Abstract
Abstract 2967
Zoledronic Acid (ZOL) has demonstrated up to half a year improvement in survival of multiple myeloma patients. This conjoins with prior experimental studies to support a potential anti-myeloma effect for bisphosphonates. However, the in vivo effect can be confounded with the concomitant use of chemotherapy and new drugs, as well as with competing risks that emerge from the beneficial effect of bisphosphonates on the skeletal related events and its relationship with survival. Accordingly, the real anti-tumor effect of ZOL remains to be elucidated in clinical practice, for example in patients free of the disturbing effect of chemotherapy. New therapies provide an 80–90% response rate in MM, although most patients ultimately relapse. During relapse, many patients show an initial re-positivization or re-growth of the M-component (biochemical relapse) that is usually not accompanied by clinical symptoms. The usual decision at this point is not to treat such patients. Thus, these asymptomatic patients represent a perfect group to explore the antitumor benefit of ZOL in the absence of any other cytotoxic therapy.
To analyze the anti-tumor effect of ZOL in the absence of any other cytotoxic drug in MM patients under asymptomatic biochemical relapse. Primary end-point was Symptomatic Progression Free Survival (sPFS). Secondary evaluation variables were response rate, skeletal related events and time to next chemotherapy.
192 patients are calculated to be recruited in a randomized, prospective, open label phase IV trial in which a group of patients receive ZOL (4 mg iv./4 wk, 12 doses) and Best Supportive Care (BSC) and the rest only BSC. All patients are monitorized every 4 wk.
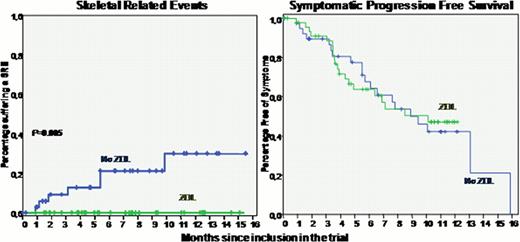
This is an interim analysis corresponding to the first 93 patients included in the trial: 49 treated with ZOL and 44 without ZOL. Asymptomatic Biochemical Relapse was confirmed in all patients who had a median age of 68 yr (40–87) and a male female distribution of 50/43. M-component distribution was IgG (69%), IgA (29%) and only light chain (2%). Relapse had presented after 1, 2 or 33 lines of therapy in 67%, 22% and 11% of cases, respectively. Prior treatment had always included transplant (65%), bortezomib (33%), IMiDs (33%), or a combination of them. Lytic bone lesions were present in 66% of patients and one or two skeletal related events (SRE) had presented prior to the inclusion in the trial in 31% of cases. FISH/cytogenetics was abnormal in 49% of cases: t(11;14) 18%, Rb deletion (alone) 16%, del(p53) 9%, t(4;14) 6% and t(14;x) 4%. After randomization, both groups of patients were well balanced in terms of prognostic features, prior response, and time from diagnosis and relapse to the inclusion in the trial. 25 patients have completed the program and four terminated before completion due to patient refusal (n=2) and development of other diseases (n=2). 31 patients are still ongoing and 38 have progressed before 12 mo of treatment, with a median sPFS of 287 days (9.4 months) with similar results between the two arms (271 vs. 308 days for patients receiving or not receiving ZA, respectively; 12-month projected sPFS was 47% vs. 42%, respectively). Interestingly, patients not treated with ZOL progressed with more advanced bone disease (8 cases of new bone lesions or re-growth of prior lesions, 1 spinal cord compression, and 2 cases of hypercalcemia) vs. patients treated with ZOL (two cases re-growth of bone lesions, p<0.01). By contrast, patients treated with ZOL progressed more frequently with decrease o Hb level below 10 g/dL (13 vs. 8 cases). There were 7 SRE that presented only in the group of patients treated without ZOL (p=0.005)
Zoledronic Acid therapy in MM with asymptomatic relapse seems to reduce the risk of progression with symptomatic bone disease and SREs. The possible antitumor effect of Zoledronic Acid alone in biochemical relapses cannot be elucidated yet. This interim analysis supports the continuation of the trial to reach a higher number of patients and longer follow-up.
García-Sanz:Novartis Oncology: Grant Support Other. De La Rubia:Celgene, Janssen: Consultancy, Speakers Bureau. Granell:Janssen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal