Abstract
A definition of response by cytoreductive therapy in essential thrombocythemia was recently provided by the European LeukemiaNet (ELN). Complete, partial, or no clinicohematologic responses were defined on the bases of platelet count, disease-related symptoms, spleen size, and white blood cell count. To provide estimates and clinical correlation of responses according to these criteria, we retrospectively examined 416 essential thrombocythemia patients treated with hydroxyurea for at least 12 months. Complete response, partial response, and no response were 25%, 58%, and 17%, respectively. Age more than 60 years and JAK2V617F mutation were significant predictors of response. After a median follow-up of 3.9 years, we registered 23 deaths, 16 hematologic transformations, and 27 thrombotic events (rate, 1.66% patients/year). Age, previous thrombosis, leukocytosis (white blood cell count > 10 × 109/L), but not ELN responses, were independently associated with higher risk of thrombosis. The actuarial probability of thrombosis was significantly influenced by leukocytosis (P = .017) and not by platelet count, indicating that platelet number does not seem of prime relevance in the definition of ELN response.
Introduction
Sound methodologic evidence for recommending therapy in essential thrombocythemia (ET) is limited, and clinical expertise still plays a major role in driving therapeutic decisions. The common attitude is to reserve chemotherapy to patients considered at high potential risk of developing major thrombotic or hemorrhagic complications, based on known clinical variables, and to adopt a more conservative approach in those at low or intermediate risk. Hydroxyurea (HU) has emerged as the treatment of choice in 2 randomized clinical trials (RCTs). The first compared HU with no myelosuppression and showed that the number of thrombotic episodes was significantly reduced in treated patients,1 whereas the second study demonstrated that HU combined with aspirin was superior to anagrelide (plus aspirin) in reducing thrombotic complications.2 In these RCTs, HU daily dose was adjusted to maintain platelet count at less than 600 × 109/L, even though the importance of the platelet count as a risk factor for thrombosis was, and still remains, unclear. More recently, several studies have addressed the relevance of leukocytosis,3-6 bone marrow reticulin grade,7 and JAK2 V617F mutational status8 as factors associated with the risk of vascular events. Such novel information suggested the opportunity for a reappraisal of the criteria commonly used for defining clinical-hematologic (CH) response in ET patients, and with the perspective to allow comparability of response evaluation in ongoing and forthcoming trials with the novel JAK2 inhibitors. Therefore, a standardized definition of response was recently provided by the European LeukemiaNet (ELN) investigators. These experts reached a consensus in defining 3 categories of response in polycythemia vera (PV) and in ET.9 In ET, CH response was defined according to the variations of 4 major criteria: platelet count, disease-related symptoms, spleen size, and white blood cell count. On this basis, complete response (CR), partial response (PR), or no CH responses (NR) were defined. However, it should be mentioned that these definitions resulted from a consensus process and were not evidence-based. Therefore, in this study, we retrospectively examined 416 ET patients treated with HU as first-line therapy aimed at providing estimates of CH responses according to ELN criteria and assessing correlations with outcomes.
Methods
Patients
From 1981 to 2009, 3 Italian centers (Bergamo, Florence, and Vicenza) treated 416 consecutive and unselected newly diagnosed patients (PVSG and WHO-2001 criteria). They were included in this study if treatment with HU had lasted at least 12 months. HU was started in patients meeting high-risk criteria (age > 60 years or/and previous thrombosis) or if they presented extreme thrombocytosis (> 1500 × 109/L), aspirin-resistant microvascular symptoms (erythromelalgia, migraine, amaurosis fugax) or progressive splenomegaly. Patients started with 0.5 to 1.0 g HU daily; then the dosage was subsequently adjusted to maintain the platelet count at less than 600 × 109/L. In each of the 3 centers, clinical features, including treatment, and laboratory tests were recorded at follow-up visits every 3 months. Low-dose aspirin (100 mg/day) was given in patients who had no contraindication to this drug.
Permissions were obtained from the Institutional Review Boards to review the medical records.
Definition of responses
According to ELN criteria, CH CR was defined as: platelet count less than or equal to 400 × 109/L, no disease-related symptoms, normal spleen size, and white blood cell count less than or equal to 10 × 109/L. PR was recognized in patients who did not fulfill the criteria for CR but presented a platelet count less than or equal to 600 × 109/L or a decrease greater than 50% from baseline. Any response that did not satisfy PR criteria was classified as NR.
Definition of events
We examined the cumulative rate of arterial and venous thrombosis, including acute myocardial infarction, ischemic stroke, cerebral transient ischemic attacks, peripheral arterial thrombosis, and venous thromboembolism. Diagnostic procedures were carried out as previously reported.4 The diagnostic criteria for myelofibrosis transformation included severe fibrosis (> grade 2 or 3 fibrosis) on trephine biopsy and clinical factors, such as progressive splenomegaly, unexplained anemia, constitutional symptoms, and characteristic blood film changes. Acute leukemia was diagnosed according to WHO criteria.
Statistical methods
ELN response criteria were assessed on the whole cohort of 416 patients at 3, 6, and 12 months of treatment. Factors associated with the achievement of responses were tested by a logistic regression model, including age, sex, time elapsed from diagnosis to HU start, JAK2V617F status, white blood cell count (WBC), and platelet count.
Major thrombotic events and hematologic transformations occurring after 12 months of therapy were calculated as rates per 100 patients per year.
The effect of ELN responses as well as other potential prognostic factors on thrombosis-free survival was evaluated by fitting various Cox regression models, considering each factor separately (univariate analysis) or adjusting for the confounding effects of: time elapsed from diagnosis to HU treatment start, sex, age, previous thrombotic event, JAK2V617F status, and WBC and platelet count measured after 12 months of HU treatment (multivariate analysis).
Thrombosis-free survival was calculated by Kaplan-Meier curves and compared by log-rank tests. Cutoffs for WBC and platelet counts were chosen according to the ELN criteria. All probability values were 2-tailed; P value less than or equal to .05 was considered significant.
Results
Patient characteristics at presentation are shown in Table 1 and reflect well the variety of clinical features found in the routine clinical practice of care of unselected ET patients. After a median of 4.9 months of observation, patients meeting the criteria of the high-risk category based on older age and occurrence of thrombohemorrhagic symptoms (83%) or with aspirin-resistant microvessel disturbances and concomitant generic risk factors (hypertension, diabetes, smoking; 17%) started HU therapy and were kept on low-dose aspirin.
Clinical characteristics at HU start
| Characteristic . | Value . |
|---|---|
| Patients, N | 416 |
| Sex, male/female, n (%) | 154/262 (37/63) |
| Median age, y (range) | 66 (18-95) |
| Less than 40 | 24 (6) |
| 40-60 | 119 (28) |
| More than 60 | 273 (66) |
| JAK2 V617F/JAK2* wt, n (%) | 231/148 (61/39) |
| Patients with thrombosis, n (%) | 161 (39) |
| AMI | 32 (20) |
| Stroke/TIA | 77 (48) |
| PAT | 16 (10) |
| VTE | 36 (22) |
| Median time from diagnosis to HU start, mo (range) | 4.9 (0-255) |
| Less than 1 y, n (%) | 258 (62) |
| 1-5 y, n (%) | 108 (26) |
| More than 5 y, n (%) | 50 (12) |
| Patients on aspirin, n (%) | 324 (78) |
| Characteristic . | Value . |
|---|---|
| Patients, N | 416 |
| Sex, male/female, n (%) | 154/262 (37/63) |
| Median age, y (range) | 66 (18-95) |
| Less than 40 | 24 (6) |
| 40-60 | 119 (28) |
| More than 60 | 273 (66) |
| JAK2 V617F/JAK2* wt, n (%) | 231/148 (61/39) |
| Patients with thrombosis, n (%) | 161 (39) |
| AMI | 32 (20) |
| Stroke/TIA | 77 (48) |
| PAT | 16 (10) |
| VTE | 36 (22) |
| Median time from diagnosis to HU start, mo (range) | 4.9 (0-255) |
| Less than 1 y, n (%) | 258 (62) |
| 1-5 y, n (%) | 108 (26) |
| More than 5 y, n (%) | 50 (12) |
| Patients on aspirin, n (%) | 324 (78) |
AMI indicates acute myocardial infarction; TIA, cerebral transient ischemic attack; PAT, peripheral arterial thrombosis; and VTE, venous thromboembolism.
Percentages calculated on 379 JAK2 V617F evaluated patients.
After completing 12 months of therapy, the number of patients alive who withdrew from HU was 18 (4%, at a median of 6 years from HU start) because of choice of the patient/enrollment in new protocols (n = 6), or side effects (leg ulcers, n = 5; lack of platelet control, n = 4; anemia, n = 2; or other reason, n = 1).
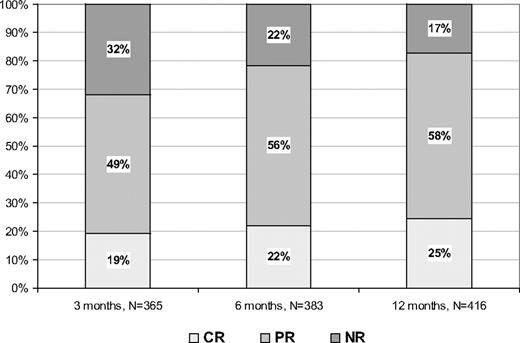
Figure 1 reports the rate of responses at 3, 6, and 12 months of treatment, respectively. The highest rates of CR and PR were obtained after 12 months and were 25% and 58%, respectively. No responses were found in 17% of patients.
Rate of responses according to ELN criteria. For some patients, data at 3 and/or 6 months of therapy were not available.
Rate of responses according to ELN criteria. For some patients, data at 3 and/or 6 months of therapy were not available.
In a multivariate logistic model, age more than or equal to 60 years and the presence of JAK2 V617F mutation were significant predictors of response evaluated at 1 year of treatment (P = .02 and P < .001, respectively).
Table 2 reports hematologic values and clinical findings before and after 12 months of HU treatment. The great majority (83%) of patients reached platelet counts less than 600 × 109/L, but platelet count normalization (< 400 × 109/L) was obtained in only 25%. Normalization of leukocyte count (< 10 × 109/L) was documented in 89% of cases. Of note is the incomplete reduction of symptoms and splenomegaly that were still present in 12% and 8% of cases, respectively.
Laboratory and clinical characteristics
| . | At baseline . | After 12 months of HU . |
|---|---|---|
| Median PLT, ×109/L (range) | 906 (258-3920) | 494 (149-1356) |
| Less than 400 × 109/L, n (%) | 5 (1) | 107 (26) |
| 400-600 × 109/L, n (%) | 16 (4) | 237 (57) |
| More than 600 × 109/L, n (%) | 395 (95) | 72 (17) |
| Median WBC, ×109/L (range) | 9.4 (4.2-34) | 6.7 (3.2-28.9) |
| Less than 10 × 109/L, n (%) | 267 (64) | 371 (89) |
| More than 10 × 109/L, n (%) | 149 (36) | 45 (11) |
| Aspirin-resistant microvascular symptoms, n (%) | ||
| No | 313 (75) | 368 (88) |
| Yes | 103 (25) | 48 (12) |
| Splenomegaly, n (%) | ||
| No | 359 (86) | 381 (92) |
| Yes | 57 (14) | 35 (8) |
| . | At baseline . | After 12 months of HU . |
|---|---|---|
| Median PLT, ×109/L (range) | 906 (258-3920) | 494 (149-1356) |
| Less than 400 × 109/L, n (%) | 5 (1) | 107 (26) |
| 400-600 × 109/L, n (%) | 16 (4) | 237 (57) |
| More than 600 × 109/L, n (%) | 395 (95) | 72 (17) |
| Median WBC, ×109/L (range) | 9.4 (4.2-34) | 6.7 (3.2-28.9) |
| Less than 10 × 109/L, n (%) | 267 (64) | 371 (89) |
| More than 10 × 109/L, n (%) | 149 (36) | 45 (11) |
| Aspirin-resistant microvascular symptoms, n (%) | ||
| No | 313 (75) | 368 (88) |
| Yes | 103 (25) | 48 (12) |
| Splenomegaly, n (%) | ||
| No | 359 (86) | 381 (92) |
| Yes | 57 (14) | 35 (8) |
PLT indicates platelets.
Six thrombotic episodes occurred within the first year of treatment and 27 events (70% arterial) were registered during follow-up (Table 3). Overall, the thrombotic rate was 1.66% patients/year, whereas the rate of hematologic transformations was 0.68% patients/year. Deaths were registered in 23 patients (5.5%) and occurred after a median of 5.7 years from HU start (rate, 0.9% patients/year).
Outcomes during follow-up
| Outcome . | Value . |
|---|---|
| Years of follow-up after 1 y of HU treatment, median (range) | 3.9 (0-20.7) |
| Less than 5 y, n (%) | 245 (59) |
| 5-10 y, n (%) | 122 (29) |
| More than 10 y, n (%) | 49 (12) |
| Thrombosis, n (%) | 27 (6.5) |
| AMI | 8 (30) |
| Stroke/TIA | 9 (33) |
| PAT | 2 (7) |
| VTE | 8 (30) |
| Rate of thrombosis, percentage of patients/y | 1.66 |
| Hematologic transformations, n (%) | 16 (3.8) |
| MF | 10 (63) |
| AML | 6 (37) |
| Rate of hematologic transformations, % of patients/y | 0.68 |
| Deaths, n (%) | 23 (5.5) |
| Thrombotic cause | 3 (13) |
| Hemorrhagic cause | 1 (4) |
| Post-ET MF and AML | 6 (26) |
| Solid tumors | 3 (13) |
| Other cause | 10 (43) |
| Rate of deaths, % of patients/y | 0.93 |
| Outcome . | Value . |
|---|---|
| Years of follow-up after 1 y of HU treatment, median (range) | 3.9 (0-20.7) |
| Less than 5 y, n (%) | 245 (59) |
| 5-10 y, n (%) | 122 (29) |
| More than 10 y, n (%) | 49 (12) |
| Thrombosis, n (%) | 27 (6.5) |
| AMI | 8 (30) |
| Stroke/TIA | 9 (33) |
| PAT | 2 (7) |
| VTE | 8 (30) |
| Rate of thrombosis, percentage of patients/y | 1.66 |
| Hematologic transformations, n (%) | 16 (3.8) |
| MF | 10 (63) |
| AML | 6 (37) |
| Rate of hematologic transformations, % of patients/y | 0.68 |
| Deaths, n (%) | 23 (5.5) |
| Thrombotic cause | 3 (13) |
| Hemorrhagic cause | 1 (4) |
| Post-ET MF and AML | 6 (26) |
| Solid tumors | 3 (13) |
| Other cause | 10 (43) |
| Rate of deaths, % of patients/y | 0.93 |
Percentages of different types of thrombosis, hematologic transformations, and cause-specific deaths relate to the total number of thromboses, hematologic transformations, and deaths, respectively.
AMI indicates acute myocardial infarction; TIA, cerebral transient ischemic attack; PAT, peripheral arterial thrombosis; VTE, venous thromboembolism; MF, myelofibrosis; and AML, acute myeloid leukemia.
Table 4 shows the results of univariate and multivariate analysis of thrombosis predictors. Age and previous thrombosis were independently associated with vascular events, whereas achievement of ELN responses, evaluated at 12 months of therapy, did not predict future vascular events. When individual variables included in the ELN response criteria were considered, we observed that different levels of platelet count did not influence the outcome; in contrast, patients with more than 10 × 109/L leucocytes had a significant higher rate of thrombosis independently from the other variables. There was no correlation between the achievement of response and hematologic transformation (data not shown).
Univariate and multivariate analysis for risk factors predicting thrombotic events in follow-up (events, n = 27)
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Time elapsed from diagnosis (> 5 vs < 5 mo) | 0.84 | 0.39-1.79 | .65 | 0.85 | 0.39-1.85 | .68 |
| Sex (male vs female) | 0.89 | 0.39-1.98 | .77 | 0.89 | 0.39-2.02 | .78 |
| Age > 60 y | 2.37 | 0.97-5.78 | .06 | 2.99 | 1.19-7.51 | .02 |
| Previous thrombosis | 3.23 | 1.45-7.18 | .004 | 3.63 | 1.61-8.16 | .002 |
| JAK2V617F | 1.17 | 0.51-2.71 | .71 | 0.96 | 0.34-2.46 | .94 |
| Response to HU | ||||||
| CR | 1 (ref) | |||||
| PR | 1.38 | 0.62-3.10 | .43 | 5.08 | 0.65-16 | .12 |
| No response | 1.00 | 0.35-2.45 | .87 | 4.47 | 0.45-25 | .20 |
| PLT,* ×109/L | ||||||
| Less than 400 | 1 (ref) | 1 (ref) | ||||
| More than 400 | 1.04 | 0.39-2.74 | .95 | 0.24 | 0.04-1.58 | .14 |
| WBC, ×109/L | ||||||
| Less than 10 | 1 (ref) | 1 (ref) | ||||
| More than 10 | 2.85 | 1.27-6.39 | .01 | 2.86 | 1.23-6.66 | .015 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Time elapsed from diagnosis (> 5 vs < 5 mo) | 0.84 | 0.39-1.79 | .65 | 0.85 | 0.39-1.85 | .68 |
| Sex (male vs female) | 0.89 | 0.39-1.98 | .77 | 0.89 | 0.39-2.02 | .78 |
| Age > 60 y | 2.37 | 0.97-5.78 | .06 | 2.99 | 1.19-7.51 | .02 |
| Previous thrombosis | 3.23 | 1.45-7.18 | .004 | 3.63 | 1.61-8.16 | .002 |
| JAK2V617F | 1.17 | 0.51-2.71 | .71 | 0.96 | 0.34-2.46 | .94 |
| Response to HU | ||||||
| CR | 1 (ref) | |||||
| PR | 1.38 | 0.62-3.10 | .43 | 5.08 | 0.65-16 | .12 |
| No response | 1.00 | 0.35-2.45 | .87 | 4.47 | 0.45-25 | .20 |
| PLT,* ×109/L | ||||||
| Less than 400 | 1 (ref) | 1 (ref) | ||||
| More than 400 | 1.04 | 0.39-2.74 | .95 | 0.24 | 0.04-1.58 | .14 |
| WBC, ×109/L | ||||||
| Less than 10 | 1 (ref) | 1 (ref) | ||||
| More than 10 | 2.85 | 1.27-6.39 | .01 | 2.86 | 1.23-6.66 | .015 |
Variables at 12 months of HU treatment. PLT indicates platelets.
For PLT > 600 × 109/L; hazard ratio = 1.05; P = .91.
By Kaplan-Meier analysis, projected thrombosis-free survival in CR patients was 90%. Thrombosis-free survival was significantly lower in the 45 patients (11%) who did not achieve leukocyte normalization, although it was independent from the control of platelet number (Figure 2).
Discussion
The results of this retrospective analysis were obtained in 3 qualified hematologic centers and well reflect clinical practice outside clinical trials. Patients were started with HU on 0.5 to 1 g daily, and doses were adjusted to maintain the platelet counts less than 600 × 109/L. The drug was continued throughout the remaining time of follow-up in the majority of patients (398 of 416). Reasons for 18 withdrawals included patient choice or other protocols and side effects in 6 and 12 cases, respectively.
The present study had 2 main purposes. The first was to estimate the frequency of CH responses, as defined by the ELN experts.9 In a large population of ET patients, we found that CR rate progressively increased over time up to a maximum of 25% after 12 months. The majority (58%) of patients receiving HU achieved a PR, as defined by a platelet count in the range of 400 to 600 × 109/L (n = 227 of 242, 94%) or a decrease of greater than or equal to 50% from baseline (6%). Among the latter, the reasons of PR were a persistence of leukocytosis (n = 28 of 242, 11%) or incomplete recovery from microvessel symptoms or splenomegaly (n = 37 of 242, 15%). No responders were 72 of 416 (17%), indicating that the proportion of HU-resistant patients, in whom second-line therapy is indicated, is relatively small. Interestingly, the probability of reaching responses was higher in patients aged 60 years or older and in those with a JAKV617F mutated status, supporting a greater sensitivity to the drug in this latter group as previously reported.10
The second aim of our analysis was to correlate the ELN responses with clinical outcomes. CH responses were not associated with hematologic transformation or death. The rate of myelofibrosis and acute leukemia found in our study (0.68% patients/year) is comparable with that of patients in the HU arm of PT1 trial (0.84% patients/year, P = .33). Deaths were registered in 23 cases (5.5%) with a rate of 0.9% patients/year and appear to be lower than in PT1 trial (rate = 2% patients/year, P = .007).
Overall, the rate of major vascular events and hematologic transformation recorded in clinical practice were comparable with the PT1 trial.2 In that RCT, the actuarial rate of first thrombosis in HU arm was 2% at first year, similar to the 1.66% patients/year rate of major vascular events measured in the present study. However, at variance with the HU arm of PT1, and in accordance to the Italian RCT,1 the rate of venous thrombosis in our cohort was significantly lower. Indeed, considering the median follow-up and the number of venous thrombosis reported in PT1 study, we have estimated that the rate of events in the HU arm was 1.4%/patients/year. This value is significantly higher than in our cohort (rate, 0.4% patients/year, P = .001) and is similar to that obtained in the PT1 anagrelide arm (0.2% patients/year, P = .83). Thus, our experience is not in keeping with the high prevalence of venous thrombosis by HU reported in the PT1 trial.
In this series, predictors of future vascular events were age and previous thrombosis, according to established criteria. However, unexpectedly, we found that the ELN responses after 12 months of HU treatment did not predict future major vascular complications in a multivariate model considering meaningful variables. In particular, the rate of major thrombosis in the 3 categories of responses was not related to the achievement of platelet targets less than 400 × 109/L or less than 600 × 109/L, indicating that normalization of platelet count did not translate in a clinical benefit in terms of major vascular events reduction. Instead, thrombosis-free survival was significantly worse in the group of patients in whom leukocyte count remained elevated after one year of treatment compared with those achieving WBC normalization (P = .017). This result confirms that leukocyte count is a prognostic factor for thrombosis and supports the recommendation that WBC should be a target of therapy. Many recent studies have provided a biologic plausibility of these epidemiologic observations.6,11,12 Both in ET and PV, an in vivo leukocyte activation has been consistently documented, in association with signs of activation of both platelets and endothelial cells, particularly in patients carrying the JAK2 V617F mutation. Thus, leukocyte and platelet activation may play a role in the generation of the prethrombotic state that characterizes these disorders.
We are aware that the present study suffers from some limitations connected with its retrospective design. Thus, we suggest that confirmation in prospective studies is necessary before recommending a revision of ELN criteria. However, our findings indicated that, at least in patients receiving HU, defining a response of platelet count may not be clinically meaningful considering the lack of correlation with vascular events, unlike the case for leukocyte count. This information can have relevance for future clinical trials with novel Jak2 inhibitors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Fondazione Italiana per la Ricerca sul Cancro (A.C. fellowship), Associazione Italiana per la Ricerca sul Cancro, European LeukemiaNet Sixth Framework Program (LSH-2002-2.2.0-3), MIUR-PRIN Projects (A.M.V.), Istituto Toscano Tumori (A.M.V.), and Associazione Italiana Lotta alla Leucemia AIL, sezione Paolo Belli, Bergamo.
Authorship
Contribution: A.C., T.B., and G.F. designed the study and wrote the manuscript; A.C. analyzed the data and designed the figures and tables; A.C., E.A., M.R., and F.D. collected clinical data and performed research; and A.M.V., G.B., F.R, A.R., and T.B. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tiziano Barbui, Divisione di Ematologia, Ospedali Riuniti, Largo Barozzi 1, 24128 Bergamo, Italy; e-mail: tbarbui@ospedaliriuniti.bergamo.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal