Abstract
We have tested whether affinity-matured TCRs that retain peptide specificity improve the ability of primary human CD8+ T cells to mount antigen-specific responses. We found that TCR affinity correlated with the speed of T-cell responses. High affinity TCR–antigen interactions rapidly initiated T-cell responses, but low affinity TCR/antigen interactions required longer time periods to elicit the same responses. Within the “natural” affinity range, increased TCR-to-antigen affinity correlated with improved ability of T cells to recognize low concentration of antigen. However, affinity-matured TCR with 700-fold enhanced affinity for MHC-to-antigen required 100-fold higher antigen-density to initiate T-cell responses than did wild-type TCR. Using modified peptides to reduce the affinity of TCR-to-antigen interaction, we demonstrate that affinity-matured TCRs are not defective, being superior to wild-type TCR in recognizing low concentration of modified peptides. These data indicate that enhancing TCR affinity can accelerate the speed of T-cell activation and reduce the ability to recognize low density of MHC-to-peptide antigen. We predict that future studies of the human T-cell repertoire will reveal 2 types of low avidity T cells: fast and slow responders, with high-affinity and low-affinity TCR, respectively.
Introduction
T cells recognize antigens using the TCR that binds peptides displayed by MHC molecules. Compared with antibodies, the affinity of TCR for MHC-to-peptide is relatively low, with dissociation constants KD ranging from 100-1μM.1-4 Unlike antibodies, TCRs do not naturally undergo somatic hypermutation to enhance affinity for the TCR-recognized antigen. The reasons for the very limited range of TCR affinities is not fully understood, but might be related to the thymic selection process that eliminates TCRs with high affinity for self MHC-to-peptide while positively selecting low-affinity interactions.5
In the field of antitumor TCR gene therapy, giving T cells high-affinity TCRs is a major goal for producing T cells capable of attacking tumor cells.6,7 As most tumor-associated targets are self-antigens that are also expressed in normal tissues, central and peripheral tolerance mechanisms are likely to delete or inactivate T cells expressing high-affinity TCR for these tumor-associated antigens.8,9 Several strategies have been explored to circumvent tolerance to tumor-associated self-antigens. This includes the use of the natural TCR repertoire of HLA-mismatched donors and HLA-transgenic mice.10-12 In the HLA-mismatched setting the TCR repertoire is not tolerant to peptides presented by non-self HLA molecules, and in the HLA-transgenic setting mice are not tolerant to human proteins and antigens that are not conserved in mice.
In vitro affinity maturation provides an additional strategy to select high-affinity TCR. In this setting affinity is not restricted to the physiologic range of naturally selected TCR, but provides an opportunity to enhance TCR affinity into the range displayed by antibodies. Yeast display, phage display, and targeted in vitro mutagenesis have been used to generate affinity-matured TCR variants.13-15 Affinity-matured versions of the murine alloreactive 2C TCR were functionally analyzed in T hybridoma cells. Hybridoma cells expressing the 2C TCR and an affinity-matured m6α TCR variant with 700-fold improved affinity recognized the allogeneic Ld plus the cognate QL9 peptide with similar efficiency. However, in CD8− hybridoma cells, only affinity-matured TCR, but not the 2C TCR, recognized peptides presented by syngeneic Kb class I molecules.16 Thus, the improved affinity was correlated with unaltered recognition of the cognate QL9/Ld antigen but enhanced cross-recognition of peptides presented by syngeneic MHC molecules. In a separate murine study, the hybridoma expression system was used to analyze TCR specific for a vesicular stomatitis virus–derived peptide presented by syngeneic Kb class I molecules. Two TCR variants with a relatively modest twofold increase in affinity displayed impaired responses against low concentration of antigen compared with the wild-type TCR. The authors suggested that optimal T-cell responses required an optimal dwell time of the TCR–antigen interaction.17
The use of hybridoma cells for TCR analysis may affect the functional outcome. For example, the peptide sensitivity of TCR-positive hybridoma cells is generally reduced, compared with the sensitivity of primary T cells expressing the same TCR, suggesting impaired TCR function in hybridoma cells. This limitation can be overcome by retroviral TCR gene transfer, which offers the opportunity to generate primary T cells with the same peptide sensitivity as unmanipulated T cells naturally expressing the same TCR.7,11,18-27 To date, few studies have been performed using primary T cells expressing affinity-matured TCR. The analysis of an HLA-A0201–restricted TCR specific for the tumor-associated antigen NY-ESO demonstrated that 2 TCR variants with 380-fold to 640-fold increase in affinity displayed nonspecific activation when introduced into primary human CD8+ T cells.28 A more recent study indicated that a relatively modest 13-fold affinity increase of the NY-ISO TCR improved the peptide-specific function of primary CD8+ T cells.15
Affinity maturation of a TCR specific for an HIV-derived Gag peptide showed that a TCR variant with 360-fold increased affinity retained peptide specificity in primary CD8+ T cells. T cells expressing this TCR more efficiently controlled the spread of HIV virus in vitro compared with T cells expressing wild-type TCR. The sensitivity and the kinetics of peptide-specific responses of wild-type and affinity-matured TCR was not determined, and it remained uncertain whether improved HIV control was caused by improved recognition of low antigen density antigen or faster kinetics of T-cell responses.29
We explored in detail how TCR-to-antigen affinities spanning the natural range and extending 700-fold into the supraphysiologic range affected the kinetics and the sensitivity of primary human CD8+ T-cell responses. We took advantage of recently generated variants of an HLA-A0201–restricted TCR specific to a peptide derived from the Tax antigen of human T lymphotopic virus I (HTLV1).14 The affinity-matured variants were generated by phage display and retained peptide specificity when introduced into primary CD8+ T cells. The functional analysis of transduced T cells revealed that although affinity-matured TCR mediated faster responses than wild-type TCR, this paradoxically led to an inability to recognize low concentration of antigen. The data strongly suggest that affinity maturation beyond the physiologic range accelerates human CD8+ T-cell responses but disrupts the serial TCR triggering that is required to maximize recognition of low-density peptide ligands.
Methods
Media, cells, antibodies, tetramer, and peptides
Unless otherwise stated, all culture medium was RPMI (Cambrex) supplemented with 10% heat inactivated FCS (Sigma-Aldrich), 1% penicillin and streptomycin (Invitrogen), and 1% l-glutamine (Invitrogen). Cell lines used were the HLA-A2+ T2 cell line that is deficient in TAP (transporter associated with antigen processing) and can be efficiently loaded with exogenous peptides and HeLa cells previously stably transduced to express HLA-A2. HLA-A2+ PBMCs were obtained from volunteer donors from the National Blood Service (London, United Kingdom). Flow cytometry antibodies were anti–human PE CD107a, PerCP CD3, and APC CD8 (BD Biosciences), PE Vβ13.1 (Immunotech), phosphor-p44/42 MAPK (ERK1/2; Thr202/Tyr204; 197G2) rabbit mAb (Cell Signaling Technology), and Alexa Fluor 488 F(ab)2 fragment of goat anti–rabbit IgG (Invitrogen). PE-labeled HLA-A2/Tax tetramers were obtained from Beckman Coulter. The peptides used in this study were the HLA-A2 binding peptides pTax (LLFGYPVYV), pHuD (LGYGFVNYI), and pWT235 (CMTWNQMNL) and were synthesised by ProImmune.
Generation of wild-type and mutated retroviral TCR constructs
Only the TCR-β chain underwent affinity maturation and was accomplished as detailed previously by Li et al.14 The TCR-α and TCR-βchain constructs were cloned together into retroviral pMP71 vectors and were linked via a viral p2A sequence. The TCR-α and TCR-β chains contained an additional interchain disulphide bound in the constant domains. This was achieved by replacing residue 48 of the α chain from a threonine to a cysteine and residue 57 of the β chain from a serine to a cysteine as previously described. The TCR construct was codon optimized.
Transduction of retroviral TCR constructs into Jurkat cells and primary T cells
For retroviral transduction, 2 × 106 phoenix amphotropic packaging cells were cultured in 10-cm culture plates for 24 hours at 37°C, 5% CO2 in DMEM supplemented with 10% heat-inactivated FCS, 1% penicillin and streptomycin, and 1% l-glutamine. The phoenix amphotropic packaging cells were cultured in fresh DMEM and transfected with 16 μg of the vector constructs using calcium phosphate precipitation (Invitrogen). Twenty-four hours after transfection, the media was replaced with RPMI culture media and incubated for a further 24 hours. The viral supernate was then harvested. Jurkat cells were split 24 hours before retroviral transduction, and PBMCs were activated for 48 hours using the anti-CD3 antibody OKT3 at 30 ng/mL and IL-2 at 600 U/mL (Chiron). Retroviral transductions were conducted on retronectin (Takara)–coated 24-well plates. One mL of virus supernate was added per well, and the plates was spun at 2000g, 32°C, for 2 hours. Wells were washed twice with PBS and cells were seeded at 5 × 105 per well in 2 mL of culture media. PBMCs were transduced in the presence of IL-2 at 600 U/mL. After 24 hours of incubation, the medium was replaced with fresh culture medium. Transduction efficiency was determined by flow cytometry analysis, conducted on a LSR II flow cytometer after a further 48-hour culture period. FACS data were analyzed using FACSdiva (Becton Dickinson).
Antigen-stimulation and expansions of TCR-transduced T cells
TCR-transduced primary T cells were stimulated and expanded every 7-10 days. The stimulations were conducted in 24-well plates in 2 mL of culture media containing 10% nonheat-inactivated FCS and 10 U/mL IL-2 (Roche), at 37°C, 5% CO2. Each well contained 5 × 105 transduced cells, 2 × 105 irradiated T2 cells loaded for 2 hours with 100μM of the peptide pTax, and 2 × 106 irradiated autologous PBMCs as feeder cells.
TCR down-regulation assay
Two × 105 TCR transduced T cells or Jurkat cells were cocultured with 2 × 105 T2 cells preloaded with peptide in round-bottom 96-well plates in 200 μL of culture media. After each coculturing time point, the reaction was immediately stopped by washing in ice-cold PBS. Jurkat cells were stained with anti-Vβ13.1 Abs, and T cells were stained with anti-CD8 Abs and tetramer and analyzed using FACS.
Phospho-ERK assays
TCR-transduced T cells (2 × 105) were cultured with 1 in 500 dilution of tetramer in 100 μL culture media for the stated times. After each time point, the reaction was immediately stopped by adding 2% paraformaldehyde for 10 minutes at 37°C. Cells were washed, permeabilized with 90% methanol (on ice, for 30 minutes), then stained with antiphosphor-p44/42 MAPK, followed by Alexa Fluor 488 F(ab)2 fragment of goat anti–rabbit IgG for FACS analysis.
CD107a up-regulation assays
For assays using T2 cells as stimulator cells, 2 × 105 T2 cells were preloaded with peptide. For assays using HLA-A2 positive HeLa cells as stimulator cells, the HeLa cells were transfected with either a pGFPC1 plasmid (Clontech) or a full-length Tax gene encoded within the pGFPC1 plasmid.30 Cells were transfected using 8μg of plasmid DNA and 24 μL of Fugene (Roche). Twenty-four hours after transfection, the GFP expression was determined and was typically 20%-25%. For stimulations, the ratio of TCR-positive T cells to GFP+ HeLa cells was 1:1. As a positive control, TCR-positive T cells were stimulated with transfected HeLa cells loaded with saturating concentration of pTax peptide (100μM) at a ratio of 1:1. T2 cells or HeLa cells were cocultured with 2 × 105 TCR transduced T cells in round-bottom 96-well plates in 250 μL culture media containing 1μg/mL brefeldin A (Sigma-Aldrich), 0.7μg/mL BD GolgiStop (BD Bioscience Pharmingen), and 2 μL of CD107a PE. After each coculturing time point the reaction was immediately stopped by washing in ice-cold PBS. T cells were stained with anti-CD8 and FACS analyzed.
ELISA
For cytokine secretion assays, 1 × 105 TCR transduced T cells were stimulated with 1 × 105 irradiated T2 cells loaded for 2 hours with peptide. Assays were conducted in triplicates in round-bottom 96-well plates in 250 μL culture media. After 18 hours of incubation at 37°C, 5% CO2 supernatant was harvested and tested for secreted IFNγ using a human ELISA kit (BD Biosciences) as per the manufacturer's instructions. The data were analyzed using Excel Version 12.2.5 software (Microsoft).
Results
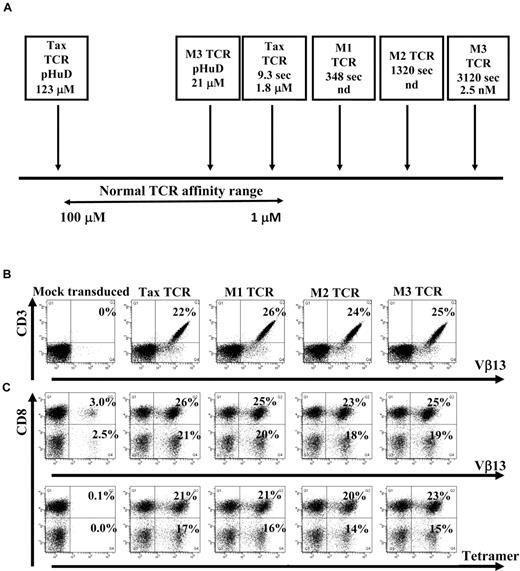
The HLA-A0201–restricted TCR specific for a peptide (pTax) derived from the Tax protein of HTLV-1 was used in this study. Recently, phage display technology was used to isolate affinity-matured versions of the Tax-TCR. Soluble TCR molecules and HLA/peptide complexes were analyzed in the surface plasmon resonance system to measure the dissociation constant KD of the TCR-HLA/peptide binding and the half-life t1/2 of the interaction.14 The binding of the wild-type Tax-TCR to HLA/pTax had a KD of 1.8μM and a t1/2 of 9.3 seconds. The t1/2 of the 3 affinity-matured versions M1-TCR, M2-TCR, and M3-TCR increased to 348 seconds, 1320 seconds, and 3120 seconds, respectively, with a KD for the M3-TCR of 2.5nM (Figure 1A). Thus, the binding of the M3-TCR to HLA/pTax was approximately 700-fold stronger and 300-fold longer than the binding of the Tax-TCR. Both the M3-TCR and the Tax-TCR bind the modified peptide pHuD with a KD of 21μM and 123μM, respectively.31 Together, the TCR molecules interacting with pTax and pHuD covered the physiologic affinity range used by naturally selected TCR, and also extended into the supra-physiologic range of affinity-matured TCR (Figure 1A).
TCR affinity ranges and human Jurkat cells and primary T cells transduced with the wild-type and affinity-matured TCR constructs. (A) Schematic diagram showing the natural TCR-to-peptide–MHC affinity range, as well as the affinities of the Tax TCR and affinity-matured TCRs to either the pTax peptide or the pHuD peptide and the positions they occupy in the affinity range. Indicated are the dissociation constant KD of the TCR-HLA/peptide binding and the half-life t1/2 (in seconds) of the interaction. Jurkat cells (B) or activated primary T cells (C) were transduced with pMP71 vectors encoding the indicated TCR. Jurkat cells were stained with anti-CD3 and Vβ13 antibodies (B) and mock transduced primary T cells or TCR transduced primary T cells were stained with anti-CD3 and CD8 antibodies and either Vβ13 antibodies or tetramer (C) 72 hours after transduction. Primary T cells were gated on the CD3+ T-cell population. The Jurkat cell transduction shown is a representative of at least 6 independent experiments that demonstrated similar results. The primary T-cell transduction shown is a representative of at least 20 independent experiments that demonstrated similar results.
TCR affinity ranges and human Jurkat cells and primary T cells transduced with the wild-type and affinity-matured TCR constructs. (A) Schematic diagram showing the natural TCR-to-peptide–MHC affinity range, as well as the affinities of the Tax TCR and affinity-matured TCRs to either the pTax peptide or the pHuD peptide and the positions they occupy in the affinity range. Indicated are the dissociation constant KD of the TCR-HLA/peptide binding and the half-life t1/2 (in seconds) of the interaction. Jurkat cells (B) or activated primary T cells (C) were transduced with pMP71 vectors encoding the indicated TCR. Jurkat cells were stained with anti-CD3 and Vβ13 antibodies (B) and mock transduced primary T cells or TCR transduced primary T cells were stained with anti-CD3 and CD8 antibodies and either Vβ13 antibodies or tetramer (C) 72 hours after transduction. Primary T cells were gated on the CD3+ T-cell population. The Jurkat cell transduction shown is a representative of at least 6 independent experiments that demonstrated similar results. The primary T-cell transduction shown is a representative of at least 20 independent experiments that demonstrated similar results.
Expression of retroviral TCR constructs in Jurkat and primary human T cells.
Human Jurkat cells and primary peripheral blood T cells were transduced with retroviral constructs encoding the Tax-TCR or the affinity-matured M1, M2, and M3-TCR. Jurkat cells were stained with anti-CD3 and Vβ13, (the β variable region segment that is used by the Tax-TCR) Abs; T cells were stained with anti-CD3 and anti-CD8, and either anti-Vβ13 or HLA-A2/pTax tetramer. TCR constructs were readily expressed in human Jurkat cells (Figure 1B). Mock-transduced T cells showed background levels of endogenous Vβ13 expression, but did not stain with HLA-A2/pTax tetramer (Figure 1C). T cells transduced with the Tax-TCR and affinity-matured TCR showed comparable percentages of CD8+ T cells and CD8− T cells expressing the Vβ13 TCR chain and capable of binding to the HLA-A2/Tax tetramer (Figure 1C). These results indicate that affinity maturation of the TCR did not affect the transduction efficiencies or the binding of the Vβ13 antibodies and tetramer to the expressed TCR molecules.
Kinetics of antigen-specific TCR down-regulation
We performed experiments to test whether TCR affinity affected the kinetics of peptide-specific immune responses. In these experiments, saturating concentrations of peptide were used to provide optimal TCR stimulation over defined periods of time.
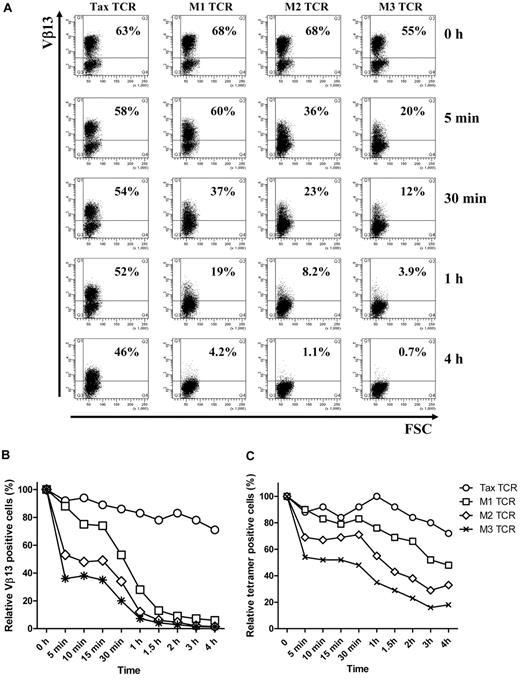
As expected, stimulation of TCR-transduced bulk Jurkat T cells with the pTax peptide resulted in down-modulation of the Tax-TCR and the affinity-matured versions. However, there was a dramatic difference in the speed of TCR down-modulation. Within 5 minutes of peptide stimulation, the affinity-matured M3-TCR showed a substantial reduction of TCR+ T cells, from 55% to 20% (Figure 2A). In addition, the 20% TCR+ T cells expressed substantially lower levels of TCR than the TCR+ T cells before peptide stimulation. After 4 hours of peptide stimulation, the M3-TCR was strongly down-modulated in all T cells. In contrast, the Tax-TCR displayed a much more subtle reduction in TCR expression over the 4-hour assay period. The level of peptide-driven reduction of TCR expression seen with the M1 and M2 TCR was between the slow reduction of the Tax-TCR and the fast reduction of the M3-TCR. The Tax-TCR and the M1, M2 and M3-TCR showed similar differences in the kinetics of down-modulation when the analysis was performed with transduced Jurkat cells or primary human T cells (compare Figure 2B and C).
T cells transduced with affinity-matured TCR down-regulate TCR faster and to a greater level than T cells transduced with Tax-TCR. (A-B) Jurkat cells and (C) primary T cells transduced with the Tax TCR and the affinity-matured TCR were cocultured with T2 stimulator cells loaded with saturating concentration of Tax peptide (100μM) for the stated time periods. Jurkat cells were stained with anti-Vβ13 antibodies and primary T cells were stained with anti-CD8 antibodies and tetramer (the axis of the FACS plots range from 101 to 105). Shown is a representative of 4 independent Jurkat experiments showing similar results. Primary T cells were gated on the CD8+ T-cell population; the percentage of tetramer-positive CD8+ T cells at time 0 was 63%, 68%, 68%, and 55% for the Tax-TCR, M1-TCR, M2-TCR, and M3-TCR, respectively. Shown is the relative loss of tetramer-positive T cells after peptide stimulation. This is a representative of 3 independent experiments showing similar results.
T cells transduced with affinity-matured TCR down-regulate TCR faster and to a greater level than T cells transduced with Tax-TCR. (A-B) Jurkat cells and (C) primary T cells transduced with the Tax TCR and the affinity-matured TCR were cocultured with T2 stimulator cells loaded with saturating concentration of Tax peptide (100μM) for the stated time periods. Jurkat cells were stained with anti-Vβ13 antibodies and primary T cells were stained with anti-CD8 antibodies and tetramer (the axis of the FACS plots range from 101 to 105). Shown is a representative of 4 independent Jurkat experiments showing similar results. Primary T cells were gated on the CD8+ T-cell population; the percentage of tetramer-positive CD8+ T cells at time 0 was 63%, 68%, 68%, and 55% for the Tax-TCR, M1-TCR, M2-TCR, and M3-TCR, respectively. Shown is the relative loss of tetramer-positive T cells after peptide stimulation. This is a representative of 3 independent experiments showing similar results.
Kinetics of antigen-specific signaling
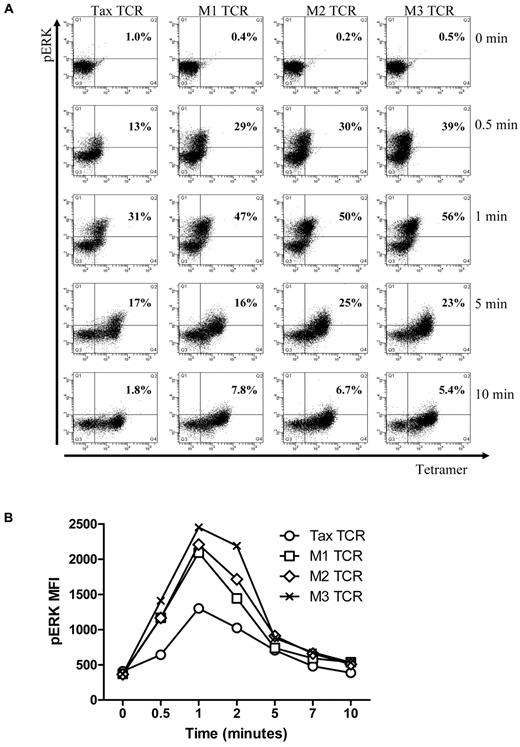
Next, we explored whether the kinetics of early signaling is different for the Tax-TCR and the affinity-matured variants. For these experiments, transduced primary human T cells were stimulated with saturating concentrations of HLA-A2/pTax tetramers for short periods, followed by intracellular staining for ERK phosphorylation, an early signaling event in T-cell activation. Tetramers were used to achieve uniform TCR stimulation within a short period, which was difficult to achieve with peptide loaded T2 cells. The transduced T cell populations used in these experiments contained similar numbers of TCR+ T cells, as determined by the percentage of tetramer-positive cells (Tax-TCR = 83%, M1-TCR = 88%, M2-TCR = 92% and M3-TCR = 94%). Unstimulated T cells showed little background phospo-ERK staining (see time point 0 in Figure 3A). After 30 seconds of tetramer stimulation, cells expressing the affinity-matured TCR showed a greater number of phospo-ERK positive cells (M1-TCR = 29%, M2-TCR = 30% and M3-TCR = 39%) compared with cells expressing Tax-TCR (13%; Figure 3A). After 1 minute of tetramer activation, the Tax-TCR reached 30% phospo-ERK+ T cells, which is the response rate seen by the M1 and M2-TCR cells after 0.5 minutes. At the 1-minute time point, the response by the M1, M2 and M3 TCR had increased to 49%, 50% and 56%, respectively. After a peak of ERK phosporylation, the signal deceased and returned to nearly baseline after 10 minutes (Figure 3A). In addition to the increased percentage of responding T cells at each time point, the intensity of ERK phosphorylation in the responding cells was greater for the affinity-matured TCR compared with the Tax-TCR (Figure 3B). Together, these data showed that affinity-matured TCR triggered faster and more potent phospo-ERK signals in the early phase of T-cell stimulation.
Up-regulation of phospho-ERK after stimulation in primary T cells transduced with the TCRs constructs and stimulated with pTax/HLA-A2 tetramer. (A) Primary T cells transduced with the Tax TCRs and having undergone 5 rounds of weekly peptide stimulation had the following percentages of CD8+tetramer+ T cells: Tax TCR = 83%; M1-TCR = 88%; M2-TCR = 92%; and M3-TCR = 94%. T cells (2 × 105) were stimulated with pTax-MHC tetramer for the stated times, and then immediately fixed, permeabilized, and stained with anti-pERK antibodies. FACS plots of tetramer binding and pERK expression at the stated times (the axis of the FACS plots range from 101 to 105). (B) Mean fluorescent intensity of pERK at the stated times. These figures are representative of 4 individual experiments that showed similar results.
Up-regulation of phospho-ERK after stimulation in primary T cells transduced with the TCRs constructs and stimulated with pTax/HLA-A2 tetramer. (A) Primary T cells transduced with the Tax TCRs and having undergone 5 rounds of weekly peptide stimulation had the following percentages of CD8+tetramer+ T cells: Tax TCR = 83%; M1-TCR = 88%; M2-TCR = 92%; and M3-TCR = 94%. T cells (2 × 105) were stimulated with pTax-MHC tetramer for the stated times, and then immediately fixed, permeabilized, and stained with anti-pERK antibodies. FACS plots of tetramer binding and pERK expression at the stated times (the axis of the FACS plots range from 101 to 105). (B) Mean fluorescent intensity of pERK at the stated times. These figures are representative of 4 individual experiments that showed similar results.
Kinetics of antigen-specific CD107 up-regulation
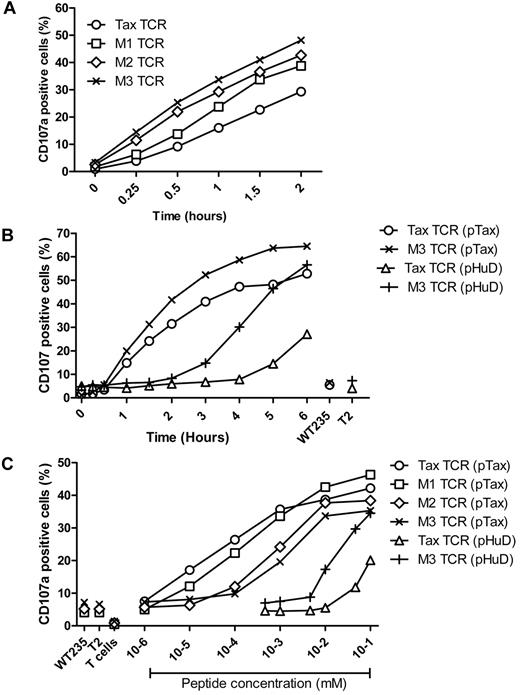
To determine whether the faster signaling and TCR down-regulation of the affinity-matured TCR correlated with accelerated antigen-specific effector function, we investigated the up-regulation of CD107, a molecule that is exposed on the surface of T cells releasing effector molecules such as perforin and cytokines from intracellular granules. Primary T cells expressing similar levels of the Tax-TCR or the affinity-matured variants were stimulated with antigen-presenting T2 cells loaded with saturating concentrations of pTax peptide. Over a 2-hour stimulation period, all TCR-transduced populations showed an increase in the percentage of CD107 positive cells (Figure 4A). However, the CD107 up-regulation for the Tax-TCR was substantially slower than up-regulation observed for the M3-TCR. At 2 hours, the number of CD107 positive cells expressing the Tax-TCR was similar to the number seen for the M3-TCR at 0.5 hours. The speed of CD107 up-regulation for the M1 and M2-TCR was between the Tax-TCR and M3-TCR, indicating a correlation between increasing half-life of TCR-antigen binding and accelerated kinetic of CD107 up-regulation.
Kinetics of antigen-specific CD107a up-regulation. (A) Primary T cells transduced with the Tax-TCR and affinity-matured TCR and having undergone 3 rounds of weekly peptide stimulation had the following CD8+ and tetramer+ expression: Tax TCR = 92%; M1-TCR = 96%; M2-TCR = 95%; and M3-TCR = 98%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with saturating concentration of pTax peptide in the presence of anti-CD107a, brefeldin A, and GolgiStop for the stated times. (A) Shown are the percentages of CD8+CD107a+ cells as a proportion of the tetramer-positive cells. The figure represents 4 individual experiments that demonstrated similar results. (B) Primary T cells transduced with the Tax-TCR and M3-TCR and having undergone 3 rounds of weekly peptide stimulation had the following percentage CD8+tetramer+ T cells: Tax TCR = 98%; M3-TCR = 99%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with saturating concentration of pTax peptide or pHuD peptide in the presence of anti-CD107a, brefeldin A, and GolgiStop for the stated times. (B) Shown are the percentages of CD8+CD107a+ cells as a proportion of the tetramer-positive cells. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide did not result in CD107 up-regulation. This figure represents 3 individual experiments that showed similar results. (C) Primary T cells transduced with the Tax-TCR and affinity-matured TCR and having undergone 5 rounds of weekly peptide stimulation had the following percentage CD8+tetramer+ T cells: Tax TCR = 91%; M1-TCR = 89%; M2-TCR = 86%; and M3-TCR = 86%. T cells (2 × 105) were stimulated with 2 × 105 cells loaded with decreasing concentrations of pTax peptide or pHuD peptide in the presence of anti-CD107a, brefeldin A, and Golgi Stop. CD107a expression was determined after a 5-hour stimulation period. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide did not result in CD107 up-regulation. Shown is a representative of 3 individual experiments that showed similar results.
Kinetics of antigen-specific CD107a up-regulation. (A) Primary T cells transduced with the Tax-TCR and affinity-matured TCR and having undergone 3 rounds of weekly peptide stimulation had the following CD8+ and tetramer+ expression: Tax TCR = 92%; M1-TCR = 96%; M2-TCR = 95%; and M3-TCR = 98%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with saturating concentration of pTax peptide in the presence of anti-CD107a, brefeldin A, and GolgiStop for the stated times. (A) Shown are the percentages of CD8+CD107a+ cells as a proportion of the tetramer-positive cells. The figure represents 4 individual experiments that demonstrated similar results. (B) Primary T cells transduced with the Tax-TCR and M3-TCR and having undergone 3 rounds of weekly peptide stimulation had the following percentage CD8+tetramer+ T cells: Tax TCR = 98%; M3-TCR = 99%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with saturating concentration of pTax peptide or pHuD peptide in the presence of anti-CD107a, brefeldin A, and GolgiStop for the stated times. (B) Shown are the percentages of CD8+CD107a+ cells as a proportion of the tetramer-positive cells. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide did not result in CD107 up-regulation. This figure represents 3 individual experiments that showed similar results. (C) Primary T cells transduced with the Tax-TCR and affinity-matured TCR and having undergone 5 rounds of weekly peptide stimulation had the following percentage CD8+tetramer+ T cells: Tax TCR = 91%; M1-TCR = 89%; M2-TCR = 86%; and M3-TCR = 86%. T cells (2 × 105) were stimulated with 2 × 105 cells loaded with decreasing concentrations of pTax peptide or pHuD peptide in the presence of anti-CD107a, brefeldin A, and Golgi Stop. CD107a expression was determined after a 5-hour stimulation period. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide did not result in CD107 up-regulation. Shown is a representative of 3 individual experiments that showed similar results.
To analyze the kinetics of CD107 up-regulation within the natural affinity range, primary T cells expressing the Tax-TCR or the M3-TCR were stimulated with T2 cells coated with saturating concentrations of the pHuD peptide. As before, the fastest response was seen when the M3-TCR was stimulated with the pTax peptide, followed by Tax-TCR stimulation with pTax peptide. The response of M3-TCR stimulated with pHuD was approximately 2 hours slower than the same TCR stimulated with pTax. The response of the Tax-TCR stimulated by pHuD was approximately 4 hours slower compared with stimulation with pTax (Figure 4B).
Affinity-matured TCR fail to respond to low concentration of antigen
We next explored whether the accelerated response displayed by affinity-matured TCR correlated with improved performance at low concentrations of peptide antigen. To investigate this, transduced T cells were stimulated with decreasing peptide concentrations for 18 hours, a period sufficient to initiate responses irrespective of the kinetics of the individual TCR-antigen combinations.
The peptide sensitivity of the CD107 response was assessed using transduced T cells expressing similar levels of the Tax-TCR or the affinity-matured versions. The T cells were stimulated with T2 cells loaded with decreasing concentrations of the pTax or pHuD peptide. The low-affinity Tax-TCR/pHuD combination required approximately 10-fold more peptides to trigger a CD107 response than the medium-affinity M3-TCR/pHuD combination (Figure 4C). The best peptide sensitivity was achieved by the Tax-TCR/pTax combination with a KD at the upper end of the natural affinity range. However, a further 700-fold increase in affinity above the natural range did not enhance the functional sensitivity of T cells, but abolished the ability to respond to the same low peptide concentrations that were recognized by the Tax-TCR (Figure 4C). The antigen concentrations required to trigger CD107 up-regulation in T cells expressing the M3-TCR was approximately 100-fold higher compared with the response by the Tax-TCR. Similarly, the M1-TCR and M2-TCR were less sensitive than the Tax-TCR, but more sensitive than the M3-TCR. This indicated that a progressive increase in TCR affinity above the natural range resulted in a progressive inability to recognize low density of HLA and peptide.
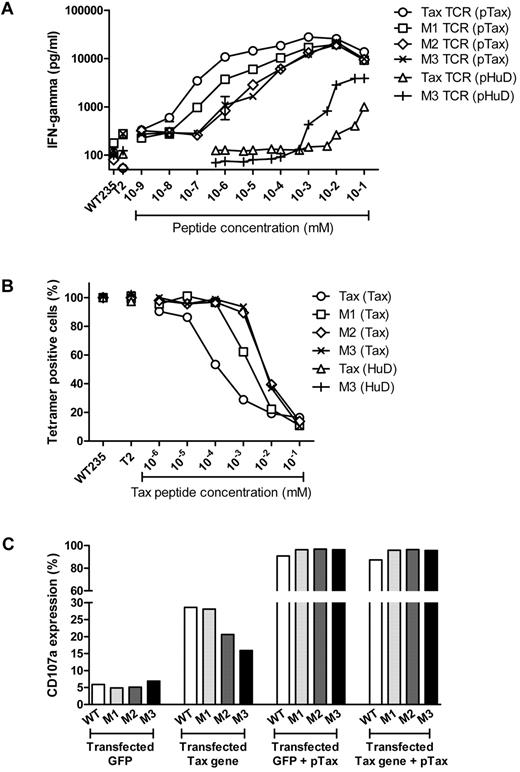
We used IFNγ production to explore if the unexpected loss in T-cell sensitivity was peculiar to CD107 up-regulation or also affected other T-cell effector functions. Again, T cells expressing the Tax-TCR produced IFNγ at lower concentrations of pTax peptide than T cells expressing the affinity-matured TCR (Figure 5A). Similar to the CD107 profile, the M3-TCR displayed lower sensitivity than the M1 and M2-TCR. However, the poor performance of the M3-TCR compared with the Tax-TCR was reversed when T cells were stimulated with the low-affinity pHuD ligand. In this situation, the M3-TCR performed approximately 10-fold better than the Tax-TCR (Figure 5A), indicating that poor recognition of low-density antigen was linked to the high-affinity M3-TCR/pTax combinations, and was not an inherent feature of the affinity-matured M3-TCR.
Primary T cells transduced with the high-affinity TCR show lower peptide sensitivity compared with Tax-TCR transduced T cells. (A) Freshly transduced primary T cells had the following percentage of CD8+Vβ13+ T cells: Tax TCR = 26%; M1-TCR = 24%; M2-TCR = 25%; and M3-TCR = 26%. Bulk transduced T cells (1 ×105) were cocultured with T2 cells at a 1:1 ratio of transduced T cells–to–T2 cells loaded with pTax peptide or pHuD peptide at the stated concentrations. After 18 hours of stimulation, the supernatant was removed to determine IFN-γ release by ELISA. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide defined the background level of IFN-γ release. This represents 4 individual experiments that demonstrated similar results. (B) Primary T cells transduced with the Tax TCR and affinity-matured versions and having undergone 5 rounds of weekly peptide stimulation had the following percentage of CD8+Vβ13+ T cells: Tax TCR = 97%; M1-TCR = 99%; M2-TCR = 99%; and M3-TCR = 99%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with decreasing concentration of pTax peptide. After an 18-hour coculturing period, cells were stained with tetramer. Shown is the relative loss of tetramer-positive T cells after peptide stimulation. The experiment represents 3 individual experiments that showed similar results. (C) Primary T cells that were transduced with the Tax TCR or the affinity-matured version and expanded using 5 rounds of weekly peptide stimulation had the following CD8+/Vβ13 expressing cells: Tax TCR = 89%; M1-TCR = 93%; M2-TCR = 91%; and M3-TCR = 90%. T cells (2 × 105) were stimulated with either 2 × 105 HeLa GFP-positive cells that had been transduced with either the GFP plasmid or the GFP-Tax gene fusion plasmid, or were stimulated with the same transduced HeLa cells that were loaded with 100μM exogenous pTax peptide. Cells were cocultured in the presence of anti-CD107a, brefeldin A, and GolgiStop for 18 hours. Shown are the percentages of CD8+ and CD107a+ T cells. The figure represents 5 independent experiments that demonstrated similar results.
Primary T cells transduced with the high-affinity TCR show lower peptide sensitivity compared with Tax-TCR transduced T cells. (A) Freshly transduced primary T cells had the following percentage of CD8+Vβ13+ T cells: Tax TCR = 26%; M1-TCR = 24%; M2-TCR = 25%; and M3-TCR = 26%. Bulk transduced T cells (1 ×105) were cocultured with T2 cells at a 1:1 ratio of transduced T cells–to–T2 cells loaded with pTax peptide or pHuD peptide at the stated concentrations. After 18 hours of stimulation, the supernatant was removed to determine IFN-γ release by ELISA. Stimulation with T2 cells pulsed with the control pWT235 peptide or without peptide defined the background level of IFN-γ release. This represents 4 individual experiments that demonstrated similar results. (B) Primary T cells transduced with the Tax TCR and affinity-matured versions and having undergone 5 rounds of weekly peptide stimulation had the following percentage of CD8+Vβ13+ T cells: Tax TCR = 97%; M1-TCR = 99%; M2-TCR = 99%; and M3-TCR = 99%. T cells (2 × 105) were stimulated with 2 × 105 T2 cells loaded with decreasing concentration of pTax peptide. After an 18-hour coculturing period, cells were stained with tetramer. Shown is the relative loss of tetramer-positive T cells after peptide stimulation. The experiment represents 3 individual experiments that showed similar results. (C) Primary T cells that were transduced with the Tax TCR or the affinity-matured version and expanded using 5 rounds of weekly peptide stimulation had the following CD8+/Vβ13 expressing cells: Tax TCR = 89%; M1-TCR = 93%; M2-TCR = 91%; and M3-TCR = 90%. T cells (2 × 105) were stimulated with either 2 × 105 HeLa GFP-positive cells that had been transduced with either the GFP plasmid or the GFP-Tax gene fusion plasmid, or were stimulated with the same transduced HeLa cells that were loaded with 100μM exogenous pTax peptide. Cells were cocultured in the presence of anti-CD107a, brefeldin A, and GolgiStop for 18 hours. Shown are the percentages of CD8+ and CD107a+ T cells. The figure represents 5 independent experiments that demonstrated similar results.
Finally, we determined the peptide concentrations required to mediate TCR down-regulation. Although the speed of down-regulation was much faster for the affinity-matured TCR (Figure 2), peptide titration clearly revealed that down-regulation of the Tax-TCR required less peptide than down-regulation of the M1-TCR, M2-TCR, and M3-TCR (Figure 5B). Consistent with the results of the IFNγ and CD107 response, 100-fold more peptide was required for down-regulation of the M3-TCR compared with the Tax-TCR. Together, this indicates that the improved speed of affinity-matured TCR was linked to a failure to recognize low concentrations of antigen.
In the final experiments, we explored the ability of the Tax-TCR and the affinity-matured TCR to recognize naturally processed Tax antigen. HLA-A2 positive HeLa cells were transfected with a plasmid vector encoding full-length Tax protein and GFP as marker, or with a control vector encoding GFP only. The transfected HeLa cells were used to stimulate T cells expressing the Tax-TCR or affinity-matured TCR, followed by measurement of Tax-specific CD107 induction. A robust CD107 up-regulation was seen when T cells expressing the Tax-TCR and the M1-TCR were stimulated with the Tax-expressing HeLa cells (Figure 5C). In contrast, impaired CD107 responses were seen when T cells expressing the M2 and M3-TCR were analyzed; M3-TCR T cells showed only 50% of the response seen with Tax-TCR T cells. Stimulation with control GFP-transfected HeLa cells showed similar low background levels of CD107 for all TCR, excluding nonspecific T-cell stimulation by transfected HeLa cells. Finally, stimulation with peptide loaded HeLa cells triggered equally strong CD107 responses in T cells expressing Tax-TCR or affinity-matured M1-TCR, M2-TCR, or M3-TCR, indicating that all T cells were fully functional when stimulated with high concentration of peptide antigen. Together, these experiments indicated that the reduced ability of the M2 and M3-TCR to recognize low concentration of peptide antigen (Figure 4C) correlated with an impaired ability to recognize cells expressing the Tax antigen endogenously (Figure 5C).
Discussion
We have used the HLA-A0201–restricted Tax-TCR and generated in vitro high-affinity versions that retained the specificity for the Tax peptide. In a previous study, the analysis of high affinity human TCR variants revealed loss of peptide-specificity in primary CD8+ T cells.28 Thus, it remained unclear whether affinity maturation is simply limited by the risk of cross reactivity, or if high affinity TCR variants that retain peptide specificity display additional functional impairments.
The data presented here showed that within the natural TCR affinity range there is a good correlation between dissociation constant KD of the TCR/ligand interaction and the speed and peptide sensitivity of T-cell responses. However, beyond the natural affinity range there is a separation of the speed and the sensitivity of CD8+ T-cell responses. Although affinity-matured TCR trigger faster responses, this was linked to a reduction in peptide sensitivity. We showed that optimal peptide sensitivity was achieved with the Tax-TCR (t1/2 9.3 seconds), and that increasing the dissociation half time t1/2 to 348, 1320, and 3120 seconds resulted in a progressive loss of peptide sensitivity. A recent study with the human NY-ESO-TCR showed that an increase in t1/2 from 2.2 seconds for the wild-type TCR to 19 seconds for an affinity-matured TCR improved the ability of TCR transduced CD8+ T cells to recognize NY-ESO–expressing tumor cells, and this improvement was not seen with an affinity-matured TCR with a t1/2 of 41 seconds.15 Together, these observations suggest that affinity maturation may be beneficial when the half-life of the TCR-HLA-to-peptide interaction is increased to more than 10 seconds, but is likely to impair peptide sensitivity when the t1/2 is enhanced into the range of minutes.
The presented data support the serial triggering model of T-cell activation, which predicts that one MHC–peptide complex can sequentially engage several TCR molecules to achieve a critical threshold of approximately 200 triggered TCRs that is required to achieve T-cell activation.32-34 In this model, the dissociation of the TCR is an essential step, which is progressively impaired when the KD and half-life of the TCR-MHC–peptide complex are increased. The experimental data presented here are fully compatible with the serial triggering model and provide an explanation for the relative low affinity and short t1/2 of the natural TCR repertoire. Our data show that affinity-matured TCR do not improve the ability of T cells to recognize small numbers of MHC-presented peptide epitopes, an essential function of high avidity T cells. The ability of T cells to recognize low concentration of peptide was found to correlate with an improved ability to control the growth of tumor cells and the spread of virus infection in vivo, supporting the biologic importance of high T-cell avidity.35,36 Similarly, we recently demonstrated a correlation between the functional avidity of Tax-specific CTLs, assayed directly ex vivo, and the efficiency of control of HTLV-1 replication in vivo.37
It is possible that the impaired avidity associated with high TCR affinity is a feature of artificially generated TCR and does not apply to the natural TCR repertoire. However, it is interesting to speculate that low-avidity T cells, that is, T cells requiring high concentration of peptide for eliciting effector function, might express TCR with higher affinity for MHC-to-peptide than the TCR used by high avidity T cells. For example, T cells expressing the Tax-TCR were of higher functional avidity than T cells expressing the M3-TCR, although the affinity of the latter was 700-fold greater. To date, there are very limited data comparing the biochemical affinities of different TCRs isolated from T cells that are specific for the same peptide antigen. Our observations predict that an identical avidity of peptide-specific T cells can be achieved by low-affinity TCR stimulated for prolonged time periods, or by high-affinity TCR stimulated for short time periods. It is conceivable that faster T-cell responses may provide improved protection against rapidly replicating viral pathogens, as was observed for an affinity-matured TCR specific for HIV.29 We anticipate that more extensive biochemical affinity measurements of TCR-to-antigen interactions will reveal examples of high-affinity TCR expressed in T cells with reduced functional avidity. In addition to mounting a faster T-cell response, the high affinity TCR might enable T cells to cross-recognize variants of the cognate peptide, thus improving protection against epitope mutations developing in the course of virus infection and tumor progression.29 In fact, it is possible that mutated epitopes may be more efficiently recognized by T cells by reducing the TCR affinity to a range that restores the capability of serial triggering, which is impaired when the TCR interacts with the cognate high-affinity peptide ligand.
It is not clear how T-cell sensitivity is affected when antigen-presenting cells present to the TCR simultaneously peptides that are high-affinity and low-affinity ligands. This situation may occur in diploid tumors expressing both cognate and variant peptides, or in infection during the development of viral escape variants. At high density of peptides, the T-cell response is expected to be fully functional and occur at the fast kinetics driven by the high-affinity TCR-to-peptide interaction. However, at low peptide densities, the response is expected to be dominated by the low TCR-to-peptide interaction capable of using serial triggering to achieve the threshold required for T-cell activation, while the high-affinity TCR-to-peptide interactions are unable to achieve the activation threshold. Whether the stable high-affinity interaction would interfere with the serial triggering of the low affinity TCR-to-peptide interaction needs to be explored experimentally. Although speculative, it is theoretically possible that low concentrations of high-affinity peptide variants might function as antagonists for T-cell activation by cognate peptides.
Recent in vivo imaging studies indicated that T-cell priming occurs in 3 stages defined by short T cell–to–dendritic cell interactions, leading to T-cell arrest and stable interactions followed by renewed T-cell motility.38 Ineffective T-cell priming leading to tolerance induction was associated with persistent short 30-90 second contact periods between T cells and dendritic cells, and a lack of T-cell arrest and formation of stable interactions.39 It is possible that productive T-cell priming is more effective for high-affinity TCR capable of responding faster to short periods of antigen presentation than for lower-affinity TCR requiring longer periods of presentation.
Together, we demonstrate that TCR with affinities beyond the natural range can initiate T cells responses faster than TCR within the natural range, although both may endow T cells with the same functional avidity. The accelerated response may facilitate T-cell priming and protection against rapidly replicating viral pathogens. The increased potential for cross-reactivity may improve recognition of viral escape variants, but it may also lead to recognition of self-antigens. Cross-recognition of self is likely to affect thymic T-cell selection and result in the removal of many high affinity TCR from the natural repertoire.40 Despite the reduced frequency it is probable that low-avidity T cells with high-affinity TCR exist in the human repertoire. Their identification will require surface plasmon resonance binding assays, as they cannot be identified using functional T-cell assays. To date, only 15 human class I–restricted TCRs have been analyzed, with a recently analyzed TCR specific for HIV-Gag displaying a 10-fold higher affinity than all previously documented human and murine TCRs.1,29 It is probable that more extensive binding studies will reveal human TCRs with substantially higher affinity range than the TCRs analyzed thus far.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Leukaemia Lymphoma Research United Kingdom program grant P3379, ATTACK Integrated Project grant LSHC-CT-2005-018914, Medical Research Council project grant G0700149, Experimental Cancer Medicine Center grant C34/A7279, and Medical Research Council Virology Center grant G0900950.
Authorship
Contribution: S.T. designed and performed experiments, analyzed data, and wrote the paper; S.-A.X. designed experiments and provided reagents; C.B. provided reagents; B.K.J. performed binding experiments and provided reagents; E.M. analyzed data; and H.J.S. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: H.J.S. is scientific adviser to Cell Medica. The remaining authors declare no competing financial interests.
Correspondence: Prof H. J. Stauss, Department of Immunology, University College London, Royal Free Hospital, London NW3 2PF, United Kingdom; e-mail: h.stauss@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal