Abstract
Essential thrombocythemia, a myeloproliferative neoplasm, is associated with increased platelet count and risk of thrombosis or hemorrhage. Cytoreductive therapy aims to normalize platelet counts despite there being only a minimal association between platelet count and complication rates. Evidence is increasing for a correlation between WBC count and thrombosis, but prospective data are lacking. In the present study, we investigated the relationship between vascular complications and 21 887 longitudinal blood counts in a prospective, multicenter cohort of 776 essential thrombocythemia patients. After correction for confounding variables, no association was seen between blood counts at diagnosis and future complications. However, platelet count outside of the normal range during follow-up was associated with an immediate risk of major hemorrhage (P = .0005) but not thrombosis (P = .7). Elevated WBC count during follow-up was correlated with thrombosis (P = .05) and major hemorrhage (P = .01). These data imply that the aim of cytoreduction in essential thrombocythemia should be to keep the platelet count, and arguably the WBC count, within the normal range. This study is registered at the International Standard Randomized Controlled Trials Number Registry (www.isrctn.org) as number 72251782.
Introduction
The myeloproliferative neoplasms (MPNs) comprise several chronic hematologic malignancies with overlapping clinical and molecular features.1 Essential thrombocythemia (ET), the most prevalent of the MPNs, is characterized by increased platelet counts, increased risk of hemorrhage and thrombosis (both arterial and venous), and long-term risk of transformation to myelofibrosis or acute leukemia. Current practice aims to reduce platelet counts in ET to the normal range in high-risk patients.2,3 However, observational studies have consistently failed to show a relationship between elevated platelet count at diagnosis or during follow-up and thrombosis risk in this disorder,4-6 and it is therefore not clear what the target platelet count should be. Furthermore, there is evidence that leukocytosis at diagnosis is a more powerful prognostic factor for complications in ET and the related MPN, polycythemia vera.4,5,7-9 These findings have generated considerable controversy and debate about the role of blood count variables in risk stratification.4,10
In the present study, we analyzed the relationship between blood counts and complications in the PT-1 trial, the largest multicenter randomized study performed in ET. This cohort of patients has been characterized extensively through molecular studies,11-14 histologic analysis,15,16 and long-term clinical follow-up.17 Longitudinal blood counts have been collected over many years.
Methods
The PT-1 trial has relevant ethics and Medicines and Healthcare Products Regulatory Agency approvals in all participating countries. Patients with ET were entered into 1 of 3 PT-1 studies depending on their risk of vascular complications. High-risk patients were treated with aspirin plus either hydroxyurea or anagrelide on a randomized basis.17 Intermediate-risk ET patients 40-60 years of age with no vascular risk factors were randomized to either hydroxyurea plus aspirin or aspirin alone. The low-risk study is a cohort study of aspirin therapy alone in patients less than 40 years of age with no vascular risk factors. Details of the trials have been described previously.17 The trial opened in 1997 and involves the United Kingdom, Ireland, France, Australia, and New Zealand. The high-risk arm closed in 2003 and the other 2 arms are still open to recruitment. The cohort comprises 776 patients who were genotyped for the JAK2 V617F mutation13 who were followed prospectively for a median of 36 months (range, 2-87). Data from 21 887 full blood counts performed during follow-up were available.
We fitted semiparametric Cox proportional hazards regression models with a penalized cubic spline basis for the blood count predictor.18 Only the first end point event per patient was included. All models included sex, JAK2 status, randomized treatment, and past history of the relevant end point as covariates. End points were thrombosis (deep or splanchnic vein thrombosis, pulmonary embolism, myocardial infarction, acute coronary syndrome, stroke, transient ischemic attack, or peripheral artery thrombosis) and major hemorrhage (intracranial, requiring transfusion > 2 units, or causing a decrease in hemoglobin (Hb) to > 2 g/dL). The hypothesis of interest was whether the inclusion of blood count variables into the model improved the fit, which was assessed with likelihood ratio tests. The relationship between complications and blood count data during follow-up was studied by treating the blood count as a time-dependent covariate, with each individual's follow-up broken into distinct, potentially discontinuous, periods of time linked to the nearest blood count immediately preceding, up to 60 days maximum.19 In addition, age and transformation to myelofibrosis were included as time-dependent covariates. Most high-risk patients remained on randomized therapy (88% of blood count intervals for hydroxyurea; 76% for anagrelide), with no material difference in results if treatment received was included in the model instead of randomization allocation.
Results and discussion
In previous studies, analyses of blood counts and risk of complications have generally either assumed the relationship to be linear or have divided the continuous blood count variable arbitrarily at specific cut points. However, the relationship may well be nonlinear and is unlikely to be discontinuous at the cut points chosen. To circumvent these issues, we fitted a flexible semiparametric family of curves, known as cubic splines,18 to model the relationship between blood count variables and risk of complications in 776 patients with ET after correction for known confounding factors. Overall, 58 patients in the cohort had at least 1 thrombosis and 31 had a major hemorrhage (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
We found no significant association between blood counts at diagnosis and future risk of thrombosis (platelets, P = .4; WBC count, P = .6; and Hb, P = 1.0), major hemorrhage (platelets, P = .6; WBC count, P = .6; and Hb, P = .6) or transformation to leukemia, myelodysplasia, or myelofibrosis (platelets, P = .5; WBC count, P = .9; and Hb, P = .8). Some previous studies in ET have demonstrated an association between leukocytosis at diagnosis and later complications,7,20 but not other studies.21 The reasons for the discrepancies among these studies include differences in statistical methodology, length of follow-up, and play of chance.
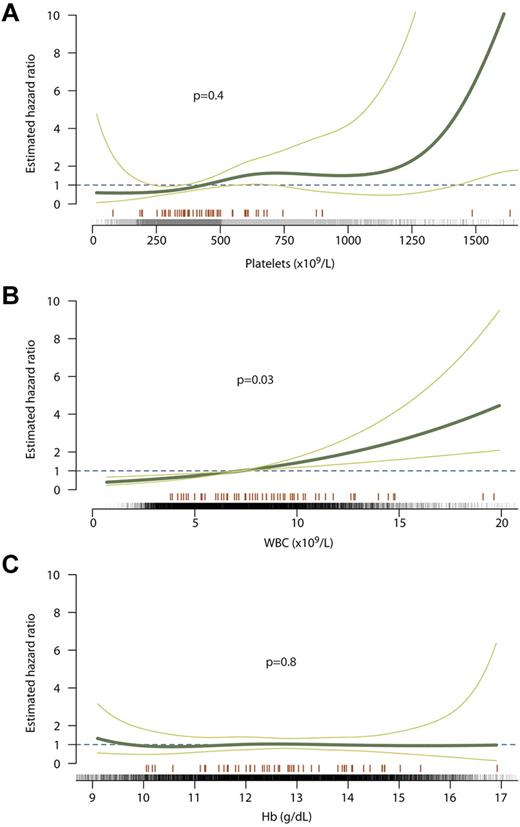
Using data from 21 887 full blood counts collected longitudinally in the 776 patients, we assessed whether blood counts during follow-up were associated with vascular complications in the 60 days after the date of the blood count (supplemental Table 2). Risk of thrombosis, comprising both arterial and venous thrombosis, was not significantly associated with platelet count during follow-up (P = .4, Figure 1A). The fitted curve in Figure 1A shows that the risk (hazard ratio) was relatively flat throughout the range of platelet counts, although confidence intervals were wide at platelet counts over 1000 × 109/L. In contrast, we found a significant association between WBC count and risk of thrombosis (P = .03, Figure 1B), with a nearly linear relationship and no particular threshold at which the risk began to increase. We found no association between Hb concentration and risk of thrombosis (P = .8, Figure 1C).
Relationship between blood counts during follow-up and risk of thrombosis. The curve of best fit is shown as a thick green line with the 95% confidence intervals for the curve shown as thin green lines. A hazard ratio of 1 (dashed line) indicates no increased risk of thrombosis, whereas > 1 indicates increased risk. Counts at which events occurred are marked with an orange line above the x axis; counts at which no events occurred are marked with black ticks. Curves are shown for platelet count (A), WBC count (B), and Hb concentration (C).
Relationship between blood counts during follow-up and risk of thrombosis. The curve of best fit is shown as a thick green line with the 95% confidence intervals for the curve shown as thin green lines. A hazard ratio of 1 (dashed line) indicates no increased risk of thrombosis, whereas > 1 indicates increased risk. Counts at which events occurred are marked with an orange line above the x axis; counts at which no events occurred are marked with black ticks. Curves are shown for platelet count (A), WBC count (B), and Hb concentration (C).
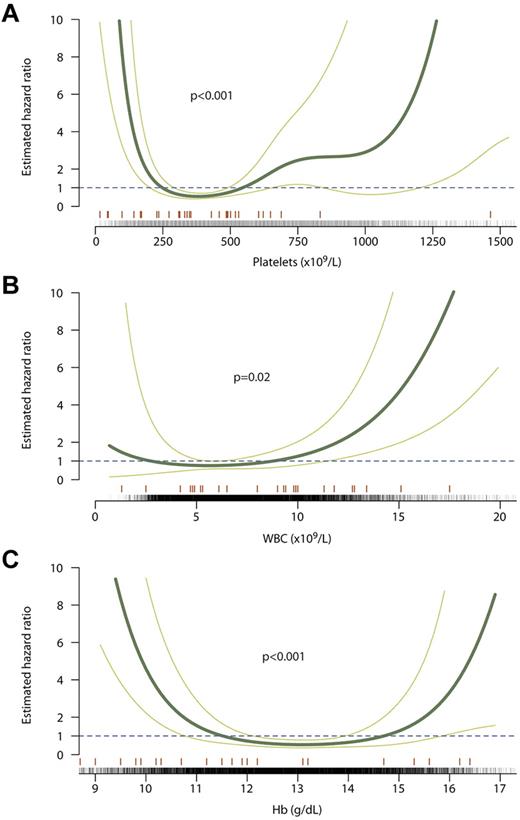
Interestingly, we found a U-shaped curve for the relationship between platelet count and risk of major hemorrhage (P = .0005, Figure 2A). There is strong evidence for an association between hemorrhage and a low platelet count, with thrombocytopenia in this situation generally resulting from excessive cytoreduction. Although confidence intervals are wide, those time periods in which platelet counts were above the normal range (> 450 × 109/L) as opposed to within normal limits were also associated with an increased risk of hemorrhage (hazard ratio, 3.7; 95% confidence interval, 1.7-8.2; P = .001). The curve for WBC count also showed a U-shaped pattern (P = .01; Figure 2B). Finally, a low Hb concentration was also correlated with major hemorrhage (P < .0001, Figure 2C). It is likely, however, that this latter association represents patients with an acute hemorrhage on the background of chronic blood loss, rather than a potentially causal link between low Hb and hemorrhage.
Relationship between blood counts during follow-up and risk of major hemorrhage. The curve of best fit is shown as a thick green line with the 95% confidence intervals for the curve shown as thin green lines. A hazard ratio of 1 (dashed line) indicates no increased risk of thrombosis, whereas > 1 indicates increased risk. Counts at which events occurred are marked with an orange line above the x axis; counts at which no events occurred are marked with black ticks. Curves are shown for platelet count (A), WBC count (B), and Hb concentration (C).
Relationship between blood counts during follow-up and risk of major hemorrhage. The curve of best fit is shown as a thick green line with the 95% confidence intervals for the curve shown as thin green lines. A hazard ratio of 1 (dashed line) indicates no increased risk of thrombosis, whereas > 1 indicates increased risk. Counts at which events occurred are marked with an orange line above the x axis; counts at which no events occurred are marked with black ticks. Curves are shown for platelet count (A), WBC count (B), and Hb concentration (C).
The results of the present study indicate that platelet count in ET is correlated with the immediate risk of major hemorrhage but not thrombosis. Strikingly, the risk of major hemorrhage is at its lowest with a platelet count in the normal range, and therefore provides evidence for the recommendation that the goal of cytoreductive therapy in this condition should be to keep the platelet count within the normal range.3 In addition, we have shown a linear association between WBC count during follow-up and risk of thrombosis, as described previously.5 There is much biologic evidence that WBCs contribute to thrombosis in these conditions,4,22,23 and the results of the present study support this. There has been some debate about whether normalizing the WBC count should be an additional aim of cytoreductive therapy in ET.4,10 Our data show a clear correlation, but do not necessarily imply that the adverse risk associated with high WBC counts is modifiable by therapy. The strength and consistency of the observational data do, however, strongly support formal testing of the hypothesis in a randomized, controlled trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Joanna Baxter and Dr Anthony Bench for sample banking and processing.
Work in the authors' laboratories is supported by Cancer Research UK, the Leukemia & Lymphoma Society, Leukemia and Lymphoma Research, the Kay Kendall Leukaemia Fund, and the Cambridge National Institute for Health Research Biomedical Research Centre. P.J.C. is funded through a Wellcome Trust Senior Clinical Research Fellowship (grant WT088340MA).
Wellcome Trust
Authorship
Contribution: P.J.C., C.M., P.A.B., G.B., and K.W. conceived and designed the study and collected the data; P.J.C. performed the statistical analysis under the supervision of K.W.; P.J.C., J.-J.K., C.F., C.N.H., and A.R.G. coordinated and oversaw the multicenter prospective PT-1 trial; and P.J.C., C.N.H., and A.R.G. wrote the manuscript with input from the other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Anthony R. Green, Cambridge Institute for Medical Research, Hills Road, Cambridge CB2 2XY, United Kingdom; e-mail: arg1000@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal