Abstract
DIPSS-plus (the Dynamic International Prognostic Scoring System-plus) includes 8 risk factors for survival in primary myelofibrosis. In the present study of 884 karyotypically annotated patients with primary myelofibrosis, we sought to identify 1 or 2 parameters that can reliably predict death in the first 2 years of disease. After a median of 8.2 years from time of referral to the Mayo Clinic, 564 deaths (64% of patients in the study) had been recorded. Risk factors associated with > 80% 2-year mortality included monosomal karyotype, inv(3)/i(17q) abnormalities, or any 2 of the following: circulating blasts > 9%, leukocytes ≥ 40 × 109/L, or other unfavorable karyotype. Patients with any 1 of these risk profiles (n = 52) displayed significantly shorter overall survival than those otherwise belonging to a high-risk category per DIPSS-plus (n = 298); respective median survivals were 9 and 23 months (hazard ratio 2.2, 95% confidence interval 1.6-3.1; P < .01). The present information complements DIPSS-plus in the selection of primary myelofibrosis patients for high-risk treatment approaches.
Introduction
A high degree of prognostic certainty is needed to recommend high-risk treatment procedures in primary myelofibrosis (PMF). DIPSS-plus (the Dynamic International Prognostic Scoring System-plus) uses 8 risk factors to predict overall survival (OS) in PMF; these include unfavorable karyotype [ie, complex or any sole abnormality or 2 abnormalities, including +8, −7/7q−, −5/5q−, inv(3), i(17q), 12p−, or 11q23 rearrangement], circulating blast percentage ≥ 1%, platelet count < 100 × 109/L, leukocyte count > 25 × 109/L, hemoglobin level < 10 g/dL, need for red blood cell transfusion, constitutional symptoms, and age > 65 years.1 The presence of 4 or more of these risk factors defines high-risk disease with a median survival of approximately 16 months and a 2-year mortality rate of 50% to 60%. The present study was undertaken to exploit recent information in PMF that suggested a more pronounced prognostic effect for monosomal karyotype (MK),2 inv(3)/i(17q) abnormalities,3,4 and additional discriminant levels for circulating blast percentage, leukocyte count, and platelet count.3,5
Methods
The present study was approved by the Mayo Clinic institutional review board. Study eligibility criteria included availability of bone marrow histology and cytogenetic information at time of referral to the Mayo Clinic. The diagnoses of PMF and leukemic transformation were according to World Health Organization criteria.6 Unfavorable karyotype designation and DIPSS-plus risk categorization were as described previously.1,7 Unfavorable karyotype included complex or any sole or 2 abnormalities that included +8, −7/7q−, −5/5q−, inv(3), i(17q), 12p−, or 11q23 rearrangement. Follow-up information was updated through the first half of 2011 by review of patient histories and correspondence or the Social Security Death Index or by making a telephone call to the patient; “date of last follow-up” reflected this time point and not the last time a patient was seen at the Mayo Clinic.
Receiver operating characteristic plots were prepared to determine the best discriminant levels for circulating blast percentage, leukocyte count, and platelet count. OS and 2-year mortality rates were calculated from time of referral to the Mayo Clinic. Variables considered for their prognostic value were those obtained at the time of referral. Differences in the distribution of continuous variables between categories were analyzed by either Mann-Whitney test (for comparison of 2 groups) or Kruskal-Wallis test (comparison of 3 or more groups). Patient groups with nominal variables were compared by χ2 test. Survival curves were prepared by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard regression model was used for multivariable analysis. P < .05 was considered significant. StatView and JMP statistical packages (both from SAS Institute) were used for all computations.
Results and discussion
The study population consisted of 884 patients with PMF seen at the Mayo Clinic from 1977 through the first half of 2011 who had bone marrow histologic and cytogenetic information at the time of their referral. Although there was significant overlap, the present study population differed from the one previously reported for the development of DIPSS-plus1 in several aspects: (1) patients first seen before 1977 were excluded, and those seen in 2010 and the first half of 2011 were included in the present study; and (2) a higher proportion of DIPSS-plus high-risk patients were included in the present study because of increased recent activity in clinical trials that target this particular patient population. A total of 517 patients were seen within the first year of their diagnosis. Table 1 depicts clinical and laboratory characteristics of the patients at time of referral and incidences of events afterward. DIPSS-plus1 risk distributions were ∼ 9% (84 patients) low, 15% (128 patients) intermediate-1, 38% (332 patients) intermediate-2, and 38% (340 patients) high.
Clinical and laboratory features of 884 karyotypically annotated patients with primary myelofibrosis at the time of referral to the Mayo Clinic, stratified according to presence or absence of predictors for early death
| Variables . | All patients (n = 884) . | Very high-risk group* (n = 52) . | Patients with MK (n = 19) . | Patients with inv(3)/i(17q) abnormalities (n = 8) . | Patients with PB blasts > 9% and without MK or inv(3)/i(17q) (n = 30) . | Patients with WBC ≥ 40 × 109/L and without PB blasts > 9%, MK, or inv(3)/i(17q) (n = 68) . | Patients with none of the above† (n = 759) . | P . |
|---|---|---|---|---|---|---|---|---|
| Median age, y (range) | 65 (14-92) | 65 (30-83) | 63 (30-83) | 72 (59-80) | 63 (44-76) | 69 (27-85) | 65 (14-92) | .06 |
| Age > 65 y, n (%) | 429 (49) | 25 (48) | 8 (42) | 5 (63) | 12 (40) | 40 (59) | 364 (48) | .31 |
| Males, n (%) | 562 (63) | 33 (64) | 9 (47) | 7 (88) | 21 (70) | 39 (57) | 486 (64) | .21 |
| Hemoglobin, g/dL, median (range) | 10.0 (5-16) | 9.3 (6-16) | 9.0 (6-11) | 9.7 (7-11.9) | 9.7 (6-12) | 9.7 (6-16) | 10 (5-16) | .03 |
| WBC, × 109/L, median (range) | 9.0 (1.0-236) | 17.9 (1.1-179) | 6.0 (2-46) | 5.0 (1.1-31) | 12 (3-179) | 56.5 (40-236) | 8 (1-39) | < .01‡ |
| Platelets, × 109/L, median (range) | 206 (6-1765) | 93 (6-885) | 52 (6-522) | 90 (33-409) | 157 (17-885) | 155 (12-1331) | 214 (7-1765) | < .01 |
| PB blast, %, median (range) | 1.0 (0-18) | 4 (0-18) | 2.0 (0-12) | 5 (0-8) | 13 (10-18) | 2 (0-9) | 1 (0-9) | < .01‡ |
| DIPSS-plus risk group, n (%) | < .01 | |||||||
| Low | 84 (9) | 0 | 0 | 0 | 0 | 0 | 84 (11) | |
| Intermediate- 1 | 128 (15) | 0 | 0 | 0 | 0 | 0 | 128 (17) | |
| Intermediate- 2 | 332 (38) | 10 (19) | 4 (21) | 1 (13) | 12 (40) | 20 (29) | 295 (39) | |
| High | 340 (38) | 42 (81) | 15 (79) | 7 (88) | 18 (60) | 48 (71) | 252 (33) | |
| Constitutional symptoms, n (%) | 300 (34) | 21 (40) | 7 (37) | 2 (25) | 16 (53) | 29 (43) | 246 (32) | .08 |
| PB blasts ≥ 1%, n (%) | 513 (58) | 45 (87) | 15 (79) | 7 (88) | 32 (100) | 59 (87) | 402 (53) | < .01‡ |
| Hemoglobin < 10 g/dL, n (%) | 491 (56) | 34 (65) | 13 (68) | 5 (63) | 22 (73) | 40 (59) | 411 (54) | .19 |
| Transfusion required, n (%) | 347 (39) | 29 (56) | 8 (42) | 7 (88) | 16 (53) | 26 (38) | 289 (38) | .29 |
| WBC > 25 × 109/L, n (%) | 145 (16) | 24 (46) | 3 (16) | 1 (13) | 8 (27) | 68 (100) | 65 (9) | < .01‡ |
| Platelets < 100 × 109/L, n (%) | 231 (26) | 29 (56) | 12 (63) | 5 (63) | 11 (37) | 29 (43) | 177 (23) | < .01 |
| WBC < 4 × 109/L, n (%) | 144 (16) | 10 (19) | 6 (32) | 3 (38) | 3 (10) | 0 (0) | 132 (17) | < .01‡ |
| JAK2V617F, n (%) (n evaluable = 514) | 308 (60) | 20/25 (80) | 8 (100) | 4 (80) | 5 (33) | 23 (70) | 268 (59) | .02 |
| MPL mutation, n (%) (n evaluable = 338) | 28 (8) | 0/14 (0) | 0 | 0 | 0 | 3 (14) | 25 (8) | .59 |
| IDH mutation, n (%) (n evaluable = 305) | 12 (4) | 0/14 (0) | 0 | 0 | 2 (14) | 1 (5) | 9 (3) | .35 |
| Splenectomy, n (%) | 164 (19) | 12 (23) | 3 (16) | 2 (25) | 10 (33) | 22 (32) | 127 (17) | < .01 |
| Cytogenetic categories, n (%) | < .01‡ | |||||||
| Normal | 516 (58) | 0 | 0 | 0 | 9 (30) | 32 (47) | 475 (63) | |
| Favorable | 239 (27) | 1 (2) | 0 | 0 | 11 (37) | 22 (32) | 206 (27) | |
| Unfavorable | 129 (15) | 51 (98) | 19 (100) | 8 (100) | 10 (33) | 14 (21) | 78 (10) | |
| Transplanted, n (%) | 33 (4) | 2 (4) | 1 (5) | 0 | 2 (7) | 2 (3) | 28 (4) | .87 |
| Deaths, n (%) | 564 (64) | 46 (89) | 16 (84) | 6 (75) | 29 (97) | 61 (90) | 452 (60) | < .01 |
| Leukemic transformations, n (%) | 60 (7) | 7 (14) | 5 (26) | 2 (25) | 7 (23) | 6 (9) | 40 (5) | < .01 |
| Variables . | All patients (n = 884) . | Very high-risk group* (n = 52) . | Patients with MK (n = 19) . | Patients with inv(3)/i(17q) abnormalities (n = 8) . | Patients with PB blasts > 9% and without MK or inv(3)/i(17q) (n = 30) . | Patients with WBC ≥ 40 × 109/L and without PB blasts > 9%, MK, or inv(3)/i(17q) (n = 68) . | Patients with none of the above† (n = 759) . | P . |
|---|---|---|---|---|---|---|---|---|
| Median age, y (range) | 65 (14-92) | 65 (30-83) | 63 (30-83) | 72 (59-80) | 63 (44-76) | 69 (27-85) | 65 (14-92) | .06 |
| Age > 65 y, n (%) | 429 (49) | 25 (48) | 8 (42) | 5 (63) | 12 (40) | 40 (59) | 364 (48) | .31 |
| Males, n (%) | 562 (63) | 33 (64) | 9 (47) | 7 (88) | 21 (70) | 39 (57) | 486 (64) | .21 |
| Hemoglobin, g/dL, median (range) | 10.0 (5-16) | 9.3 (6-16) | 9.0 (6-11) | 9.7 (7-11.9) | 9.7 (6-12) | 9.7 (6-16) | 10 (5-16) | .03 |
| WBC, × 109/L, median (range) | 9.0 (1.0-236) | 17.9 (1.1-179) | 6.0 (2-46) | 5.0 (1.1-31) | 12 (3-179) | 56.5 (40-236) | 8 (1-39) | < .01‡ |
| Platelets, × 109/L, median (range) | 206 (6-1765) | 93 (6-885) | 52 (6-522) | 90 (33-409) | 157 (17-885) | 155 (12-1331) | 214 (7-1765) | < .01 |
| PB blast, %, median (range) | 1.0 (0-18) | 4 (0-18) | 2.0 (0-12) | 5 (0-8) | 13 (10-18) | 2 (0-9) | 1 (0-9) | < .01‡ |
| DIPSS-plus risk group, n (%) | < .01 | |||||||
| Low | 84 (9) | 0 | 0 | 0 | 0 | 0 | 84 (11) | |
| Intermediate- 1 | 128 (15) | 0 | 0 | 0 | 0 | 0 | 128 (17) | |
| Intermediate- 2 | 332 (38) | 10 (19) | 4 (21) | 1 (13) | 12 (40) | 20 (29) | 295 (39) | |
| High | 340 (38) | 42 (81) | 15 (79) | 7 (88) | 18 (60) | 48 (71) | 252 (33) | |
| Constitutional symptoms, n (%) | 300 (34) | 21 (40) | 7 (37) | 2 (25) | 16 (53) | 29 (43) | 246 (32) | .08 |
| PB blasts ≥ 1%, n (%) | 513 (58) | 45 (87) | 15 (79) | 7 (88) | 32 (100) | 59 (87) | 402 (53) | < .01‡ |
| Hemoglobin < 10 g/dL, n (%) | 491 (56) | 34 (65) | 13 (68) | 5 (63) | 22 (73) | 40 (59) | 411 (54) | .19 |
| Transfusion required, n (%) | 347 (39) | 29 (56) | 8 (42) | 7 (88) | 16 (53) | 26 (38) | 289 (38) | .29 |
| WBC > 25 × 109/L, n (%) | 145 (16) | 24 (46) | 3 (16) | 1 (13) | 8 (27) | 68 (100) | 65 (9) | < .01‡ |
| Platelets < 100 × 109/L, n (%) | 231 (26) | 29 (56) | 12 (63) | 5 (63) | 11 (37) | 29 (43) | 177 (23) | < .01 |
| WBC < 4 × 109/L, n (%) | 144 (16) | 10 (19) | 6 (32) | 3 (38) | 3 (10) | 0 (0) | 132 (17) | < .01‡ |
| JAK2V617F, n (%) (n evaluable = 514) | 308 (60) | 20/25 (80) | 8 (100) | 4 (80) | 5 (33) | 23 (70) | 268 (59) | .02 |
| MPL mutation, n (%) (n evaluable = 338) | 28 (8) | 0/14 (0) | 0 | 0 | 0 | 3 (14) | 25 (8) | .59 |
| IDH mutation, n (%) (n evaluable = 305) | 12 (4) | 0/14 (0) | 0 | 0 | 2 (14) | 1 (5) | 9 (3) | .35 |
| Splenectomy, n (%) | 164 (19) | 12 (23) | 3 (16) | 2 (25) | 10 (33) | 22 (32) | 127 (17) | < .01 |
| Cytogenetic categories, n (%) | < .01‡ | |||||||
| Normal | 516 (58) | 0 | 0 | 0 | 9 (30) | 32 (47) | 475 (63) | |
| Favorable | 239 (27) | 1 (2) | 0 | 0 | 11 (37) | 22 (32) | 206 (27) | |
| Unfavorable | 129 (15) | 51 (98) | 19 (100) | 8 (100) | 10 (33) | 14 (21) | 78 (10) | |
| Transplanted, n (%) | 33 (4) | 2 (4) | 1 (5) | 0 | 2 (7) | 2 (3) | 28 (4) | .87 |
| Deaths, n (%) | 564 (64) | 46 (89) | 16 (84) | 6 (75) | 29 (97) | 61 (90) | 452 (60) | < .01 |
| Leukemic transformations, n (%) | 60 (7) | 7 (14) | 5 (26) | 2 (25) | 7 (23) | 6 (9) | 40 (5) | < .01 |
PB indicates peripheral blood; and WBC, white blood cell count.
Very high-risk group constitutes patients with MK, inv(3)/i(17q), or 2 of the following 3 risk factors: circulating blasts > 9%, leukocyte count > 40 × 109/L, or unfavorable karyotype [ie, nonmonosomal complex karyotype or any single or 2 abnormalities including +8, −7/7q−, −5/5q−, inv(3), i(17q), 12p−, or 11q23 rearrangement].
“None of the above” refers to patients who did not display MK, inv(3)/i(17q), circulating blasts > 9%, or leukocyte count > 40 × 109/L.
P value calculations excluded groups that were defined by the parameters that were being compared.
To date, 564 deaths (64% of patients in the study) have been recorded, and median time to death from first referral to the Mayo Clinic was 8.2 years. There were a total of 60 documented cases of leukemic transformations, most of which were fatal. Other known causes of death included infections, bleeding, thromboembolic events, and other malignancies. Each of the 8 DIPSS-plus risk factors was associated with a 2-year mortality rate that ranged from 42% (circulating blast count ≥ 1%) to 60% (unfavorable karyotype). High-risk disease per DIPSS-plus was associated with 57% 2-year mortality. The only single parameters that were associated with > 80% 2-year mortality were MK (n = 19) and inv(3)/i(17q) abnormalities (n = 8). Both of these adverse cytogenetic profiles were associated with significantly worse OS than other unfavorable karyotype (n = 102; hazard ratio [HR] 5.1, 95% confidence interval [CI] 3.1-8.4 and HR 3.9, 95% CI 1.7-8.8, respectively).
In the absence of MK and inv(3)/i(17q), receiver operating characteristic analysis identified circulating blast count of 9% (area under the curve 0.62) and leukocyte count of 43 × 109/L (area under the curve 0.66) as the best discriminant levels for predicting 2-year mortality; OS was significantly worse in the presence of circulating blasts > 9% (HR 4.1, 95% CI 2.8-6.1) versus 2% to 9% (HR 1.8, 95% CI 1.5-2.2) versus < 2%; the corresponding 2-year mortality rates were 73%, 46%, and 25%. OS was also significantly worse in the presence of leukocyte count ≥ 40 × 109/L (HR 2.8, 95% CI 2.2-3.6) versus 26-39 × 109/L (HR 1.6, 95% CI, 1.2-2.1) versus < 26 × 109/L; the corresponding 2-year mortality rates were 63%, 42%, and 28%. Two-year mortality rates exceeded 80% in the presence of any 2 of the following: circulating blast count > 9%, leukocyte count ≥ 40 × 109/L, or other unfavorable karyotype. The presenting clinical and laboratory features for each of the aforementioned predictors of early death are summarized in Table 1.
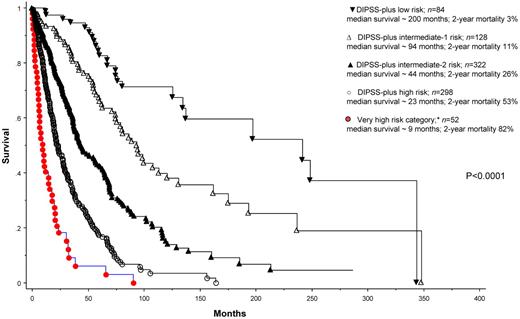
We subsequently grouped patients with MK, inv(3)/i(17q), or any 2 of circulating blast percentage > 9%, leukocyte count ≥ 40 × 109/L, and other unfavorable karyotype into a new “very high-risk” category (n = 52) that is separate from the standard DIPSS-plus high-risk category. Figure 1 illustrates the significantly worse OS (HR 2.2, 95% CI 1.6-3.1) for this subgroup of patients compared with those who otherwise belonged to the high-risk category per DIPSS-plus (n = 298). The corresponding median survival and 2-year mortality rates were 9 and 23 months and 83% and 53%, respectively. The difference in survival was explained in part by a difference in the risk of leukemic transformation: 2-year incidences of leukemic transformation were 31% and 7%, respectively (P = .002). Of note, among the 52 patients belonging to the new very high-risk category, 42 would have been assigned to the high-risk category and 10 to the intermediate-2 risk category per DIPSS-plus.1 Supplemental Figure 1 shows survival data based on DIPSS-plus risk stratification, without extraction of the patients in the very high-risk category (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Supplemental Figure 2 considers only DIPSS-plus high-risk patients and shows their survival data after further stratification into very high-risk versus simply high-risk category.
Risk-stratified survival data for 884 Mayo Clinic patients with primary myelofibrosis. *Very high-risk category includes patients with monosomal karyotype, inv(3)/i(17q) abnormalities, or any 2 of the following: Peripheral blood blasts > 9%, white blood cell ≥ 40 × 109/L, other unfavorable karyotype (ie, nonmonosomal complex karyotype or any single or 2 abnormalities, including +8, −7/7q−, −5/5q−, inv(3), i(17q), 12p−, or 11q23 rearrangement). DIPSS-plus risk categorization is as described previously.1
Risk-stratified survival data for 884 Mayo Clinic patients with primary myelofibrosis. *Very high-risk category includes patients with monosomal karyotype, inv(3)/i(17q) abnormalities, or any 2 of the following: Peripheral blood blasts > 9%, white blood cell ≥ 40 × 109/L, other unfavorable karyotype (ie, nonmonosomal complex karyotype or any single or 2 abnormalities, including +8, −7/7q−, −5/5q−, inv(3), i(17q), 12p−, or 11q23 rearrangement). DIPSS-plus risk categorization is as described previously.1
The present study provides practical information that complements DIPSS-plus in the selection of patients for high-risk treatment approaches, including allogeneic stem cell transplant. In the latter regard, one has to also take age,8,9 comorbidity score,9 HLA-matched sibling donor availability,8,10,11 and other biologic12 and molecular13,14 information into consideration during the treatment decision-making process. Regardless, it is currently not clear whether patients with the very high-risk profile would necessarily fare better with allogeneic stem cell transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T. designed the study, contributed patients, collected data, performed the statistical analysis, and wrote the paper; T.J. and D.C. collected data and participated in data analysis; N.G., R.V., and K.H.B. collected data and contributed patients; C.A.H. reviewed histopathology; D.L.V.D. reviewed cytogenetic information; A.P. contributed patients and participated in data analysis; and all authors approved the final draft of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayalew Tefferi, MD, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.