Abstract
Allogeneic stem cell transplantation (ASCT) after reduced-intensity conditioning has become a reasonable treatment option for patients with advanced myelofibrosis. The role of characteristic molecular genetic abnormalities, such as JAK2V617F on outcome of ASCT, is not yet elucidated. In 139 of 162 myelofibrosis patients with known JAK2V617F mutation status who received ASCT after reduced-intensity conditioning, the impact of JAK2 genotype, JAK2V617F allele burden, and clearance of mutation after ASCT was evaluated. Overall survival was significantly reduced in multivariate analysis in patients harboring JAK2 wild-type (hazard ratio = 2.14, P = .01) compared with JAK2 mutated patients. No significant influence on outcome was noted for the mutated allele burden analyzed either as continuous variable or after dividing into quartiles. Achievement of JAK2V617F negativity after ASCT was significantly associated with a decreased incidence of relapse (hazard ratio = 0.22, P = .04). In a landmark analysis, patients who cleared JAK2 mutation level in peripheral blood 6 months after ASCT had a significant lower risk of relapse (5% vs 35%, P = .03). We conclude that JAK2V617F-mutated status, but not allele frequency, resulted in an improved survival and rapid clearance after allografting reduces the risk of relapse.
Introduction
Primary myelofibrosis (PMF) is one of the Philadelphia chromosome–negative chronic myeloproliferative neoplasms characterized by a clonal chronic myeloproliferation and an atypical megakaryocytic hyperplasia. The clonally expanded megakaryocytes secret growth factors, which in turn induce a nonclonal proliferation of fibroblasts and result in an abnormal fibrosis in the bone marrow.1,2 This disease is clinically associated with variable degrees of cytopenias or cytoses with extramedullary hematopoiesis.3 Myelofibrosis may be also a secondary complication of 2 other myeloproliferative neoplasms: polycythemia vera (PV) and essential thrombocythemia (ET).4 The elderly group is the main affected population, and the median life expectancy ranges from a few months to many years with conservative treatment.5 Once myelofibrosis occurs, the only curative treatment is allogeneic stem cell transplantation (ASCT), which was initially associated with a significant transplantation-related mortality (TRM).6,7 The introduction of reduced-intensity conditioning regimens improved the ASCT outcome and made this treatment modality applicable in older patients.8,9 JAK2V617F mutation is an acquired point mutation in the pseudo-kinase domain of the Janus kinase-2, which confers a constitutive JAK2 pathway activation with resulting growth factor–independent proliferation of myeloid precursors.10-13 This mutation is detected in approximately 50% of PMF patients,14 approximately 100% of post-PV myelofibrosis, and approximately 30% of post-ET myelofibrosis.15 The impact of the presence of JAK2V617F mutation on disease phenotype has been so far clearly demonstrated, whereas its influence on the prognosis is controversial despite the extensive evaluation from different groups.16 JAK2V617F mutation disappears after ASCT and reappears in the case of ensuing relapse, and the value of this mutation as a minimal residual disease marker to guide adoptive immune therapy is well established.17-20 In the setting of ASCT, the prognostic impact of JAK2V617F mutation on outcome remains controversial: in one study, no impact of JAK2V617F was noted,17 whereas a recent study from the European Group for Blood and Marrow Transplantation (EBMT) indicated on inferior outcome of JAK2V617F-negative patients, but in both studies the number of patients with known JAK2 mutation status was limited to draw valid conclusions.21 The current study was conducted to validate the role of the presence of JAK2V617F mutation in a larger group of myelofibrosis patients and investigate the impact of the mutated allele burden as well as the kinetics of JAK2V617F clearance after ASCT.
Methods
A total of 162 patients with histologically proven primary or post-ET/PV myelofibrosis underwent reduced conditioning ASCT between December 1999 and May 2009. In 139 patients, the JAK2V617F mutation status was known before ASCT. Nineteen of them were treated within the pilot study of reduced-intensity conditioning and ASCT reported elsewhere,22 and further 82 within the prospective multicenter study of the Chronic Leukemia Working Party of the EBMT reported recently.21 The remaining 38 patients were treated in one center (Hamburg, Germany) according to the same treatment protocol but after completion recruiting in the previous study. Approval was obtained from the Ethics Committee Hamburg for these studies, and informed consent was provided in accordance with the Declaration of Helsinki. The characteristics of the study group are summarized in Table 1. Risk stratification for the patients before ASCT was performed using 3 different widely used scoring systems: Lille scoring system,23 Cervantes scoring system,24 and International Prognostic Scoring system (IPSS).25 The patients were as well stratified according to their cytogenetics before ASCT using data from the recent literature into 4 different groups: favorable (sole +9, 20q−, or 13q−), normal, unfavorable (complex karyotype or sole +8), and other abnormalities.26
Patient characteristics and results
| Characteristic . | Value . |
|---|---|
| No. of patients | 162 |
| Median age, y (range) | 56 (32-73) |
| Sex of patient, n | |
| Male | 96 |
| Female | 66 |
| Sex of donor, n | |
| Male | 102 |
| Female | 60 |
| Myelofibrosis, n | |
| Primary | 105 |
| Post-PV/ET | 57 |
| Median time, mo (range), for diagnosis to SCT | 50 (3-360) |
| Risk profile according to Lille score, n | |
| Low | 35 |
| Intermediate | 91 |
| High | 32 |
| Unknown | 4 |
| Risk profile according to Cervantes score, n | |
| Low | 46 |
| High | 110 |
| Unknown | 6 |
| Risk profile according to IPSS, n | |
| Low | 4 |
| Intermediate-1 | 26 |
| Intermediate-2 | 52 |
| High | 70 |
| Unknown | 10 |
| JAK2 V617F mutation status, n | |
| Positive | 95 |
| Negative | 44 |
| Unknown | 23 |
| Cytogenetics, n | |
| Favorable | 6 |
| Normal | 53 |
| Other | 23 |
| Unfavorable | 17 |
| Unknown | 63 |
| Transfusion dependency, n | |
| Not transfusion-dependent | 65 |
| Transfusion-dependent | 86 |
| Unknown | 11 |
| Splenectomy, n | 20 |
| HLA matching (10/10 alleles), n | |
| Matched | 129 |
| Mismatched | 33 |
| HLA identical sibling, n | 46 |
| Unrelated donor, n | 116 |
| Median no. (range) of CD34+ infused stem cells per kg BW | 6.9 × 106 (0.7-21.7 × 106) |
| CMV serostatus of recipients, n | |
| Positive | 83 |
| Negative | 72 |
| Unknown | 7 |
| Conditioning, n | |
| Busulfan/fludarabine based | 156 |
| Treosulfan/fludarabine | 6 |
| Primary graft failure, n (%) | 9 (6.5) |
| Median time, d (range), to leukocyte engraftment (NC > 1.0 × 109/L) | 17 (9-84) |
| Acute GVDH (n = 155), n (%) | |
| Grade 0-I | 130 (84) |
| Grade II-IV | 25 (16) |
| Grade III-IV | 9 (6) |
| Chronic GVHD (n = 136), n (%) | |
| Limited | 24 (18) |
| Extensive | 27 (20) |
| Characteristic . | Value . |
|---|---|
| No. of patients | 162 |
| Median age, y (range) | 56 (32-73) |
| Sex of patient, n | |
| Male | 96 |
| Female | 66 |
| Sex of donor, n | |
| Male | 102 |
| Female | 60 |
| Myelofibrosis, n | |
| Primary | 105 |
| Post-PV/ET | 57 |
| Median time, mo (range), for diagnosis to SCT | 50 (3-360) |
| Risk profile according to Lille score, n | |
| Low | 35 |
| Intermediate | 91 |
| High | 32 |
| Unknown | 4 |
| Risk profile according to Cervantes score, n | |
| Low | 46 |
| High | 110 |
| Unknown | 6 |
| Risk profile according to IPSS, n | |
| Low | 4 |
| Intermediate-1 | 26 |
| Intermediate-2 | 52 |
| High | 70 |
| Unknown | 10 |
| JAK2 V617F mutation status, n | |
| Positive | 95 |
| Negative | 44 |
| Unknown | 23 |
| Cytogenetics, n | |
| Favorable | 6 |
| Normal | 53 |
| Other | 23 |
| Unfavorable | 17 |
| Unknown | 63 |
| Transfusion dependency, n | |
| Not transfusion-dependent | 65 |
| Transfusion-dependent | 86 |
| Unknown | 11 |
| Splenectomy, n | 20 |
| HLA matching (10/10 alleles), n | |
| Matched | 129 |
| Mismatched | 33 |
| HLA identical sibling, n | 46 |
| Unrelated donor, n | 116 |
| Median no. (range) of CD34+ infused stem cells per kg BW | 6.9 × 106 (0.7-21.7 × 106) |
| CMV serostatus of recipients, n | |
| Positive | 83 |
| Negative | 72 |
| Unknown | 7 |
| Conditioning, n | |
| Busulfan/fludarabine based | 156 |
| Treosulfan/fludarabine | 6 |
| Primary graft failure, n (%) | 9 (6.5) |
| Median time, d (range), to leukocyte engraftment (NC > 1.0 × 109/L) | 17 (9-84) |
| Acute GVDH (n = 155), n (%) | |
| Grade 0-I | 130 (84) |
| Grade II-IV | 25 (16) |
| Grade III-IV | 9 (6) |
| Chronic GVHD (n = 136), n (%) | |
| Limited | 24 (18) |
| Extensive | 27 (20) |
BW indicates body weight; CMV, cytomegalovirus; NC, nuclear cells; and GVHD, graft-versus-host disease.
Determination of JAK2V617F status and monitoring of clearance after ASCT
Applying a highly sensitive real-time polymerase chain reaction (PCR) assay, previously reported18 DNA samples from 114 patients, which were obtained from peripheral blood preceding the date of ASCT, were tested in one study center (Hamburg) to determine the mutational state. This method uses a hematopoietic cell kinase gene as a reference gene and is known to have a very high sensitivity (0.01%) compared with only 1% to 5% sensitivity corresponding to most of the other known testing methods.10-13,27,28 Furthermore, 25 cases were tested in different participating centers using other center-selected assays. To compensate for the possibility that some patients who tested low positive in Hamburg may simultaneously test negative if another detection assay elsewhere would be applied, we considered the patients tested in Hamburg using the sensitive method to be JAK2-positive only when having a mutated allele frequency of more than or equal to 0.1% related to the control gene. Overall, 139 of 162 patients had an available JAK2V617F mutation status and thus could be further included in the analysis.
In 63 JAK2-positive cases, mutation analysis was repeated serially after ASCT from peripheral blood samples in intervals ranging between 2 and 8 weeks. The date of conversion into JAK2-negative status was considered the clearance date. The mutational state (positive vs negative) at 3 and 6 months after ASCT was checked and reported.
Determination of mutational allele burden
In 78 patients, allele burden of JAK2V617F mutation before ASCT could be determined. Using the hematopoietic cell kinase gene as a control gene may render misleading estimation of the JAK2 mutational allele burden, which is better quantified using the JAK2 wild-type as a control gene.28 Thus, a separate TaqMan PCR assay using the JAK2 wild-type (JAK2-wt) as a reference gene was used to accurately achieve this aim. Briefly, DNA extracted from peripheral blood samples obtained within the 6 months preceding ASCT was tested using the following primer/probe combinations: common forward primer, 5-TTATGG ACAACA GTCAAA CAA CAA TTC-3; common probe, 5-TTG TAC TTT TTT TTT TCC TTA GTC TTT CTT TGAAGC AGC A-3; reverse primer for JAK2V617F, 5-CTT ACT CTC GTC TCC ACA AAA-3; reverse primer for JAK2-wt, 5-CTT ACT CTC GTC TCC ACA AAC-3. Reactions were performed in duplicate for both mutated and wild-type alleles either in separate wells. Standard curves were obtained using serial dilutions of UKE1 cell line at known concentrations into DNA from healthy donors. The mutated allele burden was obtained using the following equation:
where Δct = (ct of mutated allele) − (ct of wild-type allele).29
Definition of relapse and progression
Relapse and progression were defined according to the International Working Group for Myelofibrosis Research and Treatment consensus criteria of treatment response in myelofibrosis with myeloid metaplasia published elsewhere.30 Briefly, diagnosis of clinical progress required one of the following: (1) progressive splenomegaly, which is defined by the appearance of a previously absent splenomegaly that is palpable at greater than 5 cm below the left costal margin or a minimum 100% increase in palpable distance for baseline splenomegaly of 5 to 10 cm or a minimum 50% increase in palpable distance for baseline splenomegaly of greater than 10 cm; (2) leukemic transformation confirmed by a bone marrow blast count of at least 20%; and (3) an increase in peripheral blood blast percentage of at least 20% that lasts for at least 8 weeks, whereas clinical relapse was diagnosed if loss of a previous complete remission and an increase in bone marrow fibrosis.
Statistical analysis
Endpoints were overall survival (OS), disease-free survival (DFS), TRM, and incidence of relapse. Characteristics of patients were expressed as median and range for continuous variables and frequencies for categorical variables. Categorical data were compared with by the χ2 test or Fisher exact test. Survival curves were estimated by the Kaplan-Meier method. The log-rank test was used to estimate the incidence of TRM and relapse to account for competing risk. For treatment-related death, relapse was the competing event; and for relapse, treatment-related death was competing event. The Gray test was used to compare of cumulative incidence curves.
In a first step, the following factors were included in univariate analysis: JAK2 status, JAK2V617F allele burden, age, human leukocyte antigen (HLA) match, related versus unrelated donor, Lille score, Cervantes score, IPSS score, cytogenetics, transfusion status, splenectomy, and primary versus post-ET/PV myelofibrosis.
In a second step, all variables with a P value ≤ .1 were entered in a multivariable Cox regression model (forward elimination) to determine independent predictors. Only results of the final models are presented as relative risks (hazard ratio [HR]) with respect to a reference category (HR = 1) together with the 95% confidence interval (CI) and P values.
The impact of achieving JAK2V617F negativity after ASCT on relapse incidence was analyzed using a time-dependent analysis. The same point was evaluated using a landmark analysis of 2 time points after ASCT. Every patient who was alive, relapse-free, and had a known JAK2V617F clearance status at the given time point was included. Most calculations were performed with SPSS Version 15; the competing risk analyses were done with ACCorD (V. Gebski, NHMRC Clinical Trials Center, University of Sydney).
Results
Association of JAK2V617F-mutated state and allele burden before ASCT with the clinical characteristics
JAK2-state was available in 139 patients, from whom 95 were JAK2V617F-positive and 44 JAK2-wt (Table 2). JAK2V617F mutation was detectable in approximately 65%, 100%, and 50% of PMF, post-PV myelofibrosis, and post-ET myelofibrosis, respectively. Before ASCT, patients harboring JAK2-wt and JAK2V617F mutation were equally distributed regarding age, sex, time from diagnosis to ASCT, fibrosis grade before ASCT, cytogenetics, disease stage (Lille, Cervantes, and IPSS scores), and transfusion dependency. Furthermore, relevant parameters for ASCT, such as donors (matched related donor, matched unrelated donor, and mismatched unrelated donor), conditioning regimen, and cytomegalovirus serostatus for recipients, were also similarly distributed. Median day to engraftment (15 vs 16 days, P = .2) and rates of primary graft failure (4.5% vs 5.3%, P > .999) did not differ between patients with mutated and unmutated genotype. Patients with JAK2-wt required significantly more treatments with stem cell boost for secondary graft dysfunction (20.5% vs 4.2%, P = .004). Deaths that occurred more frequently in the JAK2-wt group (50% vs 25% in mutated group, P = .006) were caused by relapse/progress (32% vs 17%) and transplantation-related events (68% vs 83%), respectively, but the differences were statistically not significant (Table 3).
Clinical features, ASCT-relevant variables, and post-ASCT events according to JAK2V617F mutational state
| Variable/category . | JAK2-wt . | JAK2V617F . | P . |
|---|---|---|---|
| No. (%) of patients | 44 (32) | 95 (68) | |
| Median age, y (range) (n = 139)* | 59 (36-73) | 56 (32-72) | .2 |
| Males, no. (%) (n = 139)* | 22 (50) | 60 (63) | .2 |
| Diagnosis (n = 132) | < .001 | ||
| PMF | 29 (69) | 53 (59) | |
| Post-PV MF | 0 (0) | 24 (27) | |
| Post-ET MF | 13 (31) | 13 (14) | |
| Median time, mo (range), from diagnosis to SCT (n = 130)* | 47 (3-360) | 61 (3-272) | .1 |
| Cytogenetics, no. (%) (n = 82)* | .4 | ||
| Favorable | 2 (40) | 3 (60) | |
| Normal | 13 (29) | 32 (71) | |
| Others | 3 (15) | 17 (85) | |
| Unfavorable | 5 (42) | 7 (58) | |
| Lille score, no. (%) (n = 135)* | .4 | ||
| Low | 7 (16) | 24 (26) | |
| Intermediate | 27 (61) | 48 (53) | |
| High | 10 (23) | 19 (21) | |
| Transfusion dependency, no. (%) (n = 131)* | .1 | ||
| No transfusions | 13 (33) | 44 (48) | |
| Transfusion-dependent | 27 (68) | 47 (52) | |
| IPSS, no. (%) (n = 131)* | .1 | ||
| Low | 1 (2) | 2 (2) | |
| Intermediate-1 | 8 (20) | 14 (16) | |
| Intermediate-2 | 9 (22) | 38 (42) | |
| High | 23 (56) | 36 (40) | |
| CMV status (n = 134)* | |||
| CMV+ recipients, no. (%) | 19 (46) | 55 (59) | |
| Donors, no. (%) (n = 139)* | .7 | ||
| MRD | 12 (27) | 28 (30) | |
| MUD | 21 (48) | 49 (51) | |
| MMUD | 11 (25) | 18 (19) | |
| Conditioning, no. (%) (n = 139)* | .5 | ||
| Busulfan/fludarabine-based | 41 (93) | 88 (93) | |
| Treosulfan/fludarabine | 1 (2) | 5 (5) | |
| Others | 2 (5) | 2 (2) | |
| Median no. (range) of infused CD34+ infused stem cells/kg BW (n = 132)* | 6.4 (0.6-13.9) | 7.9 (1-21.6) | .4 |
| Median time, d (range), to engraftment (n = 128)* | 15 (10-59) | 16 (9-47) | .2 |
| Acute GVHD (n = 99)* | .5 | ||
| Grade II-IV acute GVHD, no. (%) | 6 (22) | 22 (31) | |
| Chronic GVHD (n = 97)* | .6 | ||
| Extensive chronic GVHD, no. (%) | 8 (30) | 17 (24) | |
| Primary graft failure, no. (%) | |||
| Secondary graft dysfunction, no. (%) | 9 (21) | 4 (4) | .004 |
| Deaths, no. (%) | 22 (50) | 24 (25) | .006 |
| Cause of death, no. (%) | .4 | ||
| Relapse | 7 (32) | 4(17) | |
| Toxicity | 2 (9) | 2 (8) | |
| GVHD | 3 (14) | 8 (33) | |
| Sepsis | 8 (36) | 5 (21) | |
| Bleeding | 0 (0) | 1 (4) | |
| Unclear | 2 (9) | 4 (17) |
| Variable/category . | JAK2-wt . | JAK2V617F . | P . |
|---|---|---|---|
| No. (%) of patients | 44 (32) | 95 (68) | |
| Median age, y (range) (n = 139)* | 59 (36-73) | 56 (32-72) | .2 |
| Males, no. (%) (n = 139)* | 22 (50) | 60 (63) | .2 |
| Diagnosis (n = 132) | < .001 | ||
| PMF | 29 (69) | 53 (59) | |
| Post-PV MF | 0 (0) | 24 (27) | |
| Post-ET MF | 13 (31) | 13 (14) | |
| Median time, mo (range), from diagnosis to SCT (n = 130)* | 47 (3-360) | 61 (3-272) | .1 |
| Cytogenetics, no. (%) (n = 82)* | .4 | ||
| Favorable | 2 (40) | 3 (60) | |
| Normal | 13 (29) | 32 (71) | |
| Others | 3 (15) | 17 (85) | |
| Unfavorable | 5 (42) | 7 (58) | |
| Lille score, no. (%) (n = 135)* | .4 | ||
| Low | 7 (16) | 24 (26) | |
| Intermediate | 27 (61) | 48 (53) | |
| High | 10 (23) | 19 (21) | |
| Transfusion dependency, no. (%) (n = 131)* | .1 | ||
| No transfusions | 13 (33) | 44 (48) | |
| Transfusion-dependent | 27 (68) | 47 (52) | |
| IPSS, no. (%) (n = 131)* | .1 | ||
| Low | 1 (2) | 2 (2) | |
| Intermediate-1 | 8 (20) | 14 (16) | |
| Intermediate-2 | 9 (22) | 38 (42) | |
| High | 23 (56) | 36 (40) | |
| CMV status (n = 134)* | |||
| CMV+ recipients, no. (%) | 19 (46) | 55 (59) | |
| Donors, no. (%) (n = 139)* | .7 | ||
| MRD | 12 (27) | 28 (30) | |
| MUD | 21 (48) | 49 (51) | |
| MMUD | 11 (25) | 18 (19) | |
| Conditioning, no. (%) (n = 139)* | .5 | ||
| Busulfan/fludarabine-based | 41 (93) | 88 (93) | |
| Treosulfan/fludarabine | 1 (2) | 5 (5) | |
| Others | 2 (5) | 2 (2) | |
| Median no. (range) of infused CD34+ infused stem cells/kg BW (n = 132)* | 6.4 (0.6-13.9) | 7.9 (1-21.6) | .4 |
| Median time, d (range), to engraftment (n = 128)* | 15 (10-59) | 16 (9-47) | .2 |
| Acute GVHD (n = 99)* | .5 | ||
| Grade II-IV acute GVHD, no. (%) | 6 (22) | 22 (31) | |
| Chronic GVHD (n = 97)* | .6 | ||
| Extensive chronic GVHD, no. (%) | 8 (30) | 17 (24) | |
| Primary graft failure, no. (%) | |||
| Secondary graft dysfunction, no. (%) | 9 (21) | 4 (4) | .004 |
| Deaths, no. (%) | 22 (50) | 24 (25) | .006 |
| Cause of death, no. (%) | .4 | ||
| Relapse | 7 (32) | 4(17) | |
| Toxicity | 2 (9) | 2 (8) | |
| GVHD | 3 (14) | 8 (33) | |
| Sepsis | 8 (36) | 5 (21) | |
| Bleeding | 0 (0) | 1 (4) | |
| Unclear | 2 (9) | 4 (17) |
CMV indicates cytomegalovirus; MRD, matched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; BW, body weight; and GVHD, graft-versus-host disease.
Available cases for evaluation in every category.
Univariate and multivariate analysis of risk factors for OS and DFS (only with P ≤ .1) after ASCT in myelofibrosis
| Variable/category . | OS . | DFS . | ||||
|---|---|---|---|---|---|---|
| Kaplan-Meier 5-year OS (95% CI) . | Cox regression . | Kaplan-Meier 5-year DFS (95% CI) . | Cox regression . | |||
| HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Univariate | ||||||
| JAK2 type | ||||||
| JAK2V617F | 70% (62-78) | 1 | .007 | 50% (39-61) | 1 | .03 |
| JAK2-wt | 44% (30-58) | 2.23 (1.25-3.98) | 32% (19-45) | 1.71 (1.04-2.82) | ||
| Advanced age (continuous) | 1.07 (1.03-1.11) | .001 | 1.03 (1.00-1.06) | .05 | ||
| Lille score | .02 | |||||
| Low | 85% (75-95) | 1 | 70% (57-83) | 1 | .007 | |
| Intermediate + high | 55% (45-56) | 3.14 (1.25-7.92) | 36% (25-48) | 2.63 (1.30-5.31) | ||
| IPSS | ||||||
| Low + intermediate-1 | 86% (75-97) | 1 | .02 | 76% (61-91) | 1 | .01 |
| Intermediate-2 | 65% (54-76) | 1.64 (1.09-2.46) | 44% (28-60) | 2.96 (1.28-2.59 | ||
| High | 48% (34-62) | 33% (21-45) | ||||
| HLA match status | ||||||
| Matched | 67% (61-73) | 1 | .03 | 50% (41-59) | 1 | .05 |
| Mismatched | 36% (17-55) | 1.93 (1.05-3.53) | 26% (6-46) | 1.71 (1.00-2.92) | ||
| Multivariate, n = 135 | ||||||
| JAK2-wt | 2.14 (1.18-3.86) | .01 | ||||
| Advanced age (continuous) | 1.08 (1.04-1.13) | < .001 | ||||
| HLA mismatched | 1.95 (1.01-3.76) | .05 | 1.93 (1.08-3.49) | .03 | ||
| Lille intermediate + high score | 3.24 (1.57-6.70) | .002 | ||||
| Variable/category . | OS . | DFS . | ||||
|---|---|---|---|---|---|---|
| Kaplan-Meier 5-year OS (95% CI) . | Cox regression . | Kaplan-Meier 5-year DFS (95% CI) . | Cox regression . | |||
| HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Univariate | ||||||
| JAK2 type | ||||||
| JAK2V617F | 70% (62-78) | 1 | .007 | 50% (39-61) | 1 | .03 |
| JAK2-wt | 44% (30-58) | 2.23 (1.25-3.98) | 32% (19-45) | 1.71 (1.04-2.82) | ||
| Advanced age (continuous) | 1.07 (1.03-1.11) | .001 | 1.03 (1.00-1.06) | .05 | ||
| Lille score | .02 | |||||
| Low | 85% (75-95) | 1 | 70% (57-83) | 1 | .007 | |
| Intermediate + high | 55% (45-56) | 3.14 (1.25-7.92) | 36% (25-48) | 2.63 (1.30-5.31) | ||
| IPSS | ||||||
| Low + intermediate-1 | 86% (75-97) | 1 | .02 | 76% (61-91) | 1 | .01 |
| Intermediate-2 | 65% (54-76) | 1.64 (1.09-2.46) | 44% (28-60) | 2.96 (1.28-2.59 | ||
| High | 48% (34-62) | 33% (21-45) | ||||
| HLA match status | ||||||
| Matched | 67% (61-73) | 1 | .03 | 50% (41-59) | 1 | .05 |
| Mismatched | 36% (17-55) | 1.93 (1.05-3.53) | 26% (6-46) | 1.71 (1.00-2.92) | ||
| Multivariate, n = 135 | ||||||
| JAK2-wt | 2.14 (1.18-3.86) | .01 | ||||
| Advanced age (continuous) | 1.08 (1.04-1.13) | < .001 | ||||
| HLA mismatched | 1.95 (1.01-3.76) | .05 | 1.93 (1.08-3.49) | .03 | ||
| Lille intermediate + high score | 3.24 (1.57-6.70) | .002 | ||||
JAK2 status, age, Lille score, and match status were included in the multivariate analysis. IPSS scoring was excluded because of the transacting effect with Lille scoring.
Allele burden data were available from 78 JAK2V617F-positive patients, and the median allele burden was 35.6% (0.2%-98.4%; Table 4). This subpopulation was divided into 4 quartiles with the following frequencies: 27 patients (34.6%) in the first quartile, 26 (33.3%) in the second, 11 (14%) in the third, and 14 (17.9%) in the fourth. Table 4 summarizes the distribution of clinical and procedure-related characteristics between the 4 groups. Briefly, the patients with PMF and post-ET myelofibrosis were more presented in the lower quartiles and those with post-PV myelofibrosis in the upper quartiles (P = .03). Moreover, the time from diagnosis to ASCT in months increased significantly with increasing allele burden (median 18 months in the first quartile to 112 months in the fourth quartile, P = .03). All otherwise remaining characteristics were equally distributed between the 4 groups. In this population, 3 patients experienced secondary graft dysfunction after ASCT, and all of them had an allele burden less than 50% (P = .2).
Clinical features, ASCT-relevant variables, and post-ASCT events according to JAK2V617F allele burden
| Variable/category . | JAK2V617F allele burden . | P . | |||
|---|---|---|---|---|---|
| 0.1%-25% . | 26%-50% . | 51%-75% . | 76%-100% . | ||
| No. (%) of patients (n = 78)* | 27 (35) | 26 (33) | 11 (14) | 14 (18) | |
| Median age (range), y (n = 78)* | 54 (33-72) | 56 (32-65) | 58 (48-76) | 57 (49-70) | .4 |
| Males, no. (%) (n = 78)* | 15 (56) | 18 (69) | 7 (64) | 8 (57) | .8 |
| Diagnosis (n = 74)* | .03† | ||||
| PMF | 21 (81) | 16 (67) | 5 (46) | 5 (39) | |
| Post-PV MF | 1 (4) | 5 (20) | 4 (36) | 7 (54) | |
| Post-ET MF | 4 (15) | 3 (13) | 2 (18) | 1 (7) | |
| Median time, mo (range), from diagnosis to SCT (n = 75)* | 18 (3-231) | 51 (4-272) | 81 (13-181) | 112 (15-185) | .03† |
| Cytogenetics (n = 47)* | .8 | ||||
| Favorable | 0 (0) | 1 (6) | 0 (0) | 1 (10) | |
| Normal | 8 (62) | 7 (44) | 6 (75) | 6 (60) | |
| Others | 3 (23) | 5 (31) | 2 (25) | 2 (20) | |
| Unfavorable | 2 (15) | 3 (19) | 0 (0) | 1 (10) | |
| Lille score (n = 74)* | .7 | ||||
| Low | 7 (26) | 5 (20) | 3 (30) | 6 (50) | |
| Intermediate | 14 (52) | 14 (56) | 4 (40) | 4 (33) | |
| High | 6 (22) | 6 (24) | 3 (30) | 2 (17) | |
| IPSS score (n = 72) | .6 | ||||
| Low | 0 (0) | 2 (9) | 0 (0) | 0 (0) | |
| Intermediate-1 | 3 (13) | 3 (13) | 3 (28) | 2 (14) | |
| Intermediate-2 | 9 (37) | 10 (43) | 4 (36) | 7 (50) | |
| High | 12 (50) | 8 (35) | 4 (36) | 5 (36) | |
| Transfusion dependency, no. (%) (n = 74)* | .3 | ||||
| No transfusions | 10 (40) | 13 (54) | 6 (54) | 10 (71) | |
| Transfusion-dependent | 15 (60) | 11 (46) | 5 (46) | 4 (29) | |
| CMV status (n = 78)* | .2 | ||||
| CMV+ recipients, no. (%) | 13 (48) | 18 (69) | 6 (54) | 11 (79) | |
| Donors, no. (%) (n = 78)* | .7 | ||||
| MRD | 6 (22) | 7 (27) | 3 (27) | 2 (14) | |
| MUD | 16 (59) | 11 (42) | 5 (46) | 10 (72) | |
| MMUD | 5 (19) | 8 (31) | 3 (27) | 2 (14) | |
| Days to engraftment, median (range) (n = 70)* | 15 (10-27) | 16 (10-46) | 15 (9-26) | 16 (11-45) | .5 |
| Median time, d (range), to JAK2 clearance (n = 45)* | 117 (20-214) | 72 (27-216) | 75 (33-194) | 96 (38-312) | .4 |
| Grade II-IV acute GVHD, no. (%) (n = 74)* | 7 (27) | 8 (33) | 3 (27) | 5 (39) | .9 |
| Extensive chronic GVHD, no. (%) (n = 72)* | 8 (31) | 4 (17) | 2 (20) | 4 (31) | .7 |
| Primary graft failure, no. (%) (n = 78)* | 1 (4) | 3 (12) | 1 (9) | 0 | .3 |
| Secondary graft dysfunction, no. (%) (n = 78)* | 2 (7) | 1 (4) | 0 | 0 | .4 |
| Deaths, no. (%) (n = 20)* | 10 (37) | 3 (12) | 3 (27) | 4 (29) | .2 |
| Cause of death | .1 | ||||
| Relapse | 1 (10) | 2 (67) | 1 (33) | 0 | |
| Transplantation-related | 9 (90) | 1 (33) | 2 (67) | 3 (100) | |
| Probability of overall survival at 5 y, % (95% CI) | 62 (46-80) | 78 (57-99) | 66 (38-93) | 69 (48-90) | .3 |
| Probability of disease-free survival at 5 y, % (95% CI) | 41 (23-59) | 57 (34-81) | 53 (28-78) | 60 (38-82) | .6 |
| Cumulative incidence of relapse (%) at 3 y (95% CI) | 19 (3-35) | 26 (11-41) | 35 (9-61) | 11 (6-28) | .4 |
| Cumulative incidence of TRM (%) at 1 y (95% CI) | 30 (16-44) | 4 (0-11) | 16 (0-37) | 32 (10-54) | .2 |
| Variable/category . | JAK2V617F allele burden . | P . | |||
|---|---|---|---|---|---|
| 0.1%-25% . | 26%-50% . | 51%-75% . | 76%-100% . | ||
| No. (%) of patients (n = 78)* | 27 (35) | 26 (33) | 11 (14) | 14 (18) | |
| Median age (range), y (n = 78)* | 54 (33-72) | 56 (32-65) | 58 (48-76) | 57 (49-70) | .4 |
| Males, no. (%) (n = 78)* | 15 (56) | 18 (69) | 7 (64) | 8 (57) | .8 |
| Diagnosis (n = 74)* | .03† | ||||
| PMF | 21 (81) | 16 (67) | 5 (46) | 5 (39) | |
| Post-PV MF | 1 (4) | 5 (20) | 4 (36) | 7 (54) | |
| Post-ET MF | 4 (15) | 3 (13) | 2 (18) | 1 (7) | |
| Median time, mo (range), from diagnosis to SCT (n = 75)* | 18 (3-231) | 51 (4-272) | 81 (13-181) | 112 (15-185) | .03† |
| Cytogenetics (n = 47)* | .8 | ||||
| Favorable | 0 (0) | 1 (6) | 0 (0) | 1 (10) | |
| Normal | 8 (62) | 7 (44) | 6 (75) | 6 (60) | |
| Others | 3 (23) | 5 (31) | 2 (25) | 2 (20) | |
| Unfavorable | 2 (15) | 3 (19) | 0 (0) | 1 (10) | |
| Lille score (n = 74)* | .7 | ||||
| Low | 7 (26) | 5 (20) | 3 (30) | 6 (50) | |
| Intermediate | 14 (52) | 14 (56) | 4 (40) | 4 (33) | |
| High | 6 (22) | 6 (24) | 3 (30) | 2 (17) | |
| IPSS score (n = 72) | .6 | ||||
| Low | 0 (0) | 2 (9) | 0 (0) | 0 (0) | |
| Intermediate-1 | 3 (13) | 3 (13) | 3 (28) | 2 (14) | |
| Intermediate-2 | 9 (37) | 10 (43) | 4 (36) | 7 (50) | |
| High | 12 (50) | 8 (35) | 4 (36) | 5 (36) | |
| Transfusion dependency, no. (%) (n = 74)* | .3 | ||||
| No transfusions | 10 (40) | 13 (54) | 6 (54) | 10 (71) | |
| Transfusion-dependent | 15 (60) | 11 (46) | 5 (46) | 4 (29) | |
| CMV status (n = 78)* | .2 | ||||
| CMV+ recipients, no. (%) | 13 (48) | 18 (69) | 6 (54) | 11 (79) | |
| Donors, no. (%) (n = 78)* | .7 | ||||
| MRD | 6 (22) | 7 (27) | 3 (27) | 2 (14) | |
| MUD | 16 (59) | 11 (42) | 5 (46) | 10 (72) | |
| MMUD | 5 (19) | 8 (31) | 3 (27) | 2 (14) | |
| Days to engraftment, median (range) (n = 70)* | 15 (10-27) | 16 (10-46) | 15 (9-26) | 16 (11-45) | .5 |
| Median time, d (range), to JAK2 clearance (n = 45)* | 117 (20-214) | 72 (27-216) | 75 (33-194) | 96 (38-312) | .4 |
| Grade II-IV acute GVHD, no. (%) (n = 74)* | 7 (27) | 8 (33) | 3 (27) | 5 (39) | .9 |
| Extensive chronic GVHD, no. (%) (n = 72)* | 8 (31) | 4 (17) | 2 (20) | 4 (31) | .7 |
| Primary graft failure, no. (%) (n = 78)* | 1 (4) | 3 (12) | 1 (9) | 0 | .3 |
| Secondary graft dysfunction, no. (%) (n = 78)* | 2 (7) | 1 (4) | 0 | 0 | .4 |
| Deaths, no. (%) (n = 20)* | 10 (37) | 3 (12) | 3 (27) | 4 (29) | .2 |
| Cause of death | .1 | ||||
| Relapse | 1 (10) | 2 (67) | 1 (33) | 0 | |
| Transplantation-related | 9 (90) | 1 (33) | 2 (67) | 3 (100) | |
| Probability of overall survival at 5 y, % (95% CI) | 62 (46-80) | 78 (57-99) | 66 (38-93) | 69 (48-90) | .3 |
| Probability of disease-free survival at 5 y, % (95% CI) | 41 (23-59) | 57 (34-81) | 53 (28-78) | 60 (38-82) | .6 |
| Cumulative incidence of relapse (%) at 3 y (95% CI) | 19 (3-35) | 26 (11-41) | 35 (9-61) | 11 (6-28) | .4 |
| Cumulative incidence of TRM (%) at 1 y (95% CI) | 30 (16-44) | 4 (0-11) | 16 (0-37) | 32 (10-54) | .2 |
CMV indicates cytomegalovirus; MRD, matched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; and GVHD, graft-versus-host disease.
Available cases for evaluation in every category.
Statistically significant.
Survival
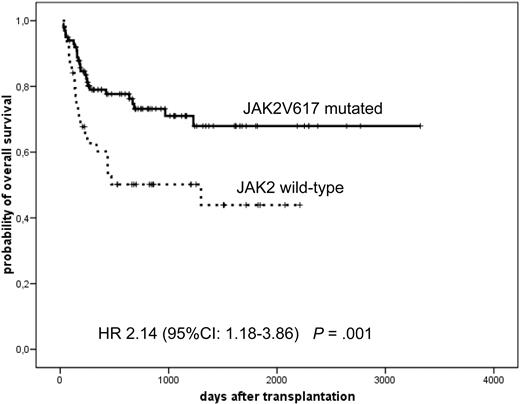
After a median follow-up of 19 months (range, 1-111 months), 5-year DFS was 46% (95% CI, 37%-55%), and 5-year OS was 62% (95% CI, 54%-70%). In univariate analysis, the following factors were found to be significantly associated with improved OS: JAK2-mutated versus -wt (70% vs 44% at 5 years, P = .007; Figure 1), advanced age as a continuous variable (HR = 1.07 per year; 95% CI, 1.03-1.11, P = .001), Lille score low versus intermediate plus high (85% vs 55%, P = .02), IPSS-low plus intermediate-1 versus intermediate-2 versus high (86% vs 65% vs 48%, P = .001), and HLA matched versus mismatched donor (67% vs 36%, P = .03). Remarkably, transfusion dependency or cytogenetic risk category did not affect the OS in this analysis (P = .9 and .6, respectively). Because of the transaction between Lille and IPSS scoring, we excluded the latter first from the multivariate analysis, which revealed the following variables as significant independent risk factors for reduced OS: JAK2-wt (HR = 2.14%; 95% CI, 1.18%-3.86%), advanced age (HR = 1.08%; 95% CI, 1.04%-1.13%), and HLA-mismatched donor (HR = 1.95%; 95% CI, 1.01%-3.76%). In a second step, we repeated multivariate analysis with IPSS scoring instead of Lille scoring demonstrating again the same findings: JAK2-wt (HR = 1.91%; 95% CI, 1.04%-3.51%), advanced age (HR = 1.09%; 95% CI, 1.05%-1.14%), and HLA-mismatched donor (HR = 2.58%; 95% CI, 1.33%-5.03%).
Probability of OS according to JAK2 status. A significantly increased survival in JAK2V617F-mutated patients.
Probability of OS according to JAK2 status. A significantly increased survival in JAK2V617F-mutated patients.
To exclude a possible impact of all JAK2-positive post-PV myelofibrosis on outcome, we further analyzed only PMF patients with known JAK2V718F status (n = 86; wt = 31 and mutated = 55). This subanalysis confirmed the worse survival of patients without JAK2V617F mutation (HR = 2.28%; 95% CI, 1.09%-4.80%, P = .03). Similarly, we explored whether the negative outcome of the JAK2-wt group could be caused by the increased incidence of secondary graft dysfunction mentioned previously. Even after excluding this subgroup of patients, the group with JAK2-wt remained significantly characterized with a negative outcome (HR = 2.81%; 95% CI, 1.53%-5.15%, P = .001).
Significant factors for improved DFS in the univariate analysis were JAK2-mutated versus -wt (50% vs 32% at 5 years, P = .03), HLA matched versus mismatched donor (50% vs 26%, P = .05), Lille score low versus intermediate plus high (70% vs 36%, P = .007), IPSS-low plus intermediate-1 versus intermediate-2 versus high (76% vs 44% vs 33%, P = .02), and advanced age evaluated as a continuous variable (HR = 1.03 per year; 95% CI, 1.00-1.06, P = .05). In the multivariate analysis, which included all these factors but IPSS, only Lille score intermediate plus high (HR = 3.23%; 95% CI, 1.57%-6.70%, P = .002) and HLA-mismatched donor (HR = 1.92%; 95% CI, 1.08%-3.43%, P = .03) were significantly independent risk factors for decreased DFS. Even after substitution of Lille score through IPSS score, the previous findings were maintained (HLA mismatch: HR = 1.86%; 95% CI, 1.04%-3.32%, P = .04; advanced IPSS stage: HR = 4.29%; 95% CI, 1.68%-10.97%, P = .03).
TRM and relapse
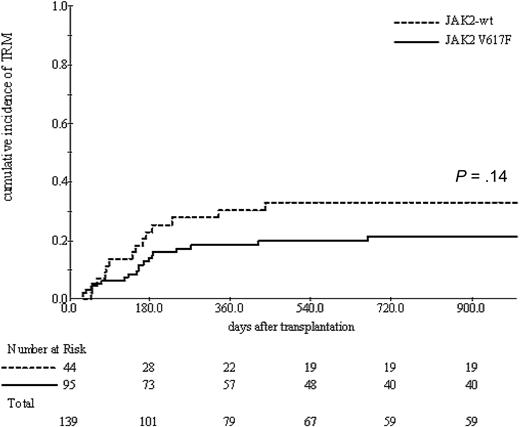
The cumulative incidence of TRM was 22% (range, 15%-29%) at 1 year. A trend toward decreased TRM was seen with JAK2-mutated patients compared with those harboring JAK2-wt (19% vs 31%, P = .1) in the univariate analysis (Figure 2), whereas TRM was significantly influenced with age (HR = 1.06 per year; 95% CI, 1.01-1.11, P = .01), HLA-mismatched versus matched donor (43% vs 20% at 1 year, P = .03).
Cumulative incidence of TRM according to JAK2 status. A nonsignificant trend toward an increased TRM in JAK2 wild-type patients.
Cumulative incidence of TRM according to JAK2 status. A nonsignificant trend toward an increased TRM in JAK2 wild-type patients.
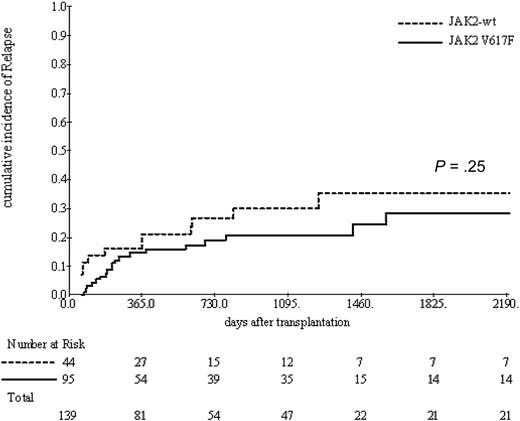
Relapse occurred in a cumulative incidence of 23% (range, 16%-30%) at 3 years after ASCT. Related donors were significantly associated with increased relapse rates compared with unrelated donors (34% vs 17%, P = .05) and with increasing IPSS score (low + intermediate-1 vs intermediate-2 vs high) (HR = 2.28%; 95% CI, 1.33%-3.91%). JAK2-wt was associated with a trend toward increased risk of relapse without reaching the statistical significance (30% vs 21%, P = .25; Figure 3). In contrast to the recently reported finding of the EBMT study,21 splenectomy before ASCT was only associated with a nonsignificant trend with respect to relapse (3-year incidence of relapse of 40% vs 25%, P = .2).
Cumulative incidence of relapse according to JAK2 status. A nonsignificant trend toward an increased relapse incidence in JAK2 wild-type patients.
Cumulative incidence of relapse according to JAK2 status. A nonsignificant trend toward an increased relapse incidence in JAK2 wild-type patients.
Impact of JAK2V617F allele burden
First, in 78 patients, JAK2V617F allele burden was examined as a continuous variable using Cox regression model for OS and DFS, and no correlation between allele burden and duration of OS and DFS could be found (P = .8 and P = .8). Similarly, no significant relation between allele burden and cumulative incidence of relapse and TRM could be identified (P = .8 and P = .6). Next we examined the patients for OS and DFS after dividing them into 4 quartiles. Duration of OS and DFS was similar between the 4 groups (P = .3 and P =.6, respectively). There was also no significant difference in cumulative incidence of relapse or progression and TRM (P = .4 and .2, respectively; Table 4). Ten deaths were reported in the group with the lowest allele burden, 9 of which for transplantation-related causes (3 graft-versus-host disease, 3 sepsis, 2 toxicity, and 1 unclear). On the other hand, 3 deaths were reported in the group with 26% to 50% allele burden, 3 in 51% to 75%, and 4 in 76% to 100%, which was not a significant difference (P = .2 and P =.1). The data of the mutational allele burden were not included in the multivariate analysis because of the small size of the group with available complete dataset.
Impact of JAK2V617F clearance
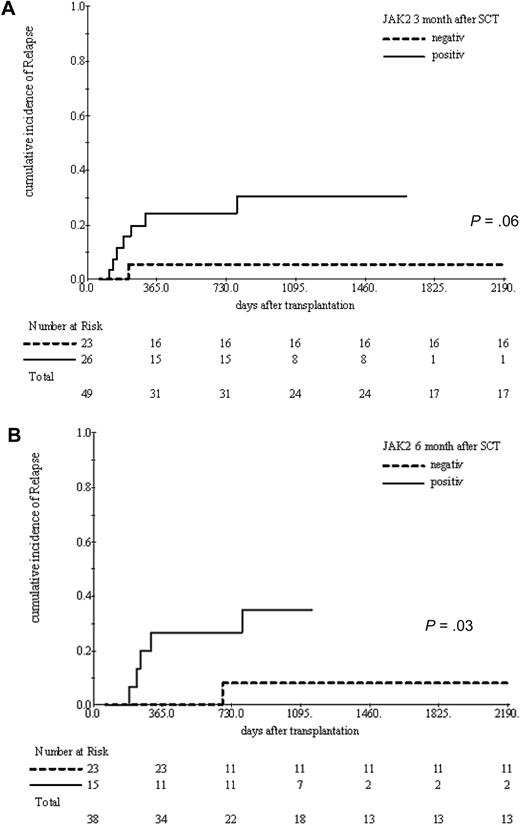
Regular post-ASCT analyses of JAK2V617F from peripheral blood samples were performed in 63 patients who presented initially with a mutated state. Forty-five of them became negative spontaneously after a median of 96 days (range, 20-427 days) after ASCT, 7 were converted to negative after application of donor lymphocyte infusions (DLIs), and 11 never became negative for the entire available follow-up. We examined the impact of JAK2V617F clearance on the cumulative incidence of relapse in a time-dependent manner. This analysis revealed that reaching JAK2V617F negativity was significantly associated with reduced risk of relapse (HR = 0.22; 95% CI, 0.05-0.93, P = .04). JAK2V617F state was also reviewed at 3 and 6 months after ASCT. The cumulative incidence of clinical relapse was tested at each time point according the JAK2V617F state (negative vs still positive). Patients who were still JAK2V617F positive at 3 months after ASCT had a borderline significant higher incidence of relapse compared with those who had already cleared the mutation (31% vs 5%, P = .06). Moreover, remaining JAK2V617F positive until 6 months is further associated with a significantly increased clinical relapse rates (35% vs 5%, P = .03; Figure 4).
Cumulative incidence of relapse at 3 and 6 months after ASCT according to JAK2V617F clearance status. Failure to clear JAK2V617F mutation until 3 months (A) and 6 months (B) after ASCT was associated with a significant increase in relapse incidence.
Cumulative incidence of relapse at 3 and 6 months after ASCT according to JAK2V617F clearance status. Failure to clear JAK2V617F mutation until 3 months (A) and 6 months (B) after ASCT was associated with a significant increase in relapse incidence.
Discussion
The discovery of JAK2V617F mutation in 2005 provided new insights for understanding the pathophysiology of Philadelphia chromosome–negative myeloproliferative neoplasms. In PMF, the phenotype associated with the JAK2V617F mutation was characterized with leukocytosis and erythrocytosis, less transfusion requirements, and more pronounced splenomegaly. The data regarding the prognostic significance of JAK2V617F mutation in PMF were more controversial, with some studies indicating a worse outcome with inferior leukemia-free survival31 and poor survival32 characterizing the mutated genotype and another one showing no significant impact.33 The question that should be answered was how a single point mutation causes or contributes to 3 clinically distinct phenotypes: PV, ET, and PMF. Some animal data suggested that the frequency of the mutated compared with the wild-type alleles “gene dosage hypothesis” in addition to some undetermined host factors may play a role in the phenotype and possibly the prognosis of these diseases.34-38
Based thereon, there was an increasingly growing interest in the concept of allele burden and its possible contribution to the understanding of pathogenesis and natural history of this heterogenic diseases group. In the case of post-PV and post-ET myelofibrosis, one retrospective study indicated that neither the phenotype nor the prognosis was influenced by the mutated allele frequency.15 The first systematic evaluation of PMF patients for the prognostic significance of the mutated allele load was performed at the Mayo Clinic, showing an inferior OS and leukemia-free survival in the cases with lower mutated allele burden compared with those with higher mutated burden or even with wild-type.39 A second evaluation done by Guglielmelli et al40 was published recently and showed no influence of the mutated state on the OS and leukemic transformation, whereas in multivariate analysis allele burden less than 25% in addition to increased age and elevated peripheral blasts were significant factors predicting reduced OS. Interestingly, the patients in the lower quartile group had shorter time to anemia and leukopenia and did not develop a splenomegaly. In contrast to the data of Mayo Clinic, the deaths in this group were mainly the result of systemic infection and not leukemic transformation. These data led the authors to suggest that lower JAK2V617F allele burden may be linked to a myelodepletive, rather than myeloproliferative, phenotype.40
The role of JAK2V617F mutation in the ASCT setting remains unclear. A small study, which included 30 patients with PMF and post-ET and post-PV myelofibrosis, found no impact of mutated status on the outcome after ASCT.17 More recently in a subanalysis of the EBMT study, including 82 patients with known JAK2V617F mutation status, wild-type was associated with an inferior survival, but this factor was not included in a multivariate analysis. Therefore, in the current study, we analyzed the impact of JAK2V617F mutation in a larger group of patients (n = 139) with known mutation status who underwent ASCT.
In this analysis, harboring JAK2 wild-type conferred an inferior survival in the study population. The same decrease in survival was noted if only PMF patients were analyzed, indicating the fact that the favorable outcome in the JAK2V617F-mutated group is not caused by the high prevalence of post-PV myelofibrosis patients. The inferior outcome of JAK2-wt group could be explained with the trend toward increased TRM and relapse rates in this group. The number of deaths was significantly higher in JAK2 wild-type group, whereas death causes were similar between the 2 groups (∼ 30% relapse-related and ∼ 70% TRM-related). The high incidence of secondary graft dysfunction in recipients negative for JAK2V617F is of special interest. There was no difference between the basis variables of JAK2V617F-positive and -negative patients, such as median number of transplanted CD34+ cells, unrelated or related donor, and Lille score that may explain this finding. Interestingly, harboring JAK2-wt retained its negative prognostic significance after removing this patient subgroup. These data suggest that the absence of JAK2V617F mutation identifies a distinct subgroup in myelofibrosis with a clearly different biologic behavior at least in the setting of ASCT.
Next, we investigated the impact of JAK2 mutational allele burden on the outcome after ASCT because it has been shown, in a nontransplantation setting, that low JAK2V617F allele burden negatively influences the therapy outcome. After examining JAK2V617F allele burden data in 75 patients either continuously or after dividing them the same way done by Guglielmelli et al,40 no correlation between mutated allele burden and OS, DFS, TRM, and relapse could be found. Remarkably, in the group with the lowest allele burden (1%-25%), more deaths through transplantation-related causes were documented, even though this finding did not reach the statistical significance. Thus, and in contrast to JAK2V617F mutation status, JAK2 mutational allele burden does not seem to have any impact on the outcome after ASCT.
In the last part of our study, we evaluated the kinetic of JAK2V617F clearance in peripheral blood after ASCT and its impact on the outcome. Reaching JAK2V617F negativity after ASCT, tested as a time-dependent variable, was associated with significantly decreased risk of relapse. Furthermore, the persistence of JAK2V617F mutation in peripheral blood until 6 months after ASCT was strongly associated with an increased risk of developing a clinical relapse. These data may lead to the recommendation of testing JAK2V617F state using a sensitive method after ASCT beginning from 3 months after ASCT in 4- to 6-week intervals. In the interval between the third and sixth months, the kinetics of JAK2V617F mutation should be used to guide therapeutic decisions, such as discontinuation of the immunosuppression. The high clinical relapse rates in the patients who still test positive at 6 months after ASCT (35%) led us to recommend considering an urgent intervention, such as stopping the immunosuppression or, if necessary, afterward application of DLIs. Our group showed recently that treatment of minimal residual disease or molecular relapse with DLIs is more effective and less toxic than applying the treatment on the incidence of clinical relapse.19
In conclusion, we were able to validate and extend a recent finding regarding the favorable impact of JAK2V617F mutation on survival after dose-reduced ASCT. Contrary to the some other previous findings regarding unfavorable prognosis associated with lower mutated allele burden in the nontransplantation setting, the current study showed no significant impact of mutated allele burden on the ASCT outcome in myelofibrosis. However, a higher number of TRM-related deaths observed with the lower quartile should be further validated in a larger group of patients, and the mechanisms that may explain this observation should be further explored. Our data strongly support the favorable impact of JAK2V617F mutation clearance after ASCT and add additional evidence supporting the value of monitoring JAK2V617F status after ASCT, which guides interventions such as DLIs to prevent clinical relapse.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by an EBMT-AMGEN fellowship grant (H.A.) and by a grant of the “Hamburger Krebsgesellschaft.” The technical equipment for the analyses was supported in part by the Rudolf Bartling Foundation (III/103).
Authorship
Contribution: H.A. collected and analyzed patient data, performed quantitative JAK2V617F PCR and statistical analysis, and wrote the manuscript; A.B., C.T., and Y.H. performed quantitative JAK2V617F PCR; G.K., J.H., M.B., D.W., W.B., and H.B. submitted patient data and DNA samples; B.F., U.B., and A.R.Z. analyzed and approved PCR results; T.Z. performed statistical analysis; N.K. designed the study and wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolaus Kröger, Department of Stem Cell Transplantation, University Hospital Hamburg-Eppendorf, Martinistr 52, D-20246 Hamburg, Germany; e-mail: nkroeger@uke.uni-hamburg.de.

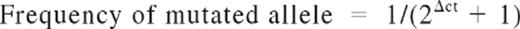
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal