Abstract
The engagement of inhibitory receptors specific for major histocompatibility complex class I (MHC-I) molecules educates natural killer (NK) cells, meaning the improvement of the response of activation receptors to subsequent stimulation. It is not known whether inhibitory MHC-I receptors educate only NK cells or whether they improve the responsiveness of all cell types, which express them. To address this issue, we analyzed the expression of inhibitory MHC-I receptors on intestinal intraepithelial lymphocytes (iIELs) and show that T-cell receptor (TCR)-αβ CD8αα iIELs express multiple inhibitory receptors specific for MHC-I molecules, including CD94/NKG2A, Ly49A, and Ly49G2. However, the presence of MHC-I ligand for these receptors did not improve the response of iIELs to activation via the TCR. The absence of iIEL education by MHC-I receptors was not related to a lack of inhibitory function of these receptors in iIELs and a failure of these receptors to couple to the TCR. Thus, unlike NK cells, iIELs do not undergo an MHC-I–guided education process. These data suggest that education is an NK cell–specific function of inhibitory MHC-I receptors.
Introduction
Natural killer (NK) cells express activating and inhibitory receptors that are specific for both major histocompatibility complex class I (MHC-I) and non–MHC-I molecules. Most activating receptors are specific for non–MHC-I ligands that are expressed on both normal and/or diseased host cells. Many inhibitory receptors are specific for MHC-I molecules. Mouse NK cells recognize classic MHC-I molecules and the nonclassic MHC-I molecule Qa-1b using inhibitory Ly49 family receptors1 and the CD94/NKG2A receptor,2 respectively. Inhibitory MHC-I receptors counteract NK-cell activation by healthy cells. Consequently, cells become sensitive to NK cell lysis when MHC-I expression is aberrantly low because of viral infection or malignant transformation. This is known as missing-self recognition.3
NK cells develop in normal numbers in MHC-I–deficient mice or patients. However, their activation receptors respond poorly to stimulation,4,5 which probably explains the lack of autoimmune-related processes in these patients. In addition, MHC-I–sufficient mice and humans have NK cells that do not express inhibitory MHC-I receptors or that express exclusively inhibitory MHC-I receptors for the wrong MHC-I. NK-cell activation receptors on such NK cells do also respond poorly to stimulation.6-8 On the other hand, activation receptors on NK cells, which express an inhibitory receptor for self-MHC-I respond efficiently to stimulation. Thus, the interaction between inhibitory receptors and MHC-I molecules mediates a functional maturation of NK cells. This is referred to as NK cell education.6-12
In addition to NK cells, inhibitory MHC-I receptors are expressed by other cell types, including intestinal intraepithelial lymphocytes (iIELs), NKT, and CD8 memory T cells.13-18 iIELs can be divided into type a or conventional T cells and type b or nonconventional T cells based on T-cell receptor (TCR) usage and expression of coreceptors. Type a mucosal T cells include MHC-I– and MHC-II–restricted T cells expressing αβ TCRs in combination with CD8αβ and CD4, respectively, whereas type b iIELs express αβ TCRs or γδ TCRs in combination with CD8αα.19 In addition, type b iIELs lack typical T-cell markers, such as Thy-1, CD2, and CD28, but they express FcϵRIγ, tactile, and several NK-cell receptors.13-15,20-22
Gut iIELs are of thymic origin and require thymic positive selection that directs lineage commitment and functional differentiation.23-25 Many TCR-αβ CD8αα iIELs are self-reactive as they undergo a self-antigen–dependent agonist selection process.26 Indeed, these cells have an antigen-experienced, memory phenotype, and they are characterized by a so-called activated yet resting state. They have a cytolytic effector phenotype, based on the expression of perforin, granzyme A and B, and Fas ligand. Finally, they constitutively express mRNA for various chemokines and immune regulators, such as macrophage inflammatory protein-1α (MIP-1α), MIP-1β, lymphotactin, regulated on activation normal T expressed and secreted, and macrophage migration inhibitory factor.14,27 However, despite their self-reactive TCR repertoire, TCR-αβ iIELs are able to prevent inflammatory responses in the intestine.28,29
In this study, we addressed the question of whether inhibitory MHC-I receptors can improve the functional properties of cell types other than NK cells. To this end, we investigated the expression of inhibitory MHC-I receptors on iIELs and tested the functional response of relevant iIEL subsets compared with NK cells. Although the engagement of MHC-I receptors enhanced the function of NK cells, it failed to improve the functional response of CD8αα iIELs. These results suggest that education by inhibitory MHC-I receptors is a specific feature of NK cells.
Methods
Mice
C57BL/6 (B6) mice were purchased from Harlan. H-2Dd (B6 Dd) heterozygous transgenic mice30 were maintained by breeding with B6 mice. H-2Dd Tg mice were identified by staining peripheral blood cells with anti–H-2Dd monoclonal antibody (mAb). H-2KbDb double-deficient mice were purchased from Taconic Farms and maintained as homozygotes. Mice were housed and bred in our animal facility, and all animal experimentation was performed after approval and according to the guidelines of the Ethical Committee for Experimental Animals at the Faculty of Medicine and Health Sciences of Ghent University.
Isolation of iIELs and splenocytes
iIELs from 6- to 10-week-old mice were isolated as described elsewhere, with few adaptations.13 Briefly, small intestines were removed and carefully cleaned from their mesentery, Peyer patches were excised, and intestines were washed of fecal contents with PBS. Intestines were subsequently opened longitudinally, cut into 0.5-cm pieces, transferred into 50-mL conical tubes, and incubated twice during 20 minutes at 37°C in Ca/Mg-free HBSS (Invitrogen) plus 5% FCS with 1mM EDTA (Invitrogen) and 1mM dithiothreitol (Sigma-Aldrich) at slow rotation. The cell suspensions were passed through a 40-μm cell strainer (Falcon) and subsequently pelleted by centrifugation at 480g. Cell pellets were resuspended in 44% Percoll (GE Healthcare), underlain with 67% Percoll, and centrifuged at 2000g for 20 minutes. iIELs from the 44%/67% interface were collected, washed twice, and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2mM glutamine, 1mM sodium pyruvate, 100μM nonessential amino acids (all from Invitrogen), and 50μM 2-mercaptoethanol (Sigma-Aldrich), unless otherwise stated.
Spleens from 6- to 10-week-old mice were removed, disrupted, and passed through a 40-μm cell strainer. Erythrocytes were lysed with ACK lysing buffer (Invitrogen), subsequently washed twice and resuspended in RPMI 1640 medium supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2mM glutamine, and 50μM 2-mercaptoethanol, unless otherwise stated.
Antibodies
mAbs used for staining/stimulation were anti-CD3 (unconjugated or FITC-conjugated, clone 145-2C11), anti-CD49b (allophycocyanin [APC]–conjugated, clone DX5), anti-CD69 (phycoerythrin [PE]–conjugated, clone H1.2F3), anti–IFN-γ (PE-conjugated, clone XMG1.2), anti-KLRG1 (biotin-conjugated, clone 2F1), anti-Ly49A (biotin-conjugated, clone A1), anti-Ly49A/D (PE-conjugated, clone 12A8), anti-Ly49C/I (biotin-conjugated, clone 5E6), anti-Ly49D (PE-conjugated, clone 4E5), anti-Ly49F (PE-conjugated or biotin-conjugated, clone HBF-719), anti-NK1.1 (unconjugated or APC-conjugated, clone PK136), anti-NKG2D (anti-CD314, PE-conjugated, clone CX5), anti–TCR-γδ (PE- or FITC-conjugated, clone GL3), anti-2B4 B6 alloantigen (anti-CD244.2, biotin-conjugated, clone 2B4), anti-Ly49G2 (APC-conjugated, clone 4D11) and mouse IgG1 and mouse IgG2a,κ (unconjugated; all from BD Biosciences). Anti-CD3 (PE/Cy7-conjugated, clone 145-2C11), anti-CD8α (PE/Cy7-conjugated, clone 53-6.7), anti-NKG2AB6 (unconjugated or biotin-conjugated, clone 16a11), and mouse IgG2b,κ (unconjugated; all from eBioscience). Anti-CD4 (peridinin chlorophyll protein/Cy5.5-conjugated, clone GK1.5), anti-CD8β (peridinin chlorophyll protein/Cy5.5-conjugated, clone YTS156.7.7), anti–TCR-β (APC/Cy7-conjugated, clone H57.597), and anti–H2-Dd (PE-conjugated, clone 34-2-12) were from BioLegend. Anti-lymphotactin (anti-ATAC, biotin-conjugated, clone 80222), anti-NKp46 (anti-CD335, PE-conjugated, clone 29A1.4), and anti–MIP-1α (anti-CCL3, PE-conjugated, clone 39624) were from R&D Systems. Anti–β2-microglobulin (PE-conjugated, clone S19.8) was from Santa Cruz Biotechnology. Anti–IL-2Rβ (anti-CD122, FITC-conjugated, clone TM-β1) was kindly provided by Dr T. Tanaka (Tokyo, Japan). Anti-Ly49A (biotin-, FITC-conjugated or unconjugated, clone JR9–318) was kindly provided by Dr J. Roland (Paris, France). Anti-Ly49E/C (biotin- or FITC-conjugated, clone 4D12) was made in-house and labeled.31 Anti-Ly49E/F (FITC-conjugated, clone CM4) was kindly provided by Dr C. G. Brooks (Newcastle on Tyne, United Kingdom).32 mAb 4D12 (Ly49C/E) plus mAb CM4 (Ly49E/F) were used to identify Ly49C-expressing cells (4D12+CM4−). In B6 (H-2b) mice, mAb 5E6 stains Ly49I, whereas Ly49C is very hard to detect.33 The lack of Ly49C staining by 5E6 using H-2b NK cells is the result of masking of the 5E6 epitope by cis interaction of Ly49C with H2-Kb, whereas 4D12 staining is not affected (W.H. laboratory, unpublished data, June 2007). Anti-Ly49G2–producing hybridoma (clone 4D11) was from ATCC and antibody was FITC-conjugated in-house. Anti-Ly49H (clone 3D10) was kindly provided by Dr W. Yokoyama, (St Louis, MO) and biotinylated in-house. Anti-NKG2A/C/E (FITC-conjugated, clone 3S9) was generated in-house and labeled,31 and Dd-HIV multimer34 was prepared in the laboratory of Dr W. Held (Epalinges, Switzerland). Biotinylated mAbs were subsequently detected by streptavidin (APC- or PE-conjugated, BD Biosciences; APC-eFluor780-conjugated, eBioscience).
Before staining, cells were blocked with anti-FcγRII/III (unconjugated, clone 2.4G2, kindly provided by Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY). Propidium iodide or the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen) was used to discriminate live and dead cells. Flow cytometry was performed using a BD LSR II flow cytometer and samples were analyzed with FACSDiva Version 6.1.2 software (BD Biosciences).
In vitro stimulation
For in vitro iIELs stimulation, 24-well tissue culture plates were coated with 10 μg/mL anti-CD3 mAb, 5 μg/mL anti-CD3 mAb plus 5 μg/mL anti-Ly49A mAb (clone JR9–318 or clone A1), or mouse IgG1/IgG2a,κ isotype control, 5 μg/mL anti-CD3 mAb plus 5 μg/mL anti-Ly49G2 mAb (clone 4D11) or mouse IgG2a,κ isotype control and with 5 μg/mL anti-CD3 mAb plus 5 μg/mL anti-NKG2A/C/E mAb (clone 3S9) or anti-NKG2B6 mAb (clone 16a11) or an IgG2b,κ isotype control, all in 625 μL PBS per well at 4°C during 16 hours. Wells were washed 3 times with PBS before iIELs were seeded at 1.25 to 1.875 × 106 cells/well. Alternatively, for Ly49A/Ly49G2/Ly49E/Ly49F sequestration experiments, iIELs were preincubated with 1 μg/mL anti-Ly49A mAb (clone JR9–318)/anti-Ly49G2 mAb (clone 4D11)/anti-Ly49E mAb (clone 4D12)/anti-Ly49F mAb (clone HBF-719) at room temperature for 15 minutes. Thereafter, cells were plated in anti-CD3–coated wells. Cells were incubated at 37°C, 5% CO2 during 15 hours, and 1 μg/mL brefeldin A (BD Biosciences) was added during the last 12 hours. For phorbol myristate acetate (PMA)/ionomycin stimulation, iIELs were seeded at 2 × 106 cells per 24-well and 50 ng/mL PMA plus 4500 ng/mL ionomycin (both from Sigma-Aldrich) was added. Cells were incubated at 37°C, 5% CO2 during 4 hours, and 1 μg/mL brefeldin A was added during the last 3 hours. Subsequently, cells were harvested and stained. To determine cytokine production, cell membrane staining was performed first, after which cells were fixed and permeabilized with the Cytofix/Cytoperm Kit (BD Biosciences) and stained for cytokines. Cytokine production is shown as the percentage of intracellular cytokine-positive cells within each CD8αα iIEL subpopulation, whereby cytokine production in the absence of TCR stimulation was subtracted. Further, to allow comparisons of independent experiments, cytokine production was normalized to that seen in CD8αα iIELs cells, which do not express inhibitory MHC-I receptors.
For in vitro splenocyte NK cell stimulation, 96-well tissue culture plates were coated with 5 μg/mL anti-NK1.1 mAb, in 100 μL PBS per well at 4°C during 16 hours. Wells were washed 3 times with PBS before splenocytes were seeded at 1 × 106 cells/well in 100 μL. Cells were incubated 5 hours at 37°C, 5% CO2 in the presence of 1 μg/mL brefeldin A and 1000 U/mL recombinant human–IL-2 (Roche Diagnostics). To determine MIP-1α and IFN-γ production, cells were fixed and permeabilized with the Cytofix/Cytoperm Kit after cell surface staining.
Determination of Ly49A-MHC-I cis interaction
Purified iIELs or spleen cell suspensions were acid stripped and stained with Dd-HIV multimer as described elsewhere.35 Briefly, cells were washed twice in PBS and resuspended in citrate buffer (0.133M citric acid/0.066M Na2HPO4, pH 3.3) at 5 × 106 cells/mL for 4 minutes at room temperature. The treatment was stopped by the addition of an excess of PBS supplemented with 1% BSA, fraction V (Roche Diagnostics) and 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen). After washing, the cells were stained for flow cytometry. Acid treatment did not affect cell viability as judged by trypan blue/propidium iodide exclusion.
Results
TCR-αβ CD8αα iIELs express inhibitory MHC-I receptors
We wanted to investigate whether inhibitory MHC-I receptors educated cell types other than NK cells. iIELs are known to express MHC-I receptors,13-15 but a detailed analysis of their distribution on the different iIEL subsets has not been reported. Marked expression of inhibitory MHC-I receptors was observed on TCR-αβ CD8αα iIELs, but not on TCR-γδ and conventional TCR-αβ CD8αβ and CD4 subsets (Figure 1A). TCR-αβ CD8αα iIELs from B6 mice (H-2b) expressed the inhibitory MHC-I receptors Ly49A, Ly49E, Ly49F, and Ly49G2, whereas the fraction of cells expressing Ly49C was very low and Ly49I was absent (Figure 1A). Note that the expression of these receptors was readily detected on spleen NK cells (Figure 1A). In addition, TCR-αβ CD8αα iIELs stained with an mAb specific for NKG2A/C/E (mAb 3S9), and approximately 80% of these cells were positive for NKG2A (mAb 16a11; Figure 1A), indicating that a considerable fraction of CD8αα iIELs expresses the inhibitory CD94/NKG2A receptor, which is specific for the nonclassic MHC-I molecule Qa-1b.2 Thus, TCR-αβ CD8αα iIELs express multiple inhibitory receptors specific for MHC-I molecules. NK cells are known to coexpress inhibitory MHC-I receptors whereby the extent of coexpression of 2 receptors corresponds well to the product of the frequencies of NK cells expressing each receptor (“product rule”).36,37 In contrast, the coexpression of 2 inhibitory MHC-I receptors on CD8αα iIELs is considerably higher than the product of the individual frequencies (Figure 1B). These data, together with the near absence of Ly49C and Ly49I expression, indicate that the expression of inhibitory MHC-I receptors on iIELs follows rules that are distinct from those of NK cells.
Inhibitory MHC-I receptors are preferentially expressed on CD8αα TCR-αβ iIELs. (A) Analysis of NK-cell receptor expression by iIELs and spleen NK cells from B6 mice. iIELs were subdivided into the following groups: (top) ■, TCR-αβ CD8αα;  , TCR-αβ CD8αβ; and □, TCR-αβ CD4; and (middle) ■, TCR-γδ CD8αα; and □, TCR-γδ CD8− CD4− (DN). The bar graph represents the mean percentage (± SEM) of cells expressing the indicated NK-cell receptor by the indicated iIEL subset (n = 3-10) or by splenic NK cells (CD3−NK1.1+; n = 4-9; bottom) in B6 mice. (B) Representative analysis of Ly49A, Ly49G2, and NKG2A/C/E coexpression by CD8αα iIELs (CD4−CD8β −CD8α+; top) and spleen NK cells (CD3−NK1.1+; bottom). Numbers indicate the percentage of cells expressing or coexpressing the specific MHC-I receptor(s). Numbers in parentheses represent the frequency of cells coexpressing 2 MHC-I receptors as predicted by the product rule.
, TCR-αβ CD8αβ; and □, TCR-αβ CD4; and (middle) ■, TCR-γδ CD8αα; and □, TCR-γδ CD8− CD4− (DN). The bar graph represents the mean percentage (± SEM) of cells expressing the indicated NK-cell receptor by the indicated iIEL subset (n = 3-10) or by splenic NK cells (CD3−NK1.1+; n = 4-9; bottom) in B6 mice. (B) Representative analysis of Ly49A, Ly49G2, and NKG2A/C/E coexpression by CD8αα iIELs (CD4−CD8β −CD8α+; top) and spleen NK cells (CD3−NK1.1+; bottom). Numbers indicate the percentage of cells expressing or coexpressing the specific MHC-I receptor(s). Numbers in parentheses represent the frequency of cells coexpressing 2 MHC-I receptors as predicted by the product rule.
Inhibitory MHC-I receptors are preferentially expressed on CD8αα TCR-αβ iIELs. (A) Analysis of NK-cell receptor expression by iIELs and spleen NK cells from B6 mice. iIELs were subdivided into the following groups: (top) ■, TCR-αβ CD8αα;  , TCR-αβ CD8αβ; and □, TCR-αβ CD4; and (middle) ■, TCR-γδ CD8αα; and □, TCR-γδ CD8− CD4− (DN). The bar graph represents the mean percentage (± SEM) of cells expressing the indicated NK-cell receptor by the indicated iIEL subset (n = 3-10) or by splenic NK cells (CD3−NK1.1+; n = 4-9; bottom) in B6 mice. (B) Representative analysis of Ly49A, Ly49G2, and NKG2A/C/E coexpression by CD8αα iIELs (CD4−CD8β −CD8α+; top) and spleen NK cells (CD3−NK1.1+; bottom). Numbers indicate the percentage of cells expressing or coexpressing the specific MHC-I receptor(s). Numbers in parentheses represent the frequency of cells coexpressing 2 MHC-I receptors as predicted by the product rule.
, TCR-αβ CD8αβ; and □, TCR-αβ CD4; and (middle) ■, TCR-γδ CD8αα; and □, TCR-γδ CD8− CD4− (DN). The bar graph represents the mean percentage (± SEM) of cells expressing the indicated NK-cell receptor by the indicated iIEL subset (n = 3-10) or by splenic NK cells (CD3−NK1.1+; n = 4-9; bottom) in B6 mice. (B) Representative analysis of Ly49A, Ly49G2, and NKG2A/C/E coexpression by CD8αα iIELs (CD4−CD8β −CD8α+; top) and spleen NK cells (CD3−NK1.1+; bottom). Numbers indicate the percentage of cells expressing or coexpressing the specific MHC-I receptor(s). Numbers in parentheses represent the frequency of cells coexpressing 2 MHC-I receptors as predicted by the product rule.
Ly49A- and Ly49G2-expressing iIELs are hyporesponsive
To investigate the potential role of inhibitory MHC-I receptors on the function of CD8αα iIELs, we stimulated purified iIELs with plate-bound anti-CD3 mAb and measured MIP-1α and lymphotactin production, the main chemokines produced by iIELs. Because iIELs do not express Ly49C and Ly49I (Figure 1A) and Ly49E and Ly49F do not or do only weakly recognize MHC-I, respectively,34,38,39 we initially concentrated on the function of iIELs expressing the inhibitory MHC-I receptors Ly49A, Ly49G2, and CD94/NKG2.
CD8αα iIELs that do not express Ly49A, Ly49G2, and CD94/NKG2 produced high amounts of MIP-1α on TCR triggering (Figure 2A). Similarly, CD8αα iIELs expressing only CD94/NKG2 produced high amounts of MIP-1α. In contrast, CD8αα iIELs expressing only Ly49A or Ly49G2 produced very little MIP-1α. Similarly, cells expressing Ly49A or Ly49G2 in combination with CD94/NKG2 were also low producers of MIP-1α (Figure 2A). Corresponding results were obtained when evaluating lymphotactin production after TCR triggering (Figure 2B). Importantly, stimulation by PMA/ionomycin induced comparable MIP-1α and lymphotactin production by the different CD8αα iIEL subpopulations (Figure 2C-D). These data show that the Ly49A+ and Ly49G2+ iIEL populations are competent to produce chemokines but that membrane proximal activation events are impaired. Indeed, the inefficient chemokine production by Ly49A+ and Ly49G2+ iIELs was associated with lack of CD69 up-regulation after TCR stimulation. In contrast, CD69 up-regulation was observed on iIEL subsets expressing no MHC-I receptor or only CD94/NKG2 (Figure 2E). Thus, CD8αα iIELs, which express inhibitory Ly49 receptors, respond poorly to TCR stimulation, whereas those expressing CD94/NKG2 respond efficiently.
Ly49A- and Ly49G2-expressing CD8αα iIELs are hyporesponsive. MIP-1α and lymphotactin production by B6 iIELs on stimulation with plate-bound anti-CD3 mAb (A-B) or on stimulation with PMA/ionomycin (C-D) as determined by intracellular staining of CD8αα iIELs (co-)expressing Ly49A, Ly49G2, and/or NKG2A/C/E. MIP-1α and lymphotactin production, corrected for background staining (no anti-CD3 mAb), is shown as the percentage of positive cells within each CD8αα cell subpopulation (mean ± SEM; n = 12). *Significant difference (P < .05) compared with CD8αα iIELs lacking inhibitory MHC-I receptors (Mann-Whitney). (E) Histograms represent CD69 expression on B6 iIELs on stimulation with plate-bound anti-CD3 mAb: grey line represents unstained control; dashed line, no stimulation; and black line, anti-CD3 stimulation. Numbers indicate the mean percentage (± SEM) of CD69high cells in the indicated iIEL subpopulation on TCR-triggering (n = 5). *Significant (P < .05) CD69 up-regulation compared with CD8αα iIELs lacking inhibitory MHC-I receptors (Mann-Whitney).
Ly49A- and Ly49G2-expressing CD8αα iIELs are hyporesponsive. MIP-1α and lymphotactin production by B6 iIELs on stimulation with plate-bound anti-CD3 mAb (A-B) or on stimulation with PMA/ionomycin (C-D) as determined by intracellular staining of CD8αα iIELs (co-)expressing Ly49A, Ly49G2, and/or NKG2A/C/E. MIP-1α and lymphotactin production, corrected for background staining (no anti-CD3 mAb), is shown as the percentage of positive cells within each CD8αα cell subpopulation (mean ± SEM; n = 12). *Significant difference (P < .05) compared with CD8αα iIELs lacking inhibitory MHC-I receptors (Mann-Whitney). (E) Histograms represent CD69 expression on B6 iIELs on stimulation with plate-bound anti-CD3 mAb: grey line represents unstained control; dashed line, no stimulation; and black line, anti-CD3 stimulation. Numbers indicate the mean percentage (± SEM) of CD69high cells in the indicated iIEL subpopulation on TCR-triggering (n = 5). *Significant (P < .05) CD69 up-regulation compared with CD8αα iIELs lacking inhibitory MHC-I receptors (Mann-Whitney).
The presence of H-2Dd does not improve the response of Ly49A+ and Ly49G2+ CD8αα iIELs
The CD8αα iIEL subsets that respond poorly to TCR stimulation express the inhibitory receptors Ly49A and Ly49G2, which have no MHC-I ligand in B6 (H-2b) mice. These data raise the possibility that, similar to NK cells,6-8 the engagement of inhibitory MHC-I receptors is needed to educate iIELs (ie, to improve their response to TCR stimulation). To test this hypothesis, we used H-2Dd transgenic mice on a B6 background (B6 Dd mice), in which Ly49A and Ly49G2 encounter an MHC-I ligand.30 Ly49A and Ly49G2 expression in B6 Dd mice is similar to Ly49A and Ly49G2 expression in B6 mice (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Although H-2Dd is expressed in the small intestine (Figure 3A), MIP-1α production by anti-CD3–stimulated Ly49A+ and Ly49G2+ CD8αα iIELs was not improved (Figure 3B). As a control, we determined whether the H-2Dd transgene improved MIP-1α production by Ly49A+ NK cells. Indeed, as shown in Figure 3C, on stimulation with NK1.1 mAb, Ly49A+ NK cells from B6 Dd mice produced significantly more MIP-1α than those from B6 mice, in agreement with previously published data.6,40 Similarly, the presence of H-2Dd improved IFN-γ production by Ly49A+ NK cells (Figure 3C). Note that iIELs do not produce IFN-γ14 (and data not shown). In conclusion, the presence of H-2Dd educates Ly49A+ NK cells but fails to educate Ly49A+ or Ly49G2+ CD8αα iIELs.
The presence of MHC-I ligand does not improve the responsiveness of Ly49A+ and Ly49G2+ CD8αα iIELs. (A) Representative histograms show H2-Dd staining of cells isolated from the small intestine (left) and spleen (right) from B6 Dd transgenic mice: grey line represents unstained control; and black line, H2-Dd stained. (B) MIP-1α production by CD8αα iIEL subpopulations from B6 (■) or B6 Dd ( ) mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb. Bar graph represents the mean (± SEM). MIP-1α production by the indicated iIEL subpopulations is shown relative to the iIEL subset lacking inhibitory MHC-I receptors for B6 Dd (n = 4) and B6 mice (n = 12). The data for B6 mice are the same as those shown in Figure 2A. *Significant difference (P < .05) in cytokine production by B6 Dd and B6 iIELs (Mann-Whitney). (C) MIP-1α and IFN-γ production by spleen NK cells from B6 (■) and B6 Dd (
) mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb. Bar graph represents the mean (± SEM). MIP-1α production by the indicated iIEL subpopulations is shown relative to the iIEL subset lacking inhibitory MHC-I receptors for B6 Dd (n = 4) and B6 mice (n = 12). The data for B6 mice are the same as those shown in Figure 2A. *Significant difference (P < .05) in cytokine production by B6 Dd and B6 iIELs (Mann-Whitney). (C) MIP-1α and IFN-γ production by spleen NK cells from B6 (■) and B6 Dd ( ) mice was determined after stimulation with plate-bound anti-NK1.1. NK cells (CD3−CD49b+) were further subdivided into Ly49A single-positive and Ly49C single-positive subsets. The mean (± SEM) cytokine production of Ly49A+ relative to Ly49C+ NK cells is shown (n = 3-5). *Significant difference (P < .05) between B6 and B6 Dd NK cells (Mann-Whitney).
) mice was determined after stimulation with plate-bound anti-NK1.1. NK cells (CD3−CD49b+) were further subdivided into Ly49A single-positive and Ly49C single-positive subsets. The mean (± SEM) cytokine production of Ly49A+ relative to Ly49C+ NK cells is shown (n = 3-5). *Significant difference (P < .05) between B6 and B6 Dd NK cells (Mann-Whitney).
The presence of MHC-I ligand does not improve the responsiveness of Ly49A+ and Ly49G2+ CD8αα iIELs. (A) Representative histograms show H2-Dd staining of cells isolated from the small intestine (left) and spleen (right) from B6 Dd transgenic mice: grey line represents unstained control; and black line, H2-Dd stained. (B) MIP-1α production by CD8αα iIEL subpopulations from B6 (■) or B6 Dd ( ) mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb. Bar graph represents the mean (± SEM). MIP-1α production by the indicated iIEL subpopulations is shown relative to the iIEL subset lacking inhibitory MHC-I receptors for B6 Dd (n = 4) and B6 mice (n = 12). The data for B6 mice are the same as those shown in Figure 2A. *Significant difference (P < .05) in cytokine production by B6 Dd and B6 iIELs (Mann-Whitney). (C) MIP-1α and IFN-γ production by spleen NK cells from B6 (■) and B6 Dd (
) mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb. Bar graph represents the mean (± SEM). MIP-1α production by the indicated iIEL subpopulations is shown relative to the iIEL subset lacking inhibitory MHC-I receptors for B6 Dd (n = 4) and B6 mice (n = 12). The data for B6 mice are the same as those shown in Figure 2A. *Significant difference (P < .05) in cytokine production by B6 Dd and B6 iIELs (Mann-Whitney). (C) MIP-1α and IFN-γ production by spleen NK cells from B6 (■) and B6 Dd ( ) mice was determined after stimulation with plate-bound anti-NK1.1. NK cells (CD3−CD49b+) were further subdivided into Ly49A single-positive and Ly49C single-positive subsets. The mean (± SEM) cytokine production of Ly49A+ relative to Ly49C+ NK cells is shown (n = 3-5). *Significant difference (P < .05) between B6 and B6 Dd NK cells (Mann-Whitney).
) mice was determined after stimulation with plate-bound anti-NK1.1. NK cells (CD3−CD49b+) were further subdivided into Ly49A single-positive and Ly49C single-positive subsets. The mean (± SEM) cytokine production of Ly49A+ relative to Ly49C+ NK cells is shown (n = 3-5). *Significant difference (P < .05) between B6 and B6 Dd NK cells (Mann-Whitney).
The Ly49A receptor on CD8αα iIELs interacts with H-2Dd in cis
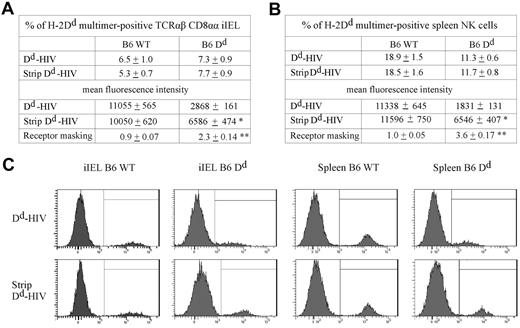
It has been shown that NK-cell education depends on the ability of the Ly49A receptor to bind H-2Dd ligand in cis (ie, in the plane of the same membrane).40 It was thus possible that deficient iIEL education was the result of lack of cis interaction between Ly49A and H-2Dd on CD8αα iIELs. Consequently, we investigated the binding of soluble H-2Dd (Dd) multimer to Ly49A expressed on CD8αα iIELs. The Dd multimer efficiently stained a subset of TCR-αβ CD8αα iIELs from H-2b mice (Figure 4A,C). Multimer binding was Ly49A-specific because it was efficiently blocked by the addition of anti-Ly49A mAb (data not shown). When iIELs were from B6 Dd mice, multimer binding to Ly49A was reduced approximately 4-fold (Figure 4A,C). In comparison, multimer binding to Ly49A was reduced 6-fold in NK cells from B6 Dd compared with B6 WT mice (Figure 4B-C).
The Ly49A receptor of TCR-αβ CD8αα iIELs interacts with MHC-I in cis. (A) iIELs isolated from B6 or B6 Dd mice were acid-treated or nontreated, and stained with Dd multimer. The percentage and the mean fluorescence intensity (MFI) of Dd multimer-binding by TCR-αβ CD8αα iIELs are shown as mean ± SEM (n = 3 for B6, n = 4 for B6 Dd). The extent of receptor masking was estimated by dividing the MFI of multimer staining after acid stripping (multimer, strip) by the MFI before acid stripping (multimer, no strip) and is presented as mean ± SEM. (B) Spleen cells from B6 or B6 Dd mice were acid-treated or nontreated, and stained with CD3, NK1.1, and Dd multimer. The percentage and the MFI of Dd multimer-binding NK cells (CD3−NK1.1+) are shown as mean ± SEM (n = 5). (A-B) *Significant difference (P < .05) in the MFI between nontreated versus acid-treated cells. **Significant difference (P < .05) in receptor masking between cells isolated from B6 versus B6 Dd mice (Mann-Whitney). (C) Representative histograms show Dd multimer staining of gated TCR-αβ CD8αα iIELs (left panels) and CD3−NK1.1+ spleen NK cells (right panels) from B6 or B6 Dd mice, as indicated.
The Ly49A receptor of TCR-αβ CD8αα iIELs interacts with MHC-I in cis. (A) iIELs isolated from B6 or B6 Dd mice were acid-treated or nontreated, and stained with Dd multimer. The percentage and the mean fluorescence intensity (MFI) of Dd multimer-binding by TCR-αβ CD8αα iIELs are shown as mean ± SEM (n = 3 for B6, n = 4 for B6 Dd). The extent of receptor masking was estimated by dividing the MFI of multimer staining after acid stripping (multimer, strip) by the MFI before acid stripping (multimer, no strip) and is presented as mean ± SEM. (B) Spleen cells from B6 or B6 Dd mice were acid-treated or nontreated, and stained with CD3, NK1.1, and Dd multimer. The percentage and the MFI of Dd multimer-binding NK cells (CD3−NK1.1+) are shown as mean ± SEM (n = 5). (A-B) *Significant difference (P < .05) in the MFI between nontreated versus acid-treated cells. **Significant difference (P < .05) in receptor masking between cells isolated from B6 versus B6 Dd mice (Mann-Whitney). (C) Representative histograms show Dd multimer staining of gated TCR-αβ CD8αα iIELs (left panels) and CD3−NK1.1+ spleen NK cells (right panels) from B6 or B6 Dd mice, as indicated.
To test whether Ly49A was masked by H-2Dd expression in cis, iIELs were exposed to acidic buffer, which disrupts MHC-I/β2-microglobulin/peptide complexes.41 Indeed, after acid treatment, the β2-microglobulin light chain could no longer be detected on the surface of iIELs (data not shown). Acid treatment of iIELs significantly improved multimer binding to Ly49A when CD8αα iIELs were from B6 Dd mice (by a factor of 2.3) but not when they were from B6 mice (factor of 0.9; Figure 4A,C). Analogous, yet somewhat more pronounced, results were obtained using NK cells. Here, acid treatment of B6 Dd NK cells improved multimer binding to Ly49A by a factor of 3.6. Thus, similar to NK cells, H-2Dd is associated with Ly49A on the surface of CD8αα iIELs. However, in contrast to NK cells, the function of Ly49A+ CD8αα iIELs does not improve in the presence of H-2Dd.
Qa-1b expression does not improve the response of CD94/NKG2+ CD8αα iIELs
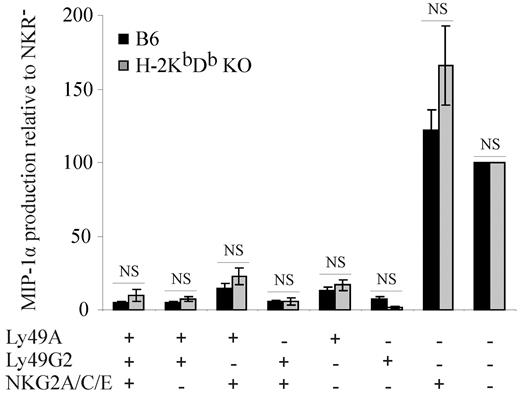
In contrast to Ly49A and Ly49G2, CD94/NKG2+ CD8αα iIELs encounter an MHC-I ligand (Qa-1b) in B6 mice, and these cells respond efficiently to TCR stimulation (Figure 2A-B). These data raised the possibility that, unlike Ly49, the engagement of CD94/NKG2A may educate iIELs. To test this hypothesis, we analyzed H-2KbDb double-deficient mice, where CD8αα iIELs develop normally42,43 but where Qa-1b expression is strongly decreased because of the lack of leader peptides from classic MHC-I molecules.2 The functional analyses show that CD94/NKG2-expressing CD8αα iIELs from H-2KbDb double-deficient and from B6 (H-2b) mice produce comparable amounts of MIP-1α (Figure 5), indicating that the presence of Qa-1b does not improve the responsiveness of CD94/NKG2+ CD8αα iIELs. In contrast, in response to NK1.1 triggering, CD94/NKG2+ NK cells from H-2b mice produced significantly more MIP-1α and IFN-γ than those from H-2KbDb double-deficient mice (data not shown), which is in agreement with published data.9 These data indicate that neither Ly49 nor CD94/NKG2 receptors educate iIELs.
The presence of MHC-I ligand does not improve the responsiveness of NKG2+ CD8αα iIELs. MIP-1α production by iIELs from B6 (■) or H-2KbDb double-deficient ( ) mice on in vitro stimulation with plate-bound anti-CD3 mAb was determined. MIP-1α production by the indicated MHC-I receptor+ CD8αα iIEL subpopulations is shown relative to CD8αα iIELs lacking inhibitory MHC-I receptors as mean ± SEM for H-2KbDb double-deficient (n = 5) and B6 mice (n = 12). The data on B6 iIELs are the same as those shown in Figure 2A. There were no significant differences (NS) in MIP-1α production between iIEL subpopulations from B6 and H-2KbDb double-deficient mice (Mann-Whitney).
) mice on in vitro stimulation with plate-bound anti-CD3 mAb was determined. MIP-1α production by the indicated MHC-I receptor+ CD8αα iIEL subpopulations is shown relative to CD8αα iIELs lacking inhibitory MHC-I receptors as mean ± SEM for H-2KbDb double-deficient (n = 5) and B6 mice (n = 12). The data on B6 iIELs are the same as those shown in Figure 2A. There were no significant differences (NS) in MIP-1α production between iIEL subpopulations from B6 and H-2KbDb double-deficient mice (Mann-Whitney).
The presence of MHC-I ligand does not improve the responsiveness of NKG2+ CD8αα iIELs. MIP-1α production by iIELs from B6 (■) or H-2KbDb double-deficient ( ) mice on in vitro stimulation with plate-bound anti-CD3 mAb was determined. MIP-1α production by the indicated MHC-I receptor+ CD8αα iIEL subpopulations is shown relative to CD8αα iIELs lacking inhibitory MHC-I receptors as mean ± SEM for H-2KbDb double-deficient (n = 5) and B6 mice (n = 12). The data on B6 iIELs are the same as those shown in Figure 2A. There were no significant differences (NS) in MIP-1α production between iIEL subpopulations from B6 and H-2KbDb double-deficient mice (Mann-Whitney).
) mice on in vitro stimulation with plate-bound anti-CD3 mAb was determined. MIP-1α production by the indicated MHC-I receptor+ CD8αα iIEL subpopulations is shown relative to CD8αα iIELs lacking inhibitory MHC-I receptors as mean ± SEM for H-2KbDb double-deficient (n = 5) and B6 mice (n = 12). The data on B6 iIELs are the same as those shown in Figure 2A. There were no significant differences (NS) in MIP-1α production between iIEL subpopulations from B6 and H-2KbDb double-deficient mice (Mann-Whitney).
Functional properties of MHC-I receptors on iIELs
The absence of iIEL education by MHC-I receptors may be related to a lack of inhibitory function in iIELs and/or a failure of these receptors to couple to the TCR. To address this possibility, we cocross-linked the TCR to MHC-I receptors using plate-bound mAbs. If MHC-I receptors are functional, this is expected to reduce iIEL activation via the TCR. Indeed, plate-bound anti-NKG2AB6 mAb reduced TCR-driven MIP-1α production by CD94/NKG2A iIELs from B6 mice (Figure 6A). MIP-1α production by MHC-I receptor-negative iIELs was not influenced (data not shown). Thus, although CD94/NKG2A can functionally couple to the TCR, it fails to educate iIELs.
Functional properties of MHC-I receptors on iIELs. (A) MIP-1α production by CD8αα iIELs from B6 mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb plus anti-NKG2A mAb (clone 16a11;  ) or an isotype control mAb (■). MIP-1α production by CD94/NKG2A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 6). *Significant difference (P < .05) between isotype control and anti-NKG2A mAb (Mann-Whitney). (B) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49A mAb clone JR9–318 (
) or an isotype control mAb (■). MIP-1α production by CD94/NKG2A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 6). *Significant difference (P < .05) between isotype control and anti-NKG2A mAb (Mann-Whitney). (B) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49A mAb clone JR9–318 ( ), anti-Ly49A mAb clone A1 (□), or an isotype control mAb (■). MIP-1α production by Ly49A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 4). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49A mAb versus isotype control (Mann-Whitney). (C) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49G2 mAb clone 4D11 (
), anti-Ly49A mAb clone A1 (□), or an isotype control mAb (■). MIP-1α production by Ly49A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 4). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49A mAb versus isotype control (Mann-Whitney). (C) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49G2 mAb clone 4D11 ( ) or an isotype control mAb (■). MIP-1α production by Ly49G2 single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 3). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49G2 mAb versus isotype control (Mann-Whitney).
) or an isotype control mAb (■). MIP-1α production by Ly49G2 single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 3). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49G2 mAb versus isotype control (Mann-Whitney).
Functional properties of MHC-I receptors on iIELs. (A) MIP-1α production by CD8αα iIELs from B6 mice was determined after in vitro stimulation with plate-bound anti-CD3 mAb plus anti-NKG2A mAb (clone 16a11;  ) or an isotype control mAb (■). MIP-1α production by CD94/NKG2A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 6). *Significant difference (P < .05) between isotype control and anti-NKG2A mAb (Mann-Whitney). (B) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49A mAb clone JR9–318 (
) or an isotype control mAb (■). MIP-1α production by CD94/NKG2A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 6). *Significant difference (P < .05) between isotype control and anti-NKG2A mAb (Mann-Whitney). (B) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49A mAb clone JR9–318 ( ), anti-Ly49A mAb clone A1 (□), or an isotype control mAb (■). MIP-1α production by Ly49A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 4). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49A mAb versus isotype control (Mann-Whitney). (C) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49G2 mAb clone 4D11 (
), anti-Ly49A mAb clone A1 (□), or an isotype control mAb (■). MIP-1α production by Ly49A single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 4). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49A mAb versus isotype control (Mann-Whitney). (C) iIELs from B6 mice were stimulated with plate-bound anti-CD3 mAb plus anti-Ly49G2 mAb clone 4D11 ( ) or an isotype control mAb (■). MIP-1α production by Ly49G2 single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 3). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49G2 mAb versus isotype control (Mann-Whitney).
) or an isotype control mAb (■). MIP-1α production by Ly49G2 single-positive CD8αα iIELs relative to the subpopulation expressing no inhibitory MHC-I receptors is shown as mean ± SEM (n = 3). There was no significant difference (NS) in MIP-1α production in the presence of plate-bound anti-Ly49G2 mAb versus isotype control (Mann-Whitney).
In contrast to CD94/NKG2A, cocross-linking of the TCR with Ly49A or Ly49G2 did not significantly reduce MIP-1α production by CD8αα iIELs (Figure 6B-C). A further inhibition might not be possible given the low responsiveness of the Ly49A- and Ly49G2-expressing iIELs. Alternatively, this might indicate that the engagement of the Ly49A and Ly49G2 receptors does not transduce inhibitory signals and/or that these receptors fail to couple to the TCR in iIELs.
Because the response of Ly49A+ and Ly49G2+ CD8αα iIELs to CD3 stimulation is low, an alternative scenario was that Ly49A and Ly49G2 inhibited these iIELs, even in the absence of cross-linking by mAb. Indeed, Ly49A has been shown to dampen NK-cell activation in the absence of MHC-I ligand.44 This was demonstrated by spatially separating (sequestering) Ly49A from the site of NK1.1 cross-linking, which improved NK-cell activation via the NK1.1 receptors.40 Consequently, we tested whether Ly49A suppressed iIEL activation via the TCR. To this end, we incubated iIELs with Ly49A mAb before the activation of iIELs with plate-bound CD3 mAb. Indeed, the sequestration of Ly49A substantially improved (2-fold) MIP-1α production by CD8αα iIELs from B6 mice (Figure 7A). Similarly, the sequestration of Ly49G2 improved the iIEL response to TCR stimulation (Figure 7B). The addition of Ly49 mAbs had no nonspecific effects on other iIEL subsets, and isotype control mAbs did not improve the response of Ly49A- and Ly49G2-expressing iIELs (Figure 7A-B). Importantly, the incubation of iIELs with Ly49A/Ly49G2 mAb did not induce chemokine production in the absence of CD3 cross-linking (data not shown), indicating that Ly49A/Ly49G2 did not activate iIELs but rather that iIELs were released from a suppressive effect of Ly49A/Ly49G2. These results show that Ly49A and Ly49G2 couple to the TCR and constitutively suppress iIEL activation through the TCR, independent of the presence of MHC-I ligand, in agreement with data obtained with NK cells.40
Sequestration of Ly49A or Ly49G2 increases the response of iIELs. (A) iIELs from B6 mice were incubated with anti-Ly49A mAb (JR9–318;  ) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 8). *Significant difference (P < .05; Mann-Whitney). (B) iIELs from B6 mice were incubated with anti-Ly49G2 mAb (4D11; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 4). *Significant difference (P < .05; Mann-Whitney). (C) iIELs from B6 mice were incubated with anti-Ly49E mAb (4D12;
) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 8). *Significant difference (P < .05; Mann-Whitney). (B) iIELs from B6 mice were incubated with anti-Ly49G2 mAb (4D11; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 4). *Significant difference (P < .05; Mann-Whitney). (C) iIELs from B6 mice were incubated with anti-Ly49E mAb (4D12;  ) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49E single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 6). *Significant difference (P < .05; Mann-Whitney). (D) iIELs from B6 mice were incubated with anti-Ly49F mAb (HBF-719; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49F single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 3). There was no significant difference (NS; Mann-Whitney).
) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49E single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 6). *Significant difference (P < .05; Mann-Whitney). (D) iIELs from B6 mice were incubated with anti-Ly49F mAb (HBF-719; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49F single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 3). There was no significant difference (NS; Mann-Whitney).
Sequestration of Ly49A or Ly49G2 increases the response of iIELs. (A) iIELs from B6 mice were incubated with anti-Ly49A mAb (JR9–318;  ) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 8). *Significant difference (P < .05; Mann-Whitney). (B) iIELs from B6 mice were incubated with anti-Ly49G2 mAb (4D11; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 4). *Significant difference (P < .05; Mann-Whitney). (C) iIELs from B6 mice were incubated with anti-Ly49E mAb (4D12;
) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 8). *Significant difference (P < .05; Mann-Whitney). (B) iIELs from B6 mice were incubated with anti-Ly49G2 mAb (4D11; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49A or Ly49G2 single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 4). *Significant difference (P < .05; Mann-Whitney). (C) iIELs from B6 mice were incubated with anti-Ly49E mAb (4D12;  ) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49E single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 6). *Significant difference (P < .05; Mann-Whitney). (D) iIELs from B6 mice were incubated with anti-Ly49F mAb (HBF-719; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49F single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 3). There was no significant difference (NS; Mann-Whitney).
) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49E single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 6). *Significant difference (P < .05; Mann-Whitney). (D) iIELs from B6 mice were incubated with anti-Ly49F mAb (HBF-719; ▨) or an isotype-matched control mAb (■). Unbound mAb was removed before stimulation with plate-bound anti-CD3 mAb. MIP-1α production by Ly49F single-positive iIELs is shown relative to iIELs lacking inhibitory MHC-I receptors as mean ± SEM (n = 3). There was no significant difference (NS; Mann-Whitney).
Large fractions of iIELs express the Ly49E or the Ly49F receptor (Figure 1A), which does not bind MHC-I or may only weakly bind the H-2d MHC-I haplotype, respectively. Similar to CD8αα iIELs expressing Ly49A or Ly49G2, those expressing only Ly49E or Ly49F responded poorly to TCR stimulation (Figure 7C-D). Sequestration of Ly49E resulted in a 2-fold increase of the response of iIELs to TCR stimulation (Figure 7C). In contrast, Ly49F sequestration did not significantly improve the iIEL response (Figure 7D), which may be the result of the inability of this specific mAb to sequester the Ly49F receptor away from the TCR or might be because of a general hyporesponsiveness of iIELs expressing Ly49F. Irrespectively, our data show that multiple Ly49 receptors suppress iIEL activation in an MHC-I independent fashion. Thus, the Ly49A and Ly49G2 and the CD94/NKG2A receptors are shown to functionally couple to the TCR. Notwithstanding, these receptors fail to educate iIELs in the presence of the respective MHC-I ligands.
Discussion
NK cells mediate an improved response to stimulation when they express an inhibitory receptor specific for self-MHC-I. In contrast, we show here that the engagement of MHC-I receptors does not improve the functional response of CD8αα iIELs.
To address the role of inhibitory MHC-I receptors on cell types other than NK cells, we first analyzed their expression on different iIEL subsets. We show that type b TCR-αβ CD8αα iIELs in B6 mice express the self-MHC-I specific CD94/NKG2A and the nonself–MHC-I–specific Ly49A and Ly49G2 receptors in a variegated and partially overlapping fashion. In contrast to published microarray data,13,14 H-2b iIELs did not express the self-MHC specific inhibitory receptor Ly49I, and Ly49C was only expressed at very low levels.
We used MHC-I transgenic and knockout mice to test whether the engagement of Ly49A, Ly49G2, or CD94/NKG2A by their MHC-I ligand improved the responsiveness of CD8αα iIELs. However, the presence of the respective MHC-I ligands failed to improve MIP-1α production by the relevant iIEL subsets. In contrast, MIP-1α production by the relevant NK-cell subset was readily increased. Importantly, the hyporesponsiveness of Ly49A- and Ly49G2-expressing CD8αα iIEL populations was not the result of an intrinsic defect in chemokine production as PMA/ionomycin stimulation resulted in significant chemokine production. These data show that inhibitory MHC-I receptors educate NK cells but fail to educate iIELs.
It could be argued that an NK-like education of iIELs is not observed because T cells do not normally respond to “self.” However, TCR-αβ CD8αα iIELs undergo a self-antigen–dependent agonist selection process and express a self-reactive TCR.26,45 This persistent stimulation may represent the basis for why these cells express Ly49 receptors. Consequently, iIELs may not be educated because inhibitory MHC-I receptors are acquired by mature rather than immature cells. However, recent studies have shown that peripheral NK cells can be educated independent of their development in the bone marrow.46,47
Education has been shown to improve the function of multiple, if not all, activation receptors expressed by NK cells. For example, NK cells produce cytokines, such as MIP-1α and IFN-γ, in response to NK1.1 cross-linking, and the response is enhanced when NK cells express an inhibitory receptor specific for self-MHC-I.6-8 In contrast, cross-linking NK1.1 on T cells, such as NKT cells, does not result in MIP-1α or IFN-γ production (data not shown). Further, because NKT cells and iIELs do not express activating NK-cell receptors, such as Ly49D, Ly49H, NK1.1, NKp46, and NKG2D (Figure 1A; and as previously described13-15 ), it is not possible to compare the responses of T cells (NKT or iIELs) and NK cells to stimulation through the same activation receptor. Because the primary activation receptor is the TCR, we tested whether inhibitory MHC-I receptors on iIELs are competent to influence signaling via the TCR. Co-cross-linking of the TCR with CD94/NKG2A provided evidence that CD94/NKG2A can inhibit the TCR on iIELs. Thus, on iIELs, CD94/NKG2A has the potential to perform a classic ligand-dependent inhibitory function. However, despite the coupling to the TCR, the CD94/NKG2A receptor does not educate iIELs.
In contrast to CD94/NKG2A, Ly49A or Ly49G2 cocross-linking to the TCR failed to inhibit iIEL activation. In agreement with these data, there is little evidence that endogenous Ly49 receptors functionally inhibit CD8 T cells.16 Thus, on T cells, inhibitory Ly49 receptors may not exert a classic ligand-dependent inhibitory function. Indeed, it was recently proposed that Ly49A may mediate a ligand-independent effect: Ly49A was shown to counteract NK-cell activation when the corresponding MHC-I ligand was absent.44 One possible explanation is that the inhibitory receptor is in proximity to activating receptors. This proximity may be sufficient to dampen NK-cell activation. Indeed when Ly49A is spatially separated from NK1.1 through sequestration via soluble mAb, NK-cell activation via NK1.1 is improved.40 This role of inhibitory MHC-I receptors may be analogous to the suppressive effect of Siglecs, such as CD22, on the activation of B cells.48 Similarly, by sequestering Ly49A and Ly49G2 (using soluble mAb), we show that Ly49A and Ly49G2 significantly suppress iIEL activation via the TCR. Thus, Ly49 receptors may not inhibit T cells in a classic ligand-dependent fashion but rather suppress T-cell activation in a ligand-independent and cell-autonomous way. The suppression of iIEL activation by the non–MHC-I–specific Ly49E receptor further emphasizes the MHC-I independent regulation of iIEL function. Irrespective of the precise mode of action, the data show that Ly49A and Ly49G2 play a functional role in iIELs and that the receptors couple to the TCR. However, unlike NK cells, Ly49A and Ly49G2 do not educate iIELs.
It has been shown that H-2Dd expression by NK cells (in cis) sequesters the majority of Ly49A receptors and that this is associated with an improved function of Ly49A+ NK cells.40 Although Ly49A and H-2Dd were also associated on the cell surface of CD8αα iIELs, the function of iIELs did not improve. However, we noted that Ly49A/H-2Ddcis association on iIELs was significantly less efficient compared with NK cells. This results in a higher fraction of unbound Ly49A on CD8αα iIELs compared with NK cells. It is thus possible that Ly49A-dependent suppression of iIELs persists although some Ly49A is sequestered by H-2Dd expression in cis. In addition, it is also possible that the TCR is more sensitive to suppression by Ly49A compared with NK-cell activation receptors. Irrespectively, the efficient sequestration of Ly49A or Ly49G2 using mAb was shown to improve the response of iIELs to TCR stimulation. Thus, the primary role of Ly49 receptors in iIELs may be to prevent the activation of self-reactive T cells. The fact that the suppressive function of Ly49A/Ly49G2 is independent of ligand may ensure proper iIELs suppression, even in mouse strains in which a Ly49A/Ly49G2 ligand is not present.
Taken together, similar to NK cells, inhibitory MHC-I receptors have the potential to inhibit iIELs in a ligand-dependent fashion or to suppress iIELs in a ligand-independent way. However, unlike NK cells, iIELs fail to undergo an MHC-I guided education process, suggesting that education is an NK cell-specific function of inhibitory MHC-I receptors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Foundation Against Cancer, a foundation of public interest, and by the Fund for Scientific Research Flanders. S.T. is supported by the Institute for the Promotion of Innovation through Science and Technology Flanders (IWT-Vlaanderen). V.D.C., T.K., and T.T. are supported by the Fund for Scientific Research Flanders. W.H. is supported in part by the Swiss National Science Foundation and the Swiss Cancer League.
Authorship
Contribution: S.T., W.H., and G.L. designed research, analyzed and interpreted data, and wrote the manuscript; S.T., J.F., and E.V.A. performed research; V.D.C., T.K., T.T., B.V., J.P., and W.H. contributed vital new reagents and analytical tools; and S.T. collected data and performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for V.D.C. is Miltenyi Biotec BV, Leiden, The Netherlands.
Correspondence: Georges Leclercq, Department of Clinical Chemistry, Microbiology and Immunology, Ghent University, De Pintelaan 185, 9000 Ghent, Belgium; e-mail: georges.leclercq@ugent.be.
References
Author notes
W.H. and G.L. contributed equally to this study and are senior authors.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal