes, and other adjuvant strategies before and after HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Helen Crawford and Bonnie Larson for help with manuscript preparation; Carlos Breton, Gary Schoch, and Elizabeth Soll for updating and maintaining the database; Dr Michael Boeckh for providing comments on the analysis; all physicians and physician assistants involved in the clinical care of patients; the referring physicians; and all patients who agreed to participate in clinical research.
This work was supported by The National Institutes of Health (grants HL036444, CA018029, CA015704, HL095999, CA078902, and HL062946).
National Institutes of Health
Authorship
Contribution: H.J.D. and T.G. designed and carried out the analysis and wrote the manuscript; M.F., H.M.S., and D.M. provided expertise on cytogenetics and hematopathology; W.A.W. verified and collated data for analysis; and B.L.S., B.G., J.M.P., U.P., A.R., J.P.R., B.M.S., D.L.S., M.S., R.S., and F.R.A. read and provided critique for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1–100, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.