Abstract
γ-Glutamylcysteine synthetase (GCS) catalyzes the initial and rate-limiting step in the biosynthesis of glutathione. γ-GCS consists of a heavy and a light subunit encoded by separate genes. Hereditary deficiency of GCS has been reported in 6 patients with hemolytic anemia and low erythrocyte levels of glutathione and γ-glutamylcysteine. In addition, 2 patients also had generalized aminoaciduria and developed neurologic symptoms. We have examined a Dutch kindred with 1 suspected case of GCS deficiency. The proband was a 68-year-old woman with a history of transient jaundice and compensated hemolytic anemia. One of her grandchildren was also GCS deficient; he was 11 years old and had a history of neonatal jaundice. The enzyme defect was confirmed and GCS activity was found to be less than 2% of normal in the erythrocytes of both patients. The complementary DNA (cDNA) for the heavy subunit of GCS was sequenced in these patients and in several members of the family. The proband and her GCS- deficient grandson were identified as homozygous for a 473C→T substitution, changing codon 158 from CCC for proline into CTC for leucine. Several family members with half-normal GCS activity in their erythrocytes were heterozygous for the mutation.
Glutathione (γ-glutamyl-cysteinyl-glycine) (GSH) is present in all mammalian tissues. It is found intracellularly in millimolar concentrations, in tissues such as the liver, kidney, brain, skeletal muscle, and erythrocytes. Glutathione plays a key role in many biologic functions, such as protein and DNA synthesis, detoxification of xenobiotics and free radicals, as well as reductive reactions.1
The turnover occurs via the γ-glutamyl cycle, which involves 6 enzymes.2 Glutathione is synthesized by the consecutive action of γ-glutamylcysteine synthetase (glutamate-cysteine ligase) (GCS) and glutathione synthetase (GS). The rate-limiting step in the biosynthesis is catalyzed by GCS, which is feedback-inhibited by GSH. This enzyme is a dimer consisting of a heavy (GCSh) and a light (GCSl) subunit. The human gene for GCSh, called GLCLC, has been localized to chromosome 6p123 and the gene for GCSl, called GLCLR, to chromosome 1p21.4 The heavy subunit (molecular weight, 72.6 kd) exhibits the catalytic activity of the native enzyme and is also responsible for the feedback inhibition by GSH. The light subunit (molecular weight, 27.7 kd) is catalytically inactive, but plays an important regulatory role by lowering the Km of GCS for glutamate and raising the Ki for GSH.5 6
Hereditary GCS deficiency has been reported in 6 patients.7-11 The sixth patient was recently reported and in this patient the first molecular diagnosis was made.11We found 2 additional patients with the same disorder in a large Dutch family with hereditary GSH deficiency in the erythrocytes, originally described by Prins et al.12 We identified the molecular mutation on the heavy subunit causing hereditary GCS deficiency in this family.
Materials and methods
This study was approved by the Ethics Committee of Karolinska Institute.
Patient history
Patient 1:2.
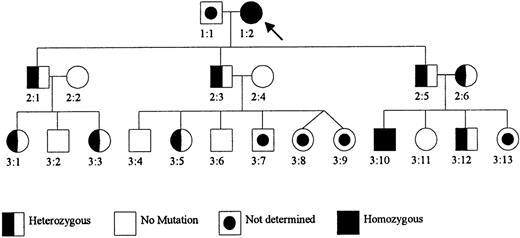
The first patient was originally reported more than 30 years ago with a GSH deficiency in her erythrocytes (Figure1).12 This proband is a Dutch woman born in 1931. Between the ages of 20 and 30, she had had at least 4 episodes of jaundice, and when investigated in her thirties she had hemolytic anemia and low erythrocyte GSH levels (2% of the normal mean). Four other members of her family had transient jaundice and marked erythrocyte GSH deficiency. One of them also had mild hepatosplenomegaly.12 Since the age of 30, the proband has had well-compensated hemolytic anemia and episodes of jaundice when eating fat food. The jaundice has no known relation to medication or infections. This patient and her family refrain from eating fava beans. She has also complained of a vestibular organ defect with equilibrium dysfunction, which was also present in some of her GSH-deficient siblings. When investigated at 68 years of age, she had the following laboratory findings: mild anemia with hemoglobin 6.5 mmol/L (reference values, 7.4-9.6 mmol/L), erythrocytes 3.4 × 109/L (reference values, 3.7-5.0 × 109/L); mean corpuscular volume 94 fL (reference values, 83-105 fL). According to the family doctor, the patient is mentally normal and has no neurologic complaints.
Pedigree of the Dutch GCS-deficient family.
Patient 1:2 was previously reported as Mrs. M.-K. (patient V:19) with GSH deficiency in the erythrocytes (Prins et al12). Her parents were consanguineous (second cousins). Subjects 2:2 and 2:6 are sisters, also related to patient 1:2 (their father is a second cousin of patient 1:2).
Pedigree of the Dutch GCS-deficient family.
Patient 1:2 was previously reported as Mrs. M.-K. (patient V:19) with GSH deficiency in the erythrocytes (Prins et al12). Her parents were consanguineous (second cousins). Subjects 2:2 and 2:6 are sisters, also related to patient 1:2 (their father is a second cousin of patient 1:2).
Patient 3:10.
This grandson of patient 1:2 is the first child of consanguineous parents (Figure 1). He had a history of neonatal jaundice requiring an exchange transfusion. Because the jaundice was more severe than normal, he was subjected to a liver biopsy, which had normal histology. During childhood, he had a well-compensated hemolytic anemia, but no other symptoms.
Analyses of γ-glutamyl cycle enzymes
Preparation of hemolysates.
Packed erythrocytes were obtained from EDTA blood samples by centrifugation at 900g for 3 minutes after washing 3 times with 5 volumes of cold isotonic NaCl solution. The packed erythrocytes were lysed by the addition of 1 volume of 50 mmol Tris-HCl buffer (pH 7.4)/L, containing 1 mmol EDTA/L, and by sonication for 2 × 20 seconds. The erythrocyte membranes were removed by centrifugation at 18,000g for 40 minutes. Hemoglobin was determined using Vanzetti's method.13
Preparation of leukocyte extracts.
Leukocyte extracts were obtained from EDTA blood samples by lysis of erythrocytes in a lysis buffer containing 155 mmol ammonium chloride/L, 10 mmol sodium bicarbonate/L, and 0.1 mmol EDTA/L. The EDTA blood sample was mixed with 40 mL cold lysis buffer and put on ice for 15 minutes. The erythrocytes were removed by centrifugation at 800g for 5 minutes. The leukocyte pellet was resuspended in 5 mL cold lysis buffer and put on ice for 10 minutes. The cell suspension was diluted to 50 mL with cold isotonic saline solution and centrifuged at 800g for 5 minutes. The pellet was again washed with isotonic saline and centrifuged at 1600g for 10 minutes. The pellet with leukocytes was analyzed.
Assay of enzyme activities and thiol-bimane adducts.
Activity of γ-GCS and levels of glutathione and γ-glutamylcysteine were assayed by the method described by Luo et al14 with the following modifications. The incubation mixture (final volume 300 μL) contained 100 mmol Tris-HCl (pH 8.2)/L, 6 mmol adenosine triphosphate (ATP)/L, 50 mmol KCl/L, 6 mmol dithiothreitol (DTT)/L, 20 mmol MgCl2/L, 3 mmol cysteine/L, and 15 mmol glutamic acid/L. The mixture was preincubated at 37°C for 15 minutes to ensure the complete reduction of disulfides to thiols. The reaction was initiated by the addition of various amounts of cell-free extracts from erythrocytes or leukocytes. After 20 minutes of incubation at 37°C, the reaction was stopped by adding 10 μL of 80% 5-sulfosalicylic acid (SSA). After removal of denaturated protein by centrifugation at 12,000g for 15 minutes, samples of the supernatant solution (100 μL) were derivatized to detect the reaction product, γ-glutamylcysteine. For the blank sample, 10 μL SSA was added to the incubation buffer before adding hemolysate or leukocyte lysate, respectively. The samples to be derivatized were added to 100 μL of 20 mmol monobromobimane/L (in 50 mmol N-ethylmorpholine/L). The mixture was placed in the dark at room temperature for 10 minutes. The reaction was stopped by adding 10 μL of 80% SSA. The high-performance liquid chromatography separation of the thiol-bimane adducts on a reversed-phase Supercosil LC-18 octadecylsilyl silica column was followed by fluorimetric detection, as reported elsewhere.15
Glutathione synthetase was determined by the method described by Wellner et al16 with the following modifications. The incubation volume was 100 μL and the mixture contained 12 mmol L-γ-glutamyl-l-α-aminobutyrate/L, 16 mmol 1-14C-glycine/L (specific activity, 9.25 MBq/mmol) including 0.4 μCy/L, 4 mmol phosphoenolpyruvate/L, 4 mmol sodium ATP/L, 8000 U pyruvate kinase/L, 100 mmol Tris HCl-buffer (pH 8.6)/L, 25 mmol KCl/L, 6 mmol MgCl2/L, and 1 g bovine serum albumin/L. The reaction was started by the addition of different amounts of cell extracts. After incubation at 37°C for 120 minutes, the reaction was stopped by the addition of 10 μL of 20% perchloric acid. Denatured protein was removed by centrifugation at 18,000g for 2 minutes, and the supernatant solution was transferred to a 0.5 (diameter) × 4 (height) cm Dowex 1 × 8 acetate ion exchange column. The remaining 1-14C-glycine was eluted with 6 mL of 20 mmol HAc/L and14C-ophthalmic acid (the product) was eluted with 6 mL of 1.5 mol ammonium acetate/L. The radioactivity was analyzed in a liquid scintillation counter.
Mutation analysis.
RNA was isolated from leukocytes by the method of Chirgwin et al.17 cDNA was synthesized, as described previously.18 The coding region of the GCShgene (GLCLC) was amplified by polymerase chain reaction (PCR) in 7 overlapping fragments with a set of oligonucleotide primers.19 Each sense-forward primer contained a “flag,” that is, a 20-nucleotide −21M13 phage sequence (5′-TGTAAAACGACGGCCAGT-3′) for BIGDYE primer cycle sequencing (ABI Prism, Applied Biosystems, Perkin-Elmer Biosystems, Foster City, CT). The positions of the primers complementary to the GLCLC cDNA sequence were:
F1 sense (4-23) 5′-flag-ACGAGGCTGAGTGTCCGTC-3′
F1 antisense (369-388) 5′-CTCCCATACTCTGGTCTCCA-3′
F2 sense (293-312) 5′-flag-GGTCCTGTCTGGGGAGAAAG-3′
F2 antisense (638-657) 5′-AGGTACTGAAGCGAGGGTGC-3′
F3 sense (581-600) 5′-flag-CCCAGTGGAAGGAGGAGCT-3′
F3 antisense (950-969) 5′-TGTCTGACACATAGCCTCGG-3′
F4 sense (923-942) 5′-flag-GGCTTTGAGTGCTGCATCTC-3′
F4 antisense (1321-1340) 5′-TGGAGGAGGGGGCTTAAATC-3′
F5 sense (1191-1210) 5′-flag-CTGGCCCAGCATGTTGCTCA-3′
F5 antisense (1572-1591) 5′-CCATCCACCACTGCATTGCC-3′
F6 sense (1519-1538) 5′-flag-GAGATGCTGTCTTGCAGGGA-3′
F6 antisense (1931-1950) 5′-CAAGTAACTCTGGGCATTCA-3′
F7 sense (1680-1699) 5′-flag-GTGTTTCCTGGACTGATCCC-3′
F7 antisense (2114-2133) 5′-ATTTCTGGCTCACTGGCCCA-3′
The PCR was performed in an Air Thermo Cycler (type 1605, Idaho Technology, Idaho Falls, ID) with cDNA as a template, 50 nmol/L (for fragment 1, 60 nmol/L) of each primer, 0.8 mmol/L of each dNTP, 1 U of Gold Taq DNA polymerase (HT Technologies, Amsterdam, The Netherlands), and 90 nmol/L antiTaq (Clontech Laboratories, Palo Alto, CA) in a buffer recommended by the manufacturer (HT Technologies), in a total volume of 15 μL in glass capillaries. Each PCR cycle consisted of a 10-second denaturation at 95°C, 45-second annealing at 55°C (fragments 2 and 3 at 60°C), and 30-second elongation at 72°C. In total, 50 cycles were run. Nucleotide sequences of the PCR products were determined directly by BigDye Primer Cycle Sequencing (Applied Biosystems) in an ABI Prism 377 sequencer (Perkin Elmer Biosystems).
Polymorphisms in codon 158 were excluded by fragment analysis after digestion of PCR product 2 with the restriction enzyme Bsa JI (New England Biolabs, Beverly, MA). The wild-type PCR product with the CCC codon 158 was digested into 3 fragments of 274, 92, and 16 nucleotides; the PCR product with the CTC codon 158 was digested into 2 fragments of 274 and 108 nucleotides. The fragments were separated by agarose-gel electrophoresis and detected under UV light.
Results
Patient 1:2 had less than 2% of the normal GCS activity in her erythrocytes (Figure 1 and Table 1). The GS activity was normal. The erythrocyte GS also had normal affinity for its substrates γ-glutamylcysteine and glycine (not shown). The erythrocyte GSH and γ-glutamylcysteine level was less than 1% of normal (Table 1). In the leukocytes of patient 1:2, the GCS activity was less than 1% of normal and the GSH level was 7% of normal.
Content of γ-glutamyl cycle enzymes and products in erythrocytes and leukocytes of 2 GCS-deficient patients
| . | Erythrocytes . | |||
|---|---|---|---|---|
| GCS Act.* . | γ-GC† . | GS Act.* . | GSH† . | |
| 1:2 | 0.412 | < 0.01 | 6.9 | 0.079 |
| 3:10 | < 0.1 | < 0.01 | 4.3 | 0.132 |
| Control range | 22.7-27.5 | 0.54-1.49 | 3.6-8.1 | 7.5-10.7 |
| (n = 6) | (n = 2) | (n = 13) | (n = 9) | |
| . | Erythrocytes . | |||
|---|---|---|---|---|
| GCS Act.* . | γ-GC† . | GS Act.* . | GSH† . | |
| 1:2 | 0.412 | < 0.01 | 6.9 | 0.079 |
| 3:10 | < 0.1 | < 0.01 | 4.3 | 0.132 |
| Control range | 22.7-27.5 | 0.54-1.49 | 3.6-8.1 | 7.5-10.7 |
| (n = 6) | (n = 2) | (n = 13) | (n = 9) | |
pkatal/mg Hb.
μmol/g Hb.
nkatal/mg protein.
μmol/mg protein.
GCS indicates γ-glutamylcysteine synthetase; γ-GC, γ-glutamylcysteine; GS, glutathione synthetase; GSH, glutathione.
The GCS activity in hemolysates from patient 1:2 did not increase markedly with an increase in glutamate concentration (data not shown). Patient 1:2 had normal levels of amino acids in her blood and urine and did not excrete excessive amounts of 5-oxoproline in her urine.
Patient 3:10 was found to have a GCS activity of less than 1% of normal in his erythrocytes (Figure 1 and Table 1). The GS activity was normal. The erythrocyte glutathione level was 1.5% of normal, and the erythrocyte γ-glutamylcysteine was less than 1% of normal.
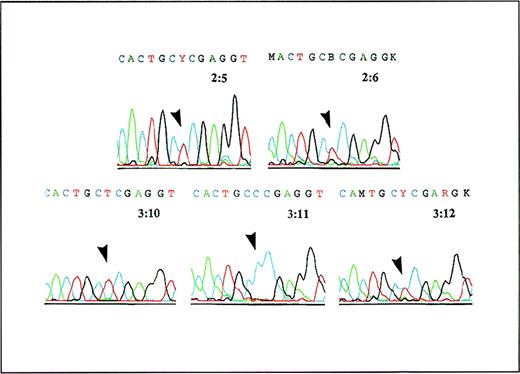
Both patients were found to be homozygous for a 473C→T mutation in GLCLC, predicting a Pro158→Leu substitution in this protein (Figure 2). This mutation was not present in 70 GCSh alleles from control subjects. The GCS activity in erythrocytes was distinctly different in family members with no mutation (23.9,22.7-27.5 pkatal/mg Hb [median, range]; n = 6) and family members who were heterozygous carriers (13.1,12.4-14.9; n = 8) or homozygous patients (< 0.1 and 0.412; n = 2) (Figure 1). The GSH content of erythrocytes in family members who were heterozygous carriers and members with no mutation was normal, as was the GS activity.
Analysis of cDNA from patient 3:10, his parents, and his siblings.
A PCR product containing the region around 473C of the GLCLC gene was generated from cDNA and was analyzed by Dye Primer Cycle sequencing. The results show (arrows) that the parents 2:5 and 2:6 are both heterozygous for the 473C→T substitution in the GLCLC gene, patient 3:10 is homozygous for this mutation, his sister 3:11 is normal, and his brother 3:12 is heterozygous.
Analysis of cDNA from patient 3:10, his parents, and his siblings.
A PCR product containing the region around 473C of the GLCLC gene was generated from cDNA and was analyzed by Dye Primer Cycle sequencing. The results show (arrows) that the parents 2:5 and 2:6 are both heterozygous for the 473C→T substitution in the GLCLC gene, patient 3:10 is homozygous for this mutation, his sister 3:11 is normal, and his brother 3:12 is heterozygous.
Discussion
We studied a large inbred family and found 2 people with GCS deficiency and hemolytic anemia. Other symptoms may have been coincidental.
We obtained indirect evidence that the hereditary defect was located in the heavy, catalytic subunit of GCS and not in the light, regulatory subunit, because the activity of the mutant GCS could not be normalized by raising the glutamate concentrations markedly6 (data not shown). Therefore, we started our molecular analysis by sequencing the cDNA of the heavy subunit. In both patients we found a homozygous 473C→T mutation in the GLCLC gene, predicting a Pro158→Leu substitution in the GCSh subunit. Genetic analysis also revealed that several other family members were heterozygous for this mutation. All of these heterozygotes had erythrocyte GCS activity reduced to about half-normal. Therefore, it is very likely that the Pro158→Leu substitution in the GCSh subunit is the cause of the decreased GCS activity. The heterozygous family members had neither decreased levels of GSH nor any clinical symptoms. For this reason we conclude that a mutation of the GLCLC gene in a heterozygous state does not cause hemolytic anemia or other symptoms.
Six patients have hitherto been reported with hereditary GCS deficiency and all show similar symptoms (Table 2). The first 2 were siblings of German descent, a brother and a sister, who in their late twenties developed hemolytic anemia, markedly decreased survival time of erythrocytes, erythrocyte GSH deficiency, and generalized aminoaciduria (patients 1 and 2 in Table 2).7 8 The sister became psychotic after treatment with sulfonamide for a urinary tract infection. In their thirties, both developed spinocerebellar degeneration and a neuromuscular disorder with peripheral neuropathy and myopathy. The sister required blood transfusions during pregnancy, but otherwise the increased rate of hemolysis was well compensated in both patients. Both showed normal activity of GS.
Summary of patients reported with GCS deficiency
| Patient no. . | Sex/age when investigated . | B-Hb g/L . | Retic % . | Ery-GSH % of normal mean . | Ery-GCS activity % of normal mean . | Reference . |
|---|---|---|---|---|---|---|
| 1 | Male 35-40 y | 132 | 8.6 | 3.1 | 13 | 7, 8 |
| 2 | Female 35-40 y | 118 | 10.3 | 2 | 9 | 7, 8 |
| 3 | Female 22 y | hematocrit 28-38% | 11.5 | 11 | 6 | 9 |
| 4 | Male 15 y | 120 | 6.8 | 4.4 | 6 | 10 |
| 5 | Female 17 y | 102 | 6.6 | 13 | 23 | 10 |
| 6 | Female 14 y | 119 | 4.9 | 3 | 10 | 11 |
| 7 | Female 68 y | 70 | 6 | < 1 | < 2 | 12; patient 1:2 in this report |
| 8 | Male 11 y | ND | ND | 1.5 | < 1 | patient 3:10 in this report |
| Patient no. . | Sex/age when investigated . | B-Hb g/L . | Retic % . | Ery-GSH % of normal mean . | Ery-GCS activity % of normal mean . | Reference . |
|---|---|---|---|---|---|---|
| 1 | Male 35-40 y | 132 | 8.6 | 3.1 | 13 | 7, 8 |
| 2 | Female 35-40 y | 118 | 10.3 | 2 | 9 | 7, 8 |
| 3 | Female 22 y | hematocrit 28-38% | 11.5 | 11 | 6 | 9 |
| 4 | Male 15 y | 120 | 6.8 | 4.4 | 6 | 10 |
| 5 | Female 17 y | 102 | 6.6 | 13 | 23 | 10 |
| 6 | Female 14 y | 119 | 4.9 | 3 | 10 | 11 |
| 7 | Female 68 y | 70 | 6 | < 1 | < 2 | 12; patient 1:2 in this report |
| 8 | Male 11 y | ND | ND | 1.5 | < 1 | patient 3:10 in this report |
B-Hb indicates blood-hemoglobin; Ery-GSH, erythrocyte glutathione; Ery-GCS, erythrocyte γ-glutamylcysteine synthetase; ND, not determined; Retic, reticulocyte.
The third patient having GCS deficiency was a woman of Polish extraction with consanguineous parents (patient 3 in Table2).9 She had a history of transient jaundice complicating an unspecified viral infection at 10 years of age. At 21, she developed anemia and reticulocytosis while pregnant and required blood transfusions. After the delivery, she was still anemic, with high reticulocyte counts. She was found to have an erythrocyte GSH deficiency and markedly diminished GCS and GSH-S-transferase activity. She had normal activity of GS. No neurologic abnormality was detected. The GSH-S-transferase deficiency has been postulated to be secondary to the lack of GSH.20
Two unrelated Japanese patients with GCS deficiency have been described (patients 4 and 5 in Table 2).10 A 15-year-old boy had a history of anemia at birth and had received a blood transfusion at 1 month of age. Thereafter he had been asymptomatic until 10 years of age, when he had an episode of anemia, reticulocytosis, jaundice, and hepatosplenomegaly. At 15 years of age, he still had mild hemolytic anemia. His growth and development were normal. He showed no signs of central nervous system involvement. He had markedly diminished activity of GCS and GSH-S-transferase, but normal activity of GS. His mother also had mild deficiencies of GCS and GSH-S-transferase. The fifth patient was a 17-year-old girl with consanguineous parents. The girl had had anemia and reticulocytosis since childhood. She had no aminoaciduria (J. Ueyama and A. Hirono, personal communication, 1998). She had a markedly decreased erythrocyte survival time. Her erythrocyte GCS activity was decreased, as also was her GSH-S-transferase activity. The activity of GS was normal. Her mother also had mild deficiencies of GCS and GSH-S-transferase.
Recently (published after submission of our manuscript) a sixth patient with GCS deficiency was described by Beutler et al (patient 6 in Table2).11 This patient was a 14-year-old white girl of Pennsylvania-Dutch/German/Swedish/Native American descent. Her parents were related to each other. The girl was examined because of menorrhagia, anemia, and reticulocytosis. She also became jaundiced 2 or 3 times per month. She had a history of neonatal hyperbilirubinemia requiring exchange transfusion. During childhood she had had 2 episodes of head trauma, 1 of which had been followed by a seizure. She was considered to have a learning disability with dyslexia and was also thought to be mentally retarded. She had, however, no abnormalities by physical examination, including neurologic evaluation. She was found to have an erythrocyte GSH deficiency and markedly diminished GCS and GSH-S-transferase activity, but normal activity of GS. Her mother and brother had intermediate activity of GCS but normal GSH levels. The patient was homozygous for an 1109A→T mutation, in the GLCLC gene, predicting a His370→Leu substitution in GCSh. The patient's mother and brother were heterozygous for the same mutation. The level of mutant messenger RNA (mRNA) in the patient's reticulocytes were normal, whereas the amount of mutant protein in her erythrocytes was decreased, indicating that the mutant protein was unstable.
Orlowski and Meister21 have suggested that the γ-glutamyl cycle is involved in the active transport of amino acids. γ-Glutamyl transpeptidase was postulated to play a key role in this process, because it is located on the outer surface of the cell membrane, especially in the epithelia of tissues extensively involved in transport, for example, the kidney. Four of the 8 patients with GCS deficiency hitherto described have been investigated with respect to urinary amino acids. Two had generalized aminoaciduria, but 2 did not. These findings may have several explanations. Assuming that the γ-glutamyl cycle is involved in amino acid transport,21the absence of aminoaciduria could be due to residual low levels of GCS being sufficient to permit function of the γ-glutamyl cycle. As with deficiencies of other erythrocyte enzymes, such as glucose-6-phosphate dehydrogenase22 and cytochrome b5 reductase,23mutations that cause instability of the enzyme may become manifest only in the red blood cells, because these cells have a rather long half-life and lack the capacity to synthesize new protein. In contrast, mutations that inactivate the catalytic capacity of GCS may lead to GSH deficiency in all tissues, with severe clinical consequences. Unfortunately, the 3-dimensional structure of GCSh is not yet known. It is, therefore, not possible to speculate about the effect of the proline-158 substitution for leucine in this protein.
Another explanation for the lack of aminoaciduria in patients with GCS deficiency may be the existence of alternative pathways for amino acid transport, not involving the γ-glutamyl cycle. The aminoaciduria reported in 2 of 8 patients may then be completely unrelated to the enzyme defect in the γ-glutamyl cycle. The present identification of the gene mutation responsible for GCS deficiency makes it possible to gain further knowledge about the functions of residual GCS activity in various tissues. The aminoaciduria may also be secondary to the low levels of GSH in the cells, similar to the decreased activity of GSH-S-transferase detected in patients with GS deficiency.20
From the findings in 8 patients with GCS deficiency, it may be concluded that all patients have hemolytic anemia and low erythrocyte levels of both GSH and γ-glutamylcysteine. Our 2 patients with GCS deficiency were homozygous for a missense mutation in the GCSh subunit. It seems important to determine the underlying mutations in the other GCS-deficient patients to learn more about the physiologic properties of GCS. The relationship between aminoaciduria, neurologic and psychiatric disorders, and the primary enzyme deficiency remains to be established.
Acknowledgments
We thank Dr H. A. Wassink for providing us with clinical data, Associate Professor Jan Wernerman for placing his laboratory facilities at our disposal, and Mrs Christina Hebert for skillful technical assistance.
Supported by the Swedish Medical Research Council (project nos. 4792 and 4210), Frimurarebarnhuset Foundation, Wera Ekström Foundation, Samariten Foundation, and Sven Jerring Foundation.
Reprints:Ellinor Ristoff, Department of Pediatrics, Karolinska Institute, Huddinge University Hospital, S-141 86 Huddinge, Sweden; e-mail: ellinor.ristoff@klinvet.ki.se.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal