Abstract
The CD30-targeting Ab-drug conjugate brentuximab vedotin (SGN-35) was recently approved for the treatment of relapsed Hodgkin lymphoma and anaplastic large-cell lymphoma by the Food and Drug Administration. In the present study, we report the experience of the German Hodgkin Study Group with brentuximab vedotin as single agent in 45 patients with refractory or relapsed CD30+ Hodgkin lymphoma who were treated either in a named patient program (n = 34) or in the context of a safety study associated with the registration program of this drug. In these very heavily pretreated patients, an objective response rate of 60%, including 22% complete remissions, could be documented. The median duration of response was 8 months. This retrospective analysis supports the previously reported excellent therapeutic efficacy of brentuximab vedotin in heavily pretreated CD30+ malignancies. This trial was registered at www.clinicaltrials.gov with identifier NCT01026233.
Introduction
The majority of Hodgkin lymphoma (HL) patients achieve long-term remission when treated with chemo- and radiotherapy. However, depending on initial risk factors and treatment, 10%-30% of patients progress or relapse. Up to 50% of these patients can still be cured with high-dose chemotherapy and autologous stem cell transplantation (ASCT).1,2 Patients with primary progressive HL or relapse within 12 months after initial therapy have a significantly poorer outcome than those with late relapse.3,4 Although salvage therapy including high-dose chemotherapy and ASCT cures up to 50% of patients, the median overall survival (OS) after ASCT failure is only approximately 2 years (Sally Arai, Michelle Fanale, Sven deVos, A.E., Tim Illidge, P.B., Anan Younes, Franck Morschhauser, Sandra J. Horning, manuscript submitted, June 2012).5,6
To improve the outcome of patients with relapsed or refractory disease and to reduce chemotherapy-associated side effects, several targeted drugs have been developed and are currently undergoing clinical evaluation in HL patients. The most advanced and promising new drug is the Ab-drug conjugate brentuximab vedotin (SGN-35).6 Brentuximab vedotin consists of the chimeric anti-CD30 mAb cAC10 conjugated to 4 molecules of monomethyl auristatin E, a synthetic antitubulin chemotherapeutic agent.7 In a phase 1 trial including 45 patients with relapsed or refractory CD30+ hematologic malignancies, an overall response rate (ORR) of 67% in patients who received the maximum tolerated dose of 1.8 mg/kg could be documented.8 For relapsed HL, these promising results were confirmed in a single-arm phase 2 trial including 102 heavily pretreated HL patients.9 The ORR was 73% with a median duration of 6.7 months, including 32% complete remissions (CRs) with a median duration of 20.5 months and 40% partial remissions (PRs) with a median duration of 3.5 months. In anaplastic large cell lymphoma, a phase 2 trial with 58 patients led to an ORR of 86% with a median duration of 12.6 months.10
In August 2011, the Food and Drug Administration granted approval of brentuximab vedotin (Adcetris) for the treatment of HL patients who fail ASCT or have had at least 2 prior multiagent chemotherapy regimens and are not candidates for ASCT. In addition, the drug was also registered for patients with systemic anaplastic large cell lymphoma after failure of at least one prior multiagent chemotherapy regimen. After registration, the companies involved (Seattle Genetics, Millennium, and Takeda) initiated a named patient program (NPP) in which more than 1000 patients have been included so far. Because very little is known about the experience with brentuximab vedotin outside of these registration trials, in the present study, we collected a total of 45 patients treated in Germany, Switzerland, and Austria. We report herein this German Hodgkin Study Group (GHSG) experience with brentuximab vedotin in patients with heavily pretreated relapsed and refractory HL.
Methods
Since March 2010, a total of 11 GHSG centers and 1 private practice used brentuximab vedotin in 45 patients with primary refractory or relapsed HL. All patients had histologically confirmed CD30+ disease. Eleven patients were included in the SGN-35-007 cardiac safety trial (clinicaltrials.gov identifier: NCT01026233); 34 patients were treated within the NPP. Two patients who were also part of the NPP were excluded from this analysis because brentuximab vedotin was given as consolidation treatment after achieving CR in one patient; another patient had an Eastern Cooperative Oncology Group (ECOG) performance status > 2. In the SGN-35-007 trial, as well as in the NPP, participants gave written informed consent in accordance with the Declaration of Helsinki, had an ECOG performance status ≤ 2, and normal organ function including peripheral blood counts within the normal range. Ethical committee approval was obtained from the University Hospital of Cologne for the SGN-35-007 study. Patients received a 30-minute infusion of brentuximab vedotin at the dose of 1.8 mg/kg of body weight every 3 weeks. No premedications were administered. Toxicities were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 and for grade 3 toxicity, dose reduction of brentuximab vedotin to 1.2 mg/kg was recommended. For all patients, computed tomography (CT) was mandatory to judge response at initial staging and restaging. Response was defined according to the revised response criteria for malignant lymphoma.11 A total of 34 patients also underwent positron emission tomography (PET) to determine the metabolic response. Treatment was continued until disease progression.
OS was defined as the time from the initiation of therapy to death from any cause and was censored at the date of last information. Progression-free survival (PFS) was measured from initiation of therapy to progression, relapse, or death from any cause and was censored at the date of last information. OS and PFS were estimated according to the method of Kaplan and Meier. Exact 95% confidence intervals (95% CIs) were used when appropriate. Analysis of the data were carried out using SAS Version 9.2 software (SAS Institute).
Results and discussion
With the aim of providing more information on the efficacy and safety of the recently registered Ab-drug conjugate brentuximab vedotin, in the present study, we analyzed the outcome and toxicity data of 45 patients with relapsed and refractory CD30+ HL. All patients had been treated within the SGN-35-007 study or the NPP. Patient characteristics are shown in Table 1. The median age was 35 years and all HL patients had classic HL with nodular sclerosis as the most frequent histological subtype. Twenty-eight patients had primary refractory disease or early relapse and 29 patients were refractory to the last treatment received. The median number of prior therapy regimen was 4 (range, 2-12), including high-dose chemotherapy and ASCT or allogenic stem cell transplantation in 39 patients. A total of 31 patients had also been treated with radiotherapy. Whereas only 15 patients had stage III/IV disease on first diagnosis, 33 patients were stage III/IV at the initiation of SGN-35 treatment. Patients who were primary refractory or had early relapse after initial treatment and in addition had refractory disease after their last treatment (n = 17) were considered very high risk.
Patient characteristics
| Patient characteristics and response to SGN-35 | |
| Median age, y | 35 |
| Male sex, n = 5 | 22/45 (49%) |
| Stage III/IV at first diagnosis, n = 5 | 15/45 (33%) |
| Previous treatment and characterization of chemotherapy sensitivity | |
| Primary refractory disease/early relapse, n (%) | 28/45 (62%) |
| Median number of previous cancer regimens, n (range) | 4 (2-12) |
| Median years between first diagnosis and SGN-35 treatment, n (range) | 4 (0-15) |
| Previous SCT (auto and/or allo SCT), n (%) | 39/45 (87%) |
| Refractory prior to SGN-35, n (%) | 29/45 (64%) |
| Disease status at initiation of SGN-35 | |
| Stage III/IV, n (%) | 33/45 (73%) |
| B-symptoms, n (%) | 20/45 (44%) |
| Extranodal manifestation, n (%) | 33/45 (73%) |
| Large mediastinal tumor, n (%) | 2/45 (4%) |
| ≥ 3 lymph node regions, n (%) | 30/44 (68%) |
| Lactate dehydrogenase > 240 U/L, n (%) | 14/37 (38%) |
| ECOG status ≤ 1, n (%) | 37/45 (82%) |
| Median number of SGN-35 courses, n (range) | 7 (1-12) |
| Best response to SGN-35, n (%) | |
| CR | 10/45 (22%) |
| PR | 17/45 (38%) |
| SD | 13/45 (29%) |
| PD | 5/45 (11%) |
| Patient characteristics and response to SGN-35 | |
| Median age, y | 35 |
| Male sex, n = 5 | 22/45 (49%) |
| Stage III/IV at first diagnosis, n = 5 | 15/45 (33%) |
| Previous treatment and characterization of chemotherapy sensitivity | |
| Primary refractory disease/early relapse, n (%) | 28/45 (62%) |
| Median number of previous cancer regimens, n (range) | 4 (2-12) |
| Median years between first diagnosis and SGN-35 treatment, n (range) | 4 (0-15) |
| Previous SCT (auto and/or allo SCT), n (%) | 39/45 (87%) |
| Refractory prior to SGN-35, n (%) | 29/45 (64%) |
| Disease status at initiation of SGN-35 | |
| Stage III/IV, n (%) | 33/45 (73%) |
| B-symptoms, n (%) | 20/45 (44%) |
| Extranodal manifestation, n (%) | 33/45 (73%) |
| Large mediastinal tumor, n (%) | 2/45 (4%) |
| ≥ 3 lymph node regions, n (%) | 30/44 (68%) |
| Lactate dehydrogenase > 240 U/L, n (%) | 14/37 (38%) |
| ECOG status ≤ 1, n (%) | 37/45 (82%) |
| Median number of SGN-35 courses, n (range) | 7 (1-12) |
| Best response to SGN-35, n (%) | |
| CR | 10/45 (22%) |
| PR | 17/45 (38%) |
| SD | 13/45 (29%) |
| PD | 5/45 (11%) |
CR indicates complete remission; PR, partial remission; SD, stable disease; and PD, progressive disease.
The HL patients reported herein had a more high-risk profile compared with those included in a recently reported phase 2 trial.9 In total, 64% of patients in the present analysis were refractory to their last treatment, compared with 42% in the phase 2 pivotal study. Moreover, the median time between the last systemic therapy and initiation of brentuximab vedotin was only 2 months (range, 0-24) in the present study, which is a clear additional indicator of disease activity. In addition, 18% of the patients reported here were classified as ECOG 2, whereas only patients with an ECOG performance status of 0-1 were treated within the phase 2 trial. Other patient characteristics, such as the median number of prior therapies (4 and 3.5), the proportion of patients with of prior radiotherapy (69% and 66%), and prior high-dose chemotherapy (87% and 100%) were similar between the present analysis and the pivotal trial.
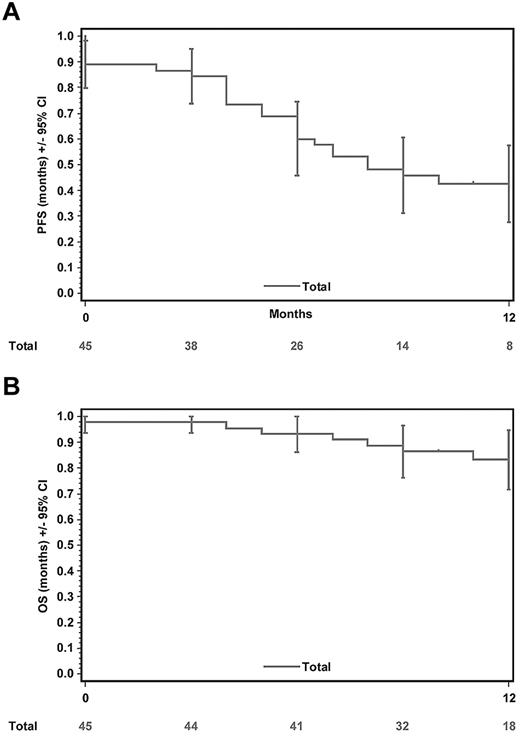
In total, 1-12 courses of brentuximab vedotin were given (median, 7) in the present study. Very similar to the pivotal studies, single-agent treatment with brentuximab vedotin demonstrated an impressive ORR of 60%, with 22% (10 patients) achieving CR. Of the 34 patients who underwent a PET-CT, 5 patients achieved a metabolic CR. PFS at 12 months was 43% (95% CI, 28%-58%) and the median PFS was 8 months (Figure 1A). A total of 37 of 45 patients were alive at the time of data collection. OS at 12 months was 83% (95% CI, 72%-95%) and the median OS has not been reached yet (Figure 1B).
Kaplan-Meier plots and 95% CIs for PFS and OS. (A) The median PFS was 8 months. (B) The OS at 12 months was 83%. The median OS has not yet been reached.
Kaplan-Meier plots and 95% CIs for PFS and OS. (A) The median PFS was 8 months. (B) The OS at 12 months was 83%. The median OS has not yet been reached.
In the subgroup of patients who achieved an objective response (n = 27), PFS at 12 months was 54% (95% CI, 34%-73%) and the median PFS was 13 months. In the 18 patients not achieving an objective response, PFS was significantly lower (P = .03 by log-rank test), with a 12-month-estimate of 27% (95% CI, 6%-48%) and a median PFS of 4.5 months. However, there was no relevant difference in OS between these groups of patients, with OS at 12 months being 87% (95% CI, 74%-100%) and 77% (95% CI, 58%-97%) in patients with and without objective response, respectively. The median OS has not yet been reached in either group.
Ten of the 17 patients considered very high-risk having primary refractory disease or early relapse plus refractory disease before brentuximab vedotin treatment achieved an objective response (ORR 59%, including 3 CRs). However, PFS of these patients was significantly lower compared with patients with lower risk (P = .001 by log-rank test). PFS at 12 months was 14% in these high-risk patients compared with 59% in those with lower risk, respectively; the median PFS was 6 and 14 months, respectively. OS of high-risk patients was considerably lower compared with low-risk patients (P = .07 by log-rank test); the OS at 12 months was 68% and 93% in high- and low-risk patients, respectively; the median OS was not reached in either group.
The toxicity profile of brentuximab vedotin in these heavily pretreated patients was also very similar to the previously published data. Dose reduction because of grade 3 toxicity was necessary in only 4 patients. No patient had to stop treatment because of toxicity. Peripheral sensory neuropathy grade 1/2 was documented in 14 patients; no case of grade 3/4 neuropathy was reported. The most common grade 3/4 adverse events were neutropenia (n = 6), thrombocytopenia (n = 3), fatigue (n = 3), and infections (n = 3). One patient developed insulin-dependent diabetes mellitus that was self-limiting after the end of treatment.
The data on brentuximab vedotin presented herein for patients treated within the NPP and the SGN-35-007 study, indicate that this drug is effective and well-tolerated in heavily pretreated and refractory HL patients. The role of brentuximab vedotin as maintenance in HL patients at high risk after ASCT is being evaluated in an ongoing phase 3 study (clinicaltrials.gov), as is the effect of combining the drug with multiagent chemotherapy (eg, AVD or modified BEACOPP).12 With respect to its application as frontline therapy, 2 cases of progressive multifocal leukoencephalopathy have been reported in HL patients treated with brentuximab vedotin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Johannes Drach and Hildegard Greinix (Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Vienna, Austria) and the staffs of all centers and private practices that participated in this data collection.
Authorship
Contribution: A.R. and S.S. coordinated the study and wrote the manuscript; H.G. performed the statistical evaluation; D.A.E., M.v.B.B., and A.E. wrote the manuscript; A.L., U.J., B.B., S.T., and P.B. contributed data; C.B. evaluated the PET-CTs; and A.E. served as the principal investigator.
Conflict-of-interest disclosure: A.E. receives research support and honoraria from Millennium and Takeda. U.J. is on the advisory board for Takeda. P.B. is on the boards of Millennium and Takeda and is sponsored by investigator-initiated trials. The remaining authors declare no competing financial interests.
Correspondence: Andreas Engert, MD, First Department of Internal Medicine, University Hospital of Cologne, Kerpener Str 62, D-50937 Cologne, Germany; e-mail: a.engert@uni-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal