Abstract
Hematologic malignancies such as acute and chronic myeloid leukemia are characterized by the malignant transformation of immature CD34+ progenitor cells. Transformation is associated with elevated expression of the Wilm's tumor gene encoded transcription factor (WT1). Here we demonstrate that WT1 can serve as a target for cytotoxic T lymphocytes (CTL) with exquisite specificity for leukemic progenitor cells. HLA-A0201– restricted CTL specific for WT1 kill leukemia cell lines and inhibit colony formation by transformed CD34+ progenitor cells isolated from patients with chronic myeloid leukemia (CML), whereas colony formation by normal CD34+ progenitor cells is unaffected. Thus, the tissue-specific transcription factor WT1 is an ideal target for CTL-mediated purging of leukemic progenitor cells in vitro and for antigen-specific therapy of leukemia and other WT1-expressing malignancies in vivo.
Cells of the hematopoietic system are derived from hematopoietic stem cells (HSC) capable of self-renewal and differentiation. Transplantation experiments in humans and mice have shown that CD34+ cell populations contain HSC capable of reconstituting the erythroid, myeloid, and lymphoid lineages in myeloablated recipients.1 In addition, HSC capable of reconstituting murine hosts were recently demonstrated in a rare population of CD34−/lin− bone marrow cells.2
There is strong evidence that the critical transformation events in CML and acute myeloid leukemia (AML) affect immature CD34+progenitor cells. Because the majority of leukemic blast cells have limited proliferative capacity, the malignant disease must be maintained by a subpopulation of leukemic progenitor cells with extensive proliferative and self-renewal capacities.3,4Transplantation studies with purified cells from patients with AML showed that only immature CD34+ cells were capable of initiating leukemia in immunocompromised murine recipients.5 Similarly, purified CD34+ cells from patients with CML efficiently initiated leukemia in murine recipients.6 7
The molecular events leading to uncontrolled progenitor cell proliferation are not fully understood. Although BCR/ABL fusion proteins associated with the t(9;22) chromosomal translocation is the hallmark of CML, BCR/ABL transcripts can also be found in healthy persons, indicating that additional factors are required for leukemia to develop.8 The Wilm's tumor gene (WT1) transcription factor is a candidate protein contributing to leukemogenesis. This transcription factor is normally expressed in immature CD34+ progenitor cells, and differentiation is associated with WT1 down-regulation.9,10 Elevated levels of WT1 expression have been observed in unseparated mononuclear cells and in purified CD34+ cells from patients with AML and CML.11,12 In vitro studies showing that increased WT1 expression can block normal differentiation and enhance proliferation of hematopoietic progenitor cells provide an explanation for the potential of WT1 to contribute to leukemogenesis.13 14
The results of a recent study suggested that T lymphocytes specific for CD34+ progenitor cells are critically important in mediating antileukemic effects in patients with CML.15However, there is no information concerning the nature of T-cell recognized target antigens expressed by CD34+ cells. In this study we explored the possibility of exploiting WT1 as a target molecule to direct cytotoxic T lymphocytes against leukemic progenitor cells. We tested the hypothesis that CML, but not normal CD34+ progenitor cells, express sufficient WT1 protein to trigger a cytotoxic T lymphocyte (CTL) attack.
Materials and methods
Cell lines
The K562 cell line was established from the pleural effusion of a female patient with CML in blast crisis.16 The BV173 cell line was established from the peripheral blood of a male patient with CML in blast crisis.17 The cell line 697 was established from the bone marrow of a 12-year-old boy with acute lymphoblastic leukemia.18 The C1R cell line is a HLA-A0201–negative Epstein-Barr virus (EBV) transformed lymphoblastoid cell line.19 The T2 cell line has been selected for loss of the genes encoding TAP (transporter associated with antigen processing), resulting in inefficient loading of human leukocyte antigen (HLA) class I molecules with endogenous peptides.20 As a consequence, the HLA-A0201 molecules of T2 cells can be efficiently loaded with exogenous peptides.Drosophila cells transfected with HLA-A0201, human β-2 microglobulin, B7.1, and ICAM-1 were a kind gift from Dr M. Jackson.
Synthetic peptides.
A peptide derived from human Wilms tumor antigen 1 P126 (RMFPNAPYL) and a control HLA-A02 01-binding peptide derived from the E7 protein of human papillomavirus type 16 were synthesized by the central peptide synthesis laboratory of the Imperial College Medical School using fluorenylmethoxycarbonyl chemistry. The quality of the peptides was assessed by high-performance liquid chromatography analysis, and the expected molecular weight was observed using matrix-assisted laser desorption mass spectrometry. The peptides were dissolved in phosphate-buffered saline (PBS; pH 7.4) to give a concentration of 2 mmol/L and were stored at −20°C.
Generation of allo–HLA–restricted CTL lines.
Peripheral blood mononuclear cells (PBMC) were separated from buffy coat packs using Ficoll gradient centrifugation and were stained with monoclonal antibodies HB54 (anti–HLA-A2, B17) and HB117 (anti–HLA-A2, A28). A2-negative PBMCs were used as responders. Peptide-coatedDrosophila cells transfected with HLA-A0201, human β2-microglobulin, B7.1, and ICAM-1 were used as initial stimulators.Drosophila cells were induced in 100 mmol/L CuSO4for 48 hours, washed 3 times with medium, and loaded with peptide at a concentration of 100 μmol/L for 4 hours. Each well of a 24-well plate received 2 × 106 responder PBMC and 2 × 105 stimulator cells in 2 mL T-cell medium. On day 5, T cells were harvested and plated in fresh T-cell medium at a density of 5 × 105 per well, with the addition of 2 × 106 autologous irradiated PBMC as feeders, 2 × 105 irradiated peptide-coated T2 cells, 10% QS4120 culture supernatant (containing anti-CD4 antibodies), and 10 U/ml hu-rIL-2 (Roche Diagnostics, Lewes, UK). The cultures were restimulated weekly using T2 cells coated with the immunizing peptide as stimulators. After 2 to 3 cycles of stimulation, the bulk cultures were cloned in 96-well plates at densities of 1, 10, and 30 cells per well; 104 peptide-coated T2 cells, 2 × 105 HLA-A2 negative PBMC feeders, and 2 U/mL IL-2 were added to each well. The cytotoxicity of each well was tested against T2 target cells coated with the immunizing peptide or a control HLA-A0201–binding peptide. Peptide-specific microcultures were expanded and restimulated weekly in 24-well plates by adding 2 × 106 feeders, 2 × 105stimulator cells, and 10 U/mL IL-2. The T-cell line 77 (Figure1B) was maintained for more than 1 year in culture and served as a source of CTL for most experiments in this study. Because this line consisted of CD4+and CD8+ T cells, CD8+ subclones were used to show that the specific killing activity was mediated by CD8+ CTL. Unlike the parental 77 line, the in vitro lifespan of CD8+ subclones was limited to a few months.
Specificity of allo-restricted CTL generated against the WT1-derived peptide P126.
CTL were isolated by limiting dilution cloning of T-lymphocyte bulk cultures from HLA-A0201− donors stimulated with HLA-A0201+ stimulator cells coated with P126 peptide. (A) Isolated CTL lines killed the TAP-deficient T2 target cells coated with the immunizing P126 peptide but not T2 cells coated with the HLA-A0201–binding E7 control peptide. (B) Peptide titration experiments showing that 3 anti-P126 CTL lines were of high avidity recognizing low picomolar concentrations of P126, and that 3 CTL lines were of low avidity because nanomolar P126 concentrations were required for target cell recognition. T2 cells coated with the indicated concentrations of P126 were used as CTL targets. High-avidity CTL were used for all subsequent experiments because low-avidity CTL did not recognize target cells expressing WT1 endogenously. (C) High-avidity CTL killed the HLA-A0201+ leukemic cell lines BV173, 697 but not the HLA-A0201+, EBV-transformed B-lymphoid cells C1R-A2. Coating of C1R-A2 with P126 resulted in efficient CTL killing. The HLA-A0201− leukemia cell line K562 was not killed by the CTL unless transfected with the HLA-A0201 gene.
Specificity of allo-restricted CTL generated against the WT1-derived peptide P126.
CTL were isolated by limiting dilution cloning of T-lymphocyte bulk cultures from HLA-A0201− donors stimulated with HLA-A0201+ stimulator cells coated with P126 peptide. (A) Isolated CTL lines killed the TAP-deficient T2 target cells coated with the immunizing P126 peptide but not T2 cells coated with the HLA-A0201–binding E7 control peptide. (B) Peptide titration experiments showing that 3 anti-P126 CTL lines were of high avidity recognizing low picomolar concentrations of P126, and that 3 CTL lines were of low avidity because nanomolar P126 concentrations were required for target cell recognition. T2 cells coated with the indicated concentrations of P126 were used as CTL targets. High-avidity CTL were used for all subsequent experiments because low-avidity CTL did not recognize target cells expressing WT1 endogenously. (C) High-avidity CTL killed the HLA-A0201+ leukemic cell lines BV173, 697 but not the HLA-A0201+, EBV-transformed B-lymphoid cells C1R-A2. Coating of C1R-A2 with P126 resulted in efficient CTL killing. The HLA-A0201− leukemia cell line K562 was not killed by the CTL unless transfected with the HLA-A0201 gene.
CTL assays.
Cytotoxic T lymphocyte assays were performed as described. Briefly, 106 T2 cells were incubated for 1 hour in 200 μL assay medium (RPMI 1640 with 5% heat inactivated fetal calf serum) with 100 μmol/L synthetic peptide at 37°C. Peptide-coated T2 cells or tumor cells were labeled with chromium 51 for l hour, washed, and added to serial 2-fold dilutions of effector cells in round-bottom, 96-well plates to obtain a total volume of 200 μL/well. Assay plates were incubated for 4 hours at 37°C, 5% CO2, and 100 μL supernatant was harvested and counted using a Wallac Gamma Counter, Wallac, Milton Keynes, UK. The specific lysis was calculated by the equation (experimental release − spontaneous release)/maximum release − spontaneous release) × 100%.
Purification of hematopoietic CD34+cells.
As a source of normal CD34+ cells, we used human bone marrow from adult healthy donors (n = 5), leukapheresis products of stem cells mobilized from solid-tumor patients in disease remission (n = 2), and cord blood (n = 1). Samples of cord blood were obtained from discarded placental and umbilical tissues by drainage of the blood into sterile collection tubes. Informed consent for use of these cells was obtained from donors or parents as appropriate. As a source of leukemic CD34+ cells, peripheral blood was obtained from patients who had CML in chronic phase and who had not been treated with interferon in at least 3 months.
Samples were diluted 1:2 in Hanks balanced salt solution and enriched for mononuclear cells by density-gradient centrifugation (Lymphoprep 1.077 g/mL; Nycromed Pharma AS, Oslo, Norway), and the recovered mononuclear fraction was subject to magnetic microbead selection for the isolation of CD34+ fraction using the Minimacs system and following the manufacturer's instructions (Miltenyi Biotec, Bisley, UK). The purity of the cell population ranged from 80% to 95% as estimated by FACS analysis using an antihuman CD34 phycoerythrin (PE) mouse monoclonal antibody (Becton Dickinson, Cowley, UK).
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR).
Total RNA of 106 cells was isolated according to RNAzol B protocol (AMS Bio, Witney, UK). cDNA synthesis of whole RNA pellet was performed in a 40μL reaction. The dissolved RNA pellet was first incubated with 2 μg oligo-dT 12-18 primer (Life Technologies, Paisley, UK) at 65°C for 10 minutes, followed by a 1-hour incubation at 42°C with a mixture of 50 U murine leukemia virus reverse transcriptase, 10 mmol/L dithiothreitol, 1 mmol/L dNTP (Roche Diagnostics), and 40 U RNase inhibitor (Promega, Southampton, UK). Five microliters cDNA preparation was used for polymerase chain reaction (PCR) amplification in a 50-μL volume of final reaction mixture containing 2.5 U Taq DNA polymerase (Qiagen, Crawley, UK), 1 mmol/L dNTP, and 20 OD/mL primer.
Amplification of the human WT1 coding region was achieved using sense primers located in exon 7 (21 mer 5′-ggc atc tga gac cag tga gaa-3′) and antisense primers in exon 10 (22 mer 5′-gag agt cag act tga aag cag t-3′). The expected size for the WT1 PCR product was 482 bp. RNA integrity was verified by amplifying the human c-ABL gene in every sample using intron-spanning primers: 22 mer sense 5′-ccc aac ctt ttc gtt gca ctg t-3′; 22 mer antisense 5′-cgg ctc tcg gag gag acg atg a-3′. The expected size of the c-ABL PCR product was 385 bp. Hot-start PCR was performed for 35 cycles with a thermal cycler (Techne Genius, Cambridge, UK) under the following conditions (same for ABL and WT1 amplification): denaturing at 95°C for 1 minute, primer annealing at 56°C for 1 minute, and chain elongation at 72°C for 2 minutes. The cycling was initiated by a 5-minute denaturation step at 95°C to heat inactivate the reverse transcriptase, and it was terminated by a 10-minute final extension at 72°C. All reverse transcription (RT)-PCRs were performed at least twice, and negative control (no cDNA) and positive control (cDNA from the WT1-expressing leukemic cell line BV173) were included in every experiment. PCR products were electrophoresed through 1.5% agarose gels.
Western blot analysis.
Separated CD34+ cells (2 × 105 cells) were washed in PBS and lysed in Laemmli buffer. The cell lysate was fractionated by a 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham, Little Chalfont, UK) by wet transfer. The membrane was then blocked in PBS containing 0.01% Tween 20 and 5% nonfat dry milk for 1 hour at room temperature and incubated first with rabbit antihuman WT-1 C19 polyclonal antibody (1:200 in blocker; Santa Cruz Biotech, Santa Cruz, CA) overnight at 4°C and then with rabbit anti-actin polyclonal serum (1:500 in blocker; Sigma, Gillingham, UK) for 30 minutes at room temperature. The signal was revealed by incubating the membrane with horseradish peroxidase-conjugated swine antirabbit antibody (1:1000; DAKO, Cambridge, UK) and ECL reaction (Amersham) according to the manufacturer's instructions.
Progenitor (CFU) assays.
Colony-forming unit (CFU) assays were performed by plating 1000 to 3000 CD34+ cells in methylcellulose medium supplemented with the following recombinant human growth factors (Stem Cell Technologies, Northampton, UK): stem cell factor (50 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), granulocyte macrophage colony-stimulating factor (20 ng/mL), and granulocyte colony-stimulating factor (20 ng/mL). The cultures were incubated for 14 days at 37°C in humidified atmosphere at 5% CO2 to allow the development of granulocyte macrophage colony-forming units.
Results
Generation of WT1-specific CTL
Expression of the WT1 transcription factor in adults is detectable in renal podocytes, testicular Sertoli cells, ovarian granulosa cells, and CD34+ bone marrow cells.21 To avoid possible immunologic tolerance to WT1, we used a previously described approach of generating peptide-specific CTL from major histocompatibility complex-mismatched donors. This approach is suitable for generating CTL against any protein overexpressed in tumor cells, independent of immunologic tolerance.22 23 A 9-amino acid-long WT1-derived peptide epitope, P126 (RMFPNAPYL), was selected as the CTL target because it bound to HLA-A0201 class I molecules, the most frequent class I allele found in white populations (not shown). Responder lymphocytes from HLA-A0201−donors were cultured in vitro with HLA-A0201+ stimulator cells presenting the P126 peptide, and limiting dilution cultures were used to isolate peptide-specific CTL lines. Experiments with peptide-coated T2 target cells showed that the CTL were highly specific for the P126 peptide (Figure 1A). Peptide titration indicated that the CTL could be divided into high-avidity lines capable of recognizing low picomolar peptide concentrations and low-avidity lines recognizing low nanomolar peptide concentrations (Figure 1B). High-avidity CTL lines were selected for further experiments.
WT1-specific CTL kill leukemia cell lines
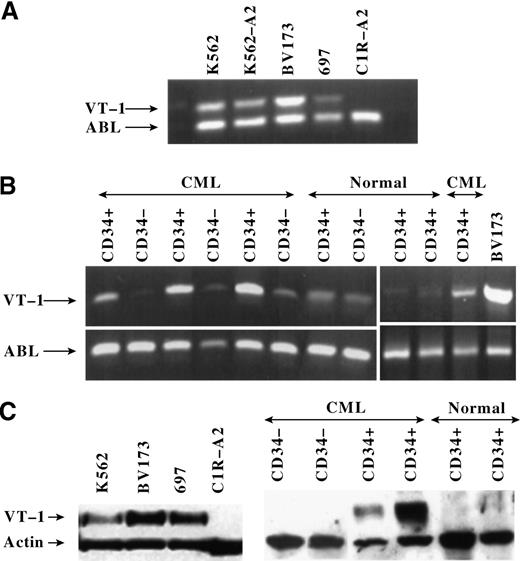
Analysis of a panel of leukemia cell lines revealed that P126-specific CTL killed the HLA-A0201+ cells BV173 and 697 (Figure 1C). The HLA-A0201− leukemia cell line K562 was only killed after transfection with HLA-A0201. In contrast, the HLA-A0201+ EBV-transformed B cell line C1R-A2 was not killed unless cells were coated with P126 peptides (Figure 1C; similar results were seen with other EBV-transformed cells). The expression of WT1 in the CTL target cells was analyzed at the RNA and protein levels. RT-PCR demonstrated that the leukemia cell lines, but not the EBV-transformed C1R-A2 cells, expressed WT1 RNA (Figure2A). Similar results were obtained by Western blot analysis showing that WT1 protein was only expressed in leukemia cells but not in C1R-A2 cells (Figure 2C). Together the data indicated that the CTL recognized A0201+ leukemia cell lines and that CTL killing correlated with WT1 expression.
WT1 RNA and protein expression in leukemic cell lines and in CD34+ and CD34− cell populations freshly isolated from patients with leukemia and normal donors.
(A) RT-PCR to measure WT1 RNA in leukemic cell lines and in the B-lymphoid cell line C1R-A2. The same cell lines were used as CTL targets in Figure 1C. The amplified WT1 product is 482 bp long. The RNA of the housekeeping ABL gene was amplified to indicate the amount of RNA in each sample. The ABL product is 385 bp long. (B) RT-PCR to measure WT1 RNA expression in purified CD34+ and CD34− cell populations from 4 patients with CML and 3 normal donors. The leukemic cell line BV173 served as a positive control for WT1 expression. Similar results were obtained with samples from 6 additional patients with CML. (C) Western blotting to measure WT1 protein expression in leukemia cell lines and in purified CD34+ and CD34− cell populations from 2 patients with CML and 2 normal donors. The expression of the housekeeping actin protein was used as an indicator to control for the amount of protein in each sample. The WT1 protein measures approximately 54 kd and the actin protein approximately 42 kd.
WT1 RNA and protein expression in leukemic cell lines and in CD34+ and CD34− cell populations freshly isolated from patients with leukemia and normal donors.
(A) RT-PCR to measure WT1 RNA in leukemic cell lines and in the B-lymphoid cell line C1R-A2. The same cell lines were used as CTL targets in Figure 1C. The amplified WT1 product is 482 bp long. The RNA of the housekeeping ABL gene was amplified to indicate the amount of RNA in each sample. The ABL product is 385 bp long. (B) RT-PCR to measure WT1 RNA expression in purified CD34+ and CD34− cell populations from 4 patients with CML and 3 normal donors. The leukemic cell line BV173 served as a positive control for WT1 expression. Similar results were obtained with samples from 6 additional patients with CML. (C) Western blotting to measure WT1 protein expression in leukemia cell lines and in purified CD34+ and CD34− cell populations from 2 patients with CML and 2 normal donors. The expression of the housekeeping actin protein was used as an indicator to control for the amount of protein in each sample. The WT1 protein measures approximately 54 kd and the actin protein approximately 42 kd.
WT1-specific CTL kill fresh leukemic CD34+ cells
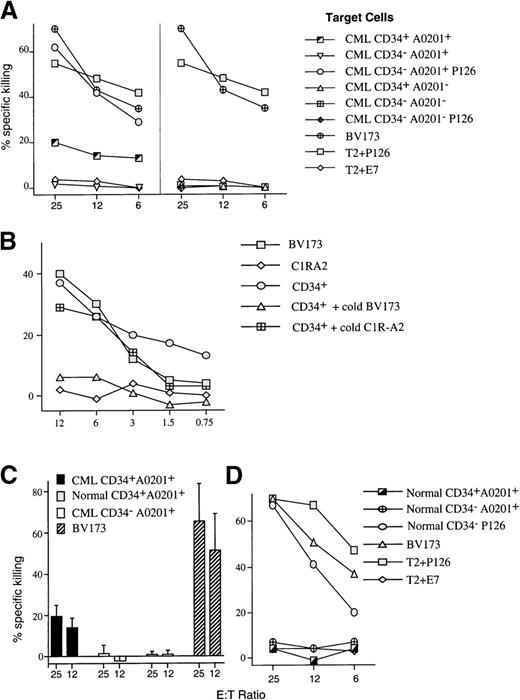
PBMCs from patients with chronic-phase CML were separated into immature CD34+ and mature CD34−populations. As expected, cells isolated from HLA-A0201− patients were not recognized by P126-specific CTL (Figure 3A). When cells from HLA-A0201+ patients with CML were analyzed, the CTL selectively recognized the CD34+ cell population, whereas no killing of the more mature CD34− population was observed (Figure 3A). Cold target competition experiments showed that the killing of CD34+ CML cells was inhibited by the leukemia cell line BV173 but not by EBV-transformed C1R-A2 cells (Figure 3B). This indicated that CD34+ cells from patients with CML and BV173 cells share the CTL-recognized target antigen and that this antigen is absent in C1R-A2 cells.
Analysis of CTL-mediated killing of CD34+cell populations purified from patients with leukemia and normal donors.
(A) Representative experiment showing the level of killing by anti-P126 CTL against purified CD34+ and CD34− cell populations isolated from patients with CML who were either HLA-A0201+ or A0201−. CD34−/A0201+ cells were not recognized by the CTL unless coated with P126 peptide. The leukemic cell line BV173 and the TAP-deficient T2 cells coated with P126 or the control E7 peptide were used as positive and negative controls in all experiments. (B) Cold target competition experiment. Shown is the killing by anti-P126 CTL against chromium-labeled CD34+ targets from an A0201+ patient with CML in the absence or presence of a 30-fold excess of cold BV173 and C1R-A2 targets. The killing of chromium-labeled BV173 and C1R-A2 is shown for comparison. (C) Average of the level of specific CTL killing of purified CD34+cells from 11 different HLA-A0201+ patients with CML and from 6 normal donors. The level of killing of CD34−cells purified from patients with CML and against the positive control cells BV173 is also shown. The figure shows the mean level and standard deviation of specific CTL killing. (D) Representative experiment showing the level of killing by anti-P126 CTL of purified CD34+ and CD34− cell populations isolated from HLA-A0201+ normal donors. No CTL killing was detectable unless target cells were coated with P126 peptide.
Analysis of CTL-mediated killing of CD34+cell populations purified from patients with leukemia and normal donors.
(A) Representative experiment showing the level of killing by anti-P126 CTL against purified CD34+ and CD34− cell populations isolated from patients with CML who were either HLA-A0201+ or A0201−. CD34−/A0201+ cells were not recognized by the CTL unless coated with P126 peptide. The leukemic cell line BV173 and the TAP-deficient T2 cells coated with P126 or the control E7 peptide were used as positive and negative controls in all experiments. (B) Cold target competition experiment. Shown is the killing by anti-P126 CTL against chromium-labeled CD34+ targets from an A0201+ patient with CML in the absence or presence of a 30-fold excess of cold BV173 and C1R-A2 targets. The killing of chromium-labeled BV173 and C1R-A2 is shown for comparison. (C) Average of the level of specific CTL killing of purified CD34+cells from 11 different HLA-A0201+ patients with CML and from 6 normal donors. The level of killing of CD34−cells purified from patients with CML and against the positive control cells BV173 is also shown. The figure shows the mean level and standard deviation of specific CTL killing. (D) Representative experiment showing the level of killing by anti-P126 CTL of purified CD34+ and CD34− cell populations isolated from HLA-A0201+ normal donors. No CTL killing was detectable unless target cells were coated with P126 peptide.
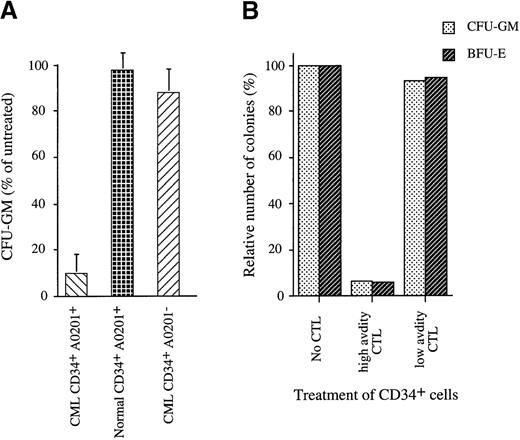
Lack of killing of CD34− cells most likely resulted from the insufficient expression of the WT1-derived target peptide because the coating of these cells with exogenous P126 peptide resulted in CTL killing (Figure 3A). RT-PCR analysis revealed strong WT1 RNA expression in CD34+ cells and low expression in CD34− cells (Figure 2B). WT1 protein expression detectable by Western blotting was restricted to CD34+, and no protein was detectable in CD34− cells (Figure 2C). Both RT-PCR and Western analysis showed variation in the level of WT1 expression in leukemic CD34+ cell populations. Thus, we explored whether the observed variation in the level of WT1 expression resulted in a variation in the level of CTL killing. However, analysis of 11 different patients with CML showed that the CTL consistently lysed approximately 20% (SD, 5%) of the CD34+ population (Figure 3C). This result raised the possibility that WT1 expression was restricted to a subpopulation of approximately 20% of CD34+ cells and that the expression level in all 11 patients with CML was sufficient to render most of these cells susceptible to CTL killing. We explored whether the subpopulation recognized by CTL included the clonogenic progenitor cells that can give rise to colonies of the granulocyte, macrophage, and erythroid lineages. When CD34+ populations isolated from 9 patients with CML were treated with P126-specific CTL, this resulted in 80% to 100% inhibition of colony formation (Figure4A). This indicated that the majority of colony-forming progenitor cells were removed by P126-specific CTL. The “escape” colonies seen in CTL-treated samples were small when compared to the colonies in untreated samples. This is consistent with the possibility that the escape colonies were derived from progenitor cells that had already initiated differentiation toward the granulocyte/myeloid lineage associated with the down-regulation of WT1 expression. Such partially differentiated progenitors might escape recognition by WT1-specific CTL, and the small size of the colonies observed in the CFU assay might reflect the reduced clonal burst size of these progenitors.
Analysis of CTL-mediated inhibition of colony formation of CD34+ cell populations purified from patients with leukemia and normal donors.
(A) CTL-mediated inhibition of colony formation by purified CD34+ cells cocultured for 4 hours with CTL at an effector/target cell ratio of 10:1. Untreated control CD34+cells were cultured under the same conditions without CTL. CTL treated and untreated control cells were then plated in methylcellulose, and after 14 days the numbers of granulocyte macrophage colony-forming units (GM-CFU) were counted. Shown is the percentage of GM-CFU after CTL treatment using the GM-CFU observed in the untreated controls as 100% reference. This figure shows the mean and standard deviation of independent experiments with CD34+ cells from 9 HLA-A0201+ patients with CML and 7 normal donors and with CD34+ cells from 5 HLA-A0201− patients with CML. (B) Colony formation by A0201+/CD34+CML cells that were untreated or treated for 4 hours with high-avidity P126-specific CTL (line 81) or with low-avidity CTL (line 85) before plating. Shown are GM-CFU and BFU-E using the number of colonies observed in the untreated controls as 100% reference.
Analysis of CTL-mediated inhibition of colony formation of CD34+ cell populations purified from patients with leukemia and normal donors.
(A) CTL-mediated inhibition of colony formation by purified CD34+ cells cocultured for 4 hours with CTL at an effector/target cell ratio of 10:1. Untreated control CD34+cells were cultured under the same conditions without CTL. CTL treated and untreated control cells were then plated in methylcellulose, and after 14 days the numbers of granulocyte macrophage colony-forming units (GM-CFU) were counted. Shown is the percentage of GM-CFU after CTL treatment using the GM-CFU observed in the untreated controls as 100% reference. This figure shows the mean and standard deviation of independent experiments with CD34+ cells from 9 HLA-A0201+ patients with CML and 7 normal donors and with CD34+ cells from 5 HLA-A0201− patients with CML. (B) Colony formation by A0201+/CD34+CML cells that were untreated or treated for 4 hours with high-avidity P126-specific CTL (line 81) or with low-avidity CTL (line 85) before plating. Shown are GM-CFU and BFU-E using the number of colonies observed in the untreated controls as 100% reference.
Importantly, colony formation by CD34+ cells from HLA-A0201− patients with CML was unaffected, indicating that the elimination of progenitors with colony-forming activity was dependent on HLA-restricted antigen recognition by the CTL. Furthermore, the elimination of colonies of the granulocyte, macrophage, and erythroid lineages in CD34+ cells from HLA-A0201+ patients with CML was dependent on high-avidity recognition of the P126 peptide. Only high-avidity CTL eliminated the progenitors of CFU-GM and BFU-E, whereas low-avidity CTL had no effect (Figure 4B).
Finally, we explored whether high-avidity CTL discriminated between leukemic and normal CD34+ progenitor cells. Normal CD34+ cells were isolated from bone marrow, peripheral blood, or umbilical cord blood of HLA-A0201+ donors and were used as CTL targets. Independent of the source of CD34+ cells, anti-P126 CTL did not inhibit colony formation by these cells (Figure 4A). Furthermore, normal CD34+ cells were not killed when used as targets in cytotoxicity assays (Figures 3C and 3D). The selective CTL killing of leukemic versus normal CD34+ cells can be explained by differences in WT1 expression. WT1 RNA expression was higher in leukemic than in normal CD34+ cells (Figure 2B), and Western blot analysis detected WT1 protein only in leukemic but not in normal CD34+ cell populations (Figure 2C).
Discussion
The infusion of donor lymphocytes to patients in relapse after previous allogeneic stem cell transplantation can engender strong graft-versus-leukemia effects.24,25 A recent study showed that complete remission in patients with CML undergoing donor lymphocyte infusion was associated with an increased frequency of T cells recognizing leukemic CD34+ progenitor cells.15 In contrast, T-cell recognition of more mature CD34− cells in patients with CML was not associated with a favorable clinical response. This suggested that T lymphocytes with specificity for CD34+ CML progenitor cells were critically important in mediating antileukemic effects in vivo. However, there is no information concerning the nature of target antigens that can direct T-cell responses selectively against leukemic CD34+ progenitor cells.
To identify such antigens, we used the allo-restricted CTL approach that, independent of immunologic tolerance, is suitable for raising CTL against any protein expressed at elevated levels in transformed cells.22,23,26 Because the triggering of the cytotoxic effector function is a threshold phenomenon, it is possible to select CTL that are only triggered by elevated target protein levels in transformed cells but not by physiological levels of protein in normal cells.23
The transcription factor WT1 was chosen as a candidate target protein for several reasons. There is evidence of overexpression in leukemic CD34+ cells,12 elevated WT1 expression can contribute to transformation,13,14 normal WT1 expression is restricted to a small number of cells in postnatal life,21and, in addition to leukemia, elevated WT1 expression has been observed in renal cell carcinoma, ovarian cancer, advanced breast cancer, and melanoma.27-30 Therefore, CTL selectively recognizing WT1 overexpressing malignant cells are invaluable reagents for antigen-specific therapy of leukemia and other more common malignancies. Furthermore, tumor escape by down-regulation of WT1 expression is unlikely to occur if overexpression is required to maintain the transformed phenotype.31-33
The allo-restricted CTL described here were isolated from HLA-A0201− donors, and they were specific for leukemic progenitor cells presenting the WT1-derived P126 peptide in the context of HLA-A0201 class I molecules. The P126 peptide was highly immunogenic because in vitro stimulation of lymphocytes from different HLA-A0201− donors consistently induced peptide-specific, HLA-A0201–restricted CTL. Therefore, P126-specific CTL are novel reagents for antigen-specific therapy of HLA-A0201+ patients with leukemia undergoing stem cell transplantation from donors displaying a 1-locus HLA-mismatch involving the HLA-A0201 allele. A 1-locus HLA mismatch is clinically acceptable, as demonstrated in a recent study showing comparable prognoses in patients with leukemia receiving transplants from 1-locus–mismatched and HLA-matched unrelated donors.34 Thus, a 1-locus–mismatch transplant provides an ideal setting for antigen-specific therapy with allo-restricted CTL derived from the donor. The in vitro stimulation protocol described here, in combination with the selection of relevant CTL by staining with HLA-A0201 tetramers containing P126 peptides, will allow rapid isolation of P126-specific CTL for adoptive therapy.
In addition, it is possible that WT1 can be exploited for antigen-specific therapy in the autologous setting. This is supported by our observation that P126-specific CTL can be isolated from HLA-A0201+ donors (unpublished data). Because WT1 expression in adults is restricted to a relatively small number of cells (eg, CD34+ bone marrow cells, renal podocytes, testicular Sertoli cells, and ovarian granulosa cells), tolerance of autologous T lymphocytes to WT1 is probably incomplete. Therefore, it may be possible to exploit the identified P126 epitope for the design of anti-WT1 vaccine preparations aimed at stimulating CTL responses against leukemia and other malignancies with elevated WT1 expression, such as renal cell carcinoma, ovarian cancer, melanoma, and breast cancer.
In addition to in vivo therapy, the WT1-specific CTL provides a tool for in vitro purging of autologous bone marrow cells harvested from patients with leukemia. The CTL removed leukemic progenitors of the granulocyte/macrophage lineage (Figure 4A) and also progenitors of the erythroid lineage (Figure 4B). In contrast, the CTL did not recognize normal progenitors of the 3 lineages. The selective removal of transformed CD34+ progenitor cells should reduce the risk for reinfusing leukemic progenitor cells, thus overcoming a major limitation of autologous stem cell transplantation.35
To date, tissue-specific minor histocompatibility antigens and lineage-specific antigens, such as proteinase 3, have been studied as potential targets for leukemia-reactive CTL.36-39 The WT-1 transcription factor is the first target antigen capable of directing CTL responses selectively against leukemic progenitor cells.
Acknowledgments
We thank Drs E. Simpson and R. I. Lechler for critically reading the manuscript and Dr F. Dazzi and F. Grant for useful discussions and practical help. We also thank Prof Fisk and Dr C. Campagnoli for help with cord blood samples and Robert J. Davidson for excellent technical support.
Supported by a project and program grant from the Leukemia Research Fund.
L.G. and I.B. contributed equally to this work.
Reprints:Hans J. Stauss, Department of Immunology, Imperial College School of Medicine, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK; e-mail: h.stauss@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal