Abstract
Abstract 2966
Poor survival in AL amyloidosis is largely driven by outcomes in patients with advanced cardiac disease. To date, the Mayo cardiac staging system is the most widely used tool to identify these high risk patients. For stage III patients few treatment options exist to modify the natural history of this disease with up to 50% dying within the first 6 months. Moreover, there are currently no studies comparing different regimens in the novel agent era specifically addressing this group. Here we present a matched comparison examining response and survival endpoints after upfront treatment in Mayo stage III patients using either Cyclophosphamide, Bortezomib and Dexamethasone (CVD) or Cyclophosphamide, Thalidomide and Dexamethasone (CTD), the current standard of care for this disease in the United Kingdom.
The primary cohort comprises 78 patients (39 in each arm) referred to the National Amyloidosis Centre in London between 2008–2012. All patients had cardiac involvement by the 2005 consensus criteria and all were Mayo stage III. The CVD cohort reflects all patients seen at the NAC with Mayo stage III disease treated with this regimen upfront. Based on baseline NT-proBNP (>8000ng/L) and dFLC (>180mg/L) the patients were then matched with a recent cohort treated with CTD as first line therapy. The CVD and CTD regimens were recommended as previously described (1, 2 ). Dose modifications were at the discretion of the treating haematologist. Both conventional haematologic responses and dFLC responses were examined (3, 4 ). Overall survival (OS) was calculated by the Kaplan-Meier method and calculated from the start of treatment until death or last follow-up. To correct for the influence of early deaths on response rates a landmark analysis was performed in patients surviving at least 3 months from treatment (n=21 (CVD) and n=30 (CTD)).
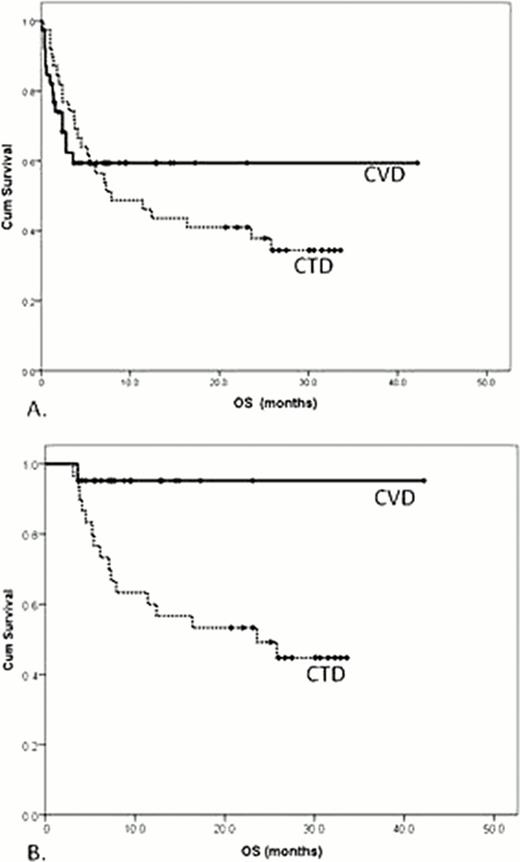
In the intention-to-treat (ITT) cohort response rates are comparable although there was a trend to higher CR rates with the CVD regimen (table 1). On an ITT basis, there was no statistically significant difference in the 1-year OS (59.4% vs 46.2% for CVD and CTD respectively, p = 0.9, figure 1a). A high rate of early deaths is noted. 23.7% of CVD and 13.1% of CTD patients died within 6 weeks (p = 0.24). 36.8% of CVD and 23.7% of CTD patients died within 3 months (p = 0.22). In the landmark analysis upfront therapy with CVD correlated with an improved 1-year OS (94% vs 62.1%, p = 0.01, figure 1 b). This may be partly driven by the increased CR rate in the CVD cohort compared to those receiving CTD (47% vs 24% respectively, p = 0.03, table 1).
Compared with CTD, treatment with CVD was not associated with a reduction in the high rate of early deaths often seen in patients with Mayo cardiac stage III disease. However, these data suggest that survival of patients treated with CVD upfront may be superior among those who remain alive after the first 3 months, consistent with the higher CR rates achieved. While it did not reach statistical significance the high rate of early deaths indicates that further optimisation and better supportive care strategies are required during the early stages of treatment especially with CVD. Ongoing phase III trials are currently underway to address these issues in a prospective manner.
The ITT cohort is shown in (A) and the landmark cohort is shown in (B). Solid and dashed lines reflect CVD and CTD treated patients respectively.
Wechalekar:Janssen-Cilag: Honoraria.
REFERENCES
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal