Abstract
The median survival of patients with primary myelofibrosis ranges from 3.5 to 5.5 years, and most patients die from cause related to the disease, including blast phase (BP, in 5%-30% of cases). Because identification of high-risk patients might use information collected during the clinical course, we assessed the prognostic value of time-dependent covariates for 2 competing risks (death and BP) in a series of 172 patients. Significant (P < .01) adverse time-dependent prognostic factors for the risk of death were the time to onset of anemia (hemoglobin < 100 g/L [10 g/dL]), leukocytosis (leukocyte count > 30 × 109/L), thrombocytopenia (platelet count < 150 × 109/L), presence of circulating blasts, intermediate-high or high International Working Group for Myelofibrosis Research and Treatment score, and time to splenectomy. The first 3 dependent covariates and the time to chemotherapy initiation (P = .05) were prognostic factors for the risk of BP. The prognostic effect of onset of leukocytosis was significantly more pronounced for BP than for death. Thus, occurrence during the follow-up of characteristics associated with an adverse prognostic value at diagnosis also influenced the risks of death and BP. Patients with leukocytosis should be closely monitored. These data might efficiently help to evaluate the severity of the disease before treatment decision during the clinical course.
Introduction
Primary myelofibrosis (PMF) is a rare and complex chronic myeloproliferative neoplasia characterized by myelofibrosis and extramedullary hematopoiesis.1,2 Despite individual clinical courses lasting up to 30 years, the prognosis is poor, with median survival ranging from 3.5 to 5.5 years.1-5 Most PMF patients will progress and finally die from cause related to the disease, including bone marrow failure, thromboembolic events, portal hypertension, cardiac failure, and blast phase (BP).4 The reported incidence of BP ranges from 5% to 30%.4,5 Although acute leukemia is part of the natural history of PMF, some cases may be related to prior therapy.
Multivariate analyses of overall survival identified the adverse prognostic value of the following initial characteristics, namely hemoglobin less than 100 g/L (10 g/dL),4,5 leukopenia with white blood cell count (WBC) less than 4 × 109/L,4,5 leukocyte count greater than 25 × 109/L5 or greater than 30 × 109/L,4 constitutional symptoms, presence of circulating blasts, age older than 65 years,5 and abnormal karyotype.3,4,6 On the basis of various combinations of these variables, several models have been developed.4,5 These models can identify 22% to 47% low-risk PMF patients with an expected 93- to 135-month median survival.4,5 Low-risk patients are more frequently younger than 557 or 655 years, with few deaths within the first 5 years and median survival from 135 to 176 months.5,7-9 Thus, it is unlikely that the introduction of other initial covariates in new prognostic systems will identify a large number of patients requiring therapy at diagnosis. Therefore, the identification of additional high-risk patients might rest on criteria observed during follow-up. Using a multivariate Cox proportional hazard regression with time-dependent covariates in a series of 64 patients, Passamonti et al10 found that the acquisition of a hemoglobin level less than 100 g/L (10 g/dL), a platelet count less than 100 × 109/L, and a leukocyte count more than 30 × 109/L retained statistical significance on overall survival of post–polycythemia vera myelofibrosis, and they proposed a score based on these 3 dynamic covariates. More recently, the presence of thrombocytopenia, excess of blood or bone marrow blasts, or chromosome 17 aberration were used to define an accelerated phase in patients with PMF or secondary myelofibrosis.11 Median subsequent survival of PMF patients with accelerated phase at diagnosis or later during the follow-up was 12 months. The occurrence of accelerated phase was associated with an increased risk of BP. In the present study, we assessed the role of time-dependent covariates in competing risk analyses because we analyzed the time to event of 2 types: death and BP, the observation of one type of failure preventing the observation of the other. For the purpose, we reviewed 2073 outpatient visits performed every 4 to 6 months during the follow-up of 172 patients with PMF (median number of visits for each patient: 11). Indeed, making treatment decisions during the clinical course of patients with PMF is often challenging. The aim of the present study was to identify prognostic factors that would facilitate individual patient selection during follow-up for allogeneic stem cell transplantation, the only curative approach,12 or for targeted therapies including antiangiogenic13-15 and antifibrosing16 agents, epigenetic modifiers, or Jak2 inhibitors.17
Methods
Patients
A total of 172 patients with PMF diagnosed between June 1963 and January 1995 were included in this study because results of follow-up procedures repeated at least every 6 months during the first 4 years were available. Median follow-up was 112 months in surviving patients (range, 44-276 months). Only 6 patients (4%) were lost to follow-up 44 to 162 months after diagnosis.
The diagnosis of PMF was made in accordance with the criteria in use at the time of the first observation, and all patients fulfilled the criteria of either the Polycythemia Vera Study Group or the recently published World Health Organization classification.18 Patients with post–polycythemia vera or post–essential thrombocythemia myelofibrosis, acute (malignant) myelofibrosis, and myelodysplastic syndrome with myelofibrosis were excluded. The study was approved by the institutional ethics committee of Center Hospitalier Schaffner.
Bone marrow trephine biopsy was performed at diagnosis in all patients and stained with standard and silver stain for reticulin fibers; 3 stages were defined according to the usual classification.19
Cytogenetic analysis was available at diagnosis in 89 patients. Metaphases were obtained from bone marrow or blood samples, after short-term (24 hours) culture without stimulation. Chromosomes were analyzed with conventional R banding technique, and classified according to the International System for Cytogenetic Nomenclature.20
Treatment
Treatment decisions were based on age, performance status, and degrees of cytopenias and myeloproliferation, and guided by erythrokinetic studies for splenectomy. A total of 84 patients (49%) received one or more single-agent oral chemotherapy treatment (hydroxyurea, busulfan, or pipobroman) 1 to 124 months after diagnosis (median, 15.2 months). A total of 28 patients underwent splenectomy 2 to 130 months after diagnosis (median, 42 months). No patient received allogeneic bone marrow transplant during the study period.
Prognostic factors analyzed
The following initial characteristics were analyzed: age, sex, spleen size, hepatomegaly, weight loss, hemoglobin concentration, WBC, platelet count, percentage of circulating blasts, percentage of immature granulocytes (apart from blasts), reticulocyte count, bone marrow staging, cytogenetics, and Lille4 and International Working Group for Myelofibrosis Research and Treatment (IWGMRT)5 scoring systems. The intermediate- and high-risk subgroups of the Lille scoring system were merged in a single category as suggested recently.5,9
The following adverse characteristics were assessed during follow-up and analyzed as time-dependent covariates21 : splenomegaly (more than 10 cm below the costal margin), the presence of hepatomegaly, weight loss (at least 10% of baseline weight during the last 6 months), anemia (hemoglobin less than 100 g/L [10 g/dL]), leukocytosis (WBC greater than 30 × 109/L), thrombocytopenia (less than 150 × 109/L), and the presence of circulating blasts (1% or more). The scores (Lille and IWGMRT) evolved also and they were updated at each visit. The changes in scores observed during the follow-up were also analyzed as time-dependent covariates. Our continuously updated PMF database was interrogated to determine, among the 172 patients with available information, the timing of onset of an unfavorable value for each characteristic. The first occurrence and the total duration during follow-up of each adverse characteristic were analyzed as time-dependent covariates. The former time-dependent covariate took the value zero until the first notification of the unfavorable value of the parameter. The latter time-dependent covariate evaluated the total length of follow-up while the adverse value of the characteristic was observed. It took the value 0 as long as the favorable value of the parameter was observed during the follow-up and the value 1 as long as the unfavorable value of the parameter was recorded during the follow-up. Similarly, we analyzed the time to splenectomy, the time to initiation of chemotherapy, and the total duration of follow-up while chemotherapy was delivered. Because of the wide variability of the value of circulating blast count during follow-up examinations, we could assess only the prognostic value of the time to first occurrence of circulating blasts.
Statistical analysis
All Cox models were fitted after checking the proportional hazards model assumption with the graphs of the Schoenfeld residuals22 and the test proposed by Therneau and Grambsch.22 Treating onset and duration of change in the value of covariates as time-dependent variables in the Cox regression models enabled us to analyze the impact of these events on the entire population, whereas landmarking techniques consider only a subset of the population. All analyses were performed with SAS Version 8.02 software (SAS Institute Inc) using the time-dependent and the counting process forms of the Cox model22 and results were confirmed using S-PLUS Version 6.2 (Insightful Corp) with the counting process form of the Cox model.
The response variable was time to 1 of the 2 following competing events: death or BP, defined by an excess of blasts of 20% or more in blood or bone marrow, according to the IWGMRT criteria.23 To evaluate the prognostic role of each characteristic listed in the previous section for these 2 competing risks (death and BP), we used the proportional hazards regression model proposed by Wei et al.24 For each covariate, the results were expressed by cause-specific hazard ratio (CSHR; with a test for statistical significance) of each competing risk and a test for a difference of effect of the covariate between the 2 competing risks.25
Finally, to illustrate the practical consequences, we performed landmark analyses26 for 2 particular subgroups of patients, namely low-risk patients (Lille score) still alive 3 years after diagnosis and intermediate- and high-risk patients still alive at 18 months. These time points were chosen to ensure a sufficient sample size. In the landmark method,26 patients who died before the time point were excluded.
Results
Initial characteristics and clinical course
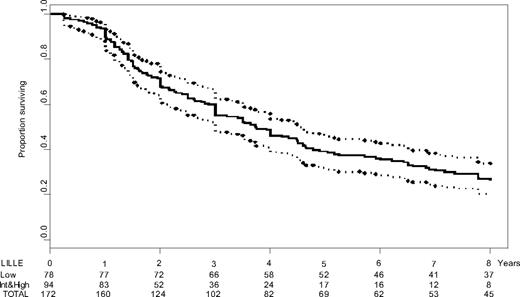
Median age was 65 years (range, 35-83 years; male/female ratio = 1.1:1). Median overall survival was estimated at 44.7 months (95% confidence interval [CI], 36-55.1; Figure 1). Twenty-five patients died from BP (splenectomy had been done in 6 of these 25 patients); 34 from bone marrow failure without BP; 14 from portal hypertension; 6 from cachexia; 16 from cardiovascular complication; 20 from unrelated cause; and 41 from unknown cause. Initial characteristics associated with a prognostic value for survival are reported in Table 1. During follow-up, hemoglobin level dropped to less than 100 g/L (10 g/dL) in 146 (85%) patients, platelet count declined to less than 150 × 109/L in 109 (63%), and WBC exceeded 30 × 109/L in 86 (50%).
Survival of 172 patients with primary myelofibrosis. Kaplan-Meier estimates of the overall survival of the patient population (solid), along with pointwise 95% confidence intervals. The time axis legend includes the numbers of patients at risk for the subgroups of patients with initial low-risk Lille score, initial intermediate- or high-risk Lille score, and for the whole series. Int indicates intermediate.
Survival of 172 patients with primary myelofibrosis. Kaplan-Meier estimates of the overall survival of the patient population (solid), along with pointwise 95% confidence intervals. The time axis legend includes the numbers of patients at risk for the subgroups of patients with initial low-risk Lille score, initial intermediate- or high-risk Lille score, and for the whole series. Int indicates intermediate.
Initial characteristics of the 172 patients with primary myelofibrosis
| Characteristic . | Value . |
|---|---|
| Patients, no. | 172 |
| Age, y, median ± SD | 65 ± 9.69 |
| Hemoglobin, g/L, median ± SD | 104 ± 24.5 |
| White blood cell count, ×109/L, median ± SD | 11.25 ± 14.26 |
| Platelet count, ×109/L, median ± SD | 297 ± 276 |
| Abnormal cytogenetics* (%) | 30 of 89 (34) |
| Weight loss, n (%) | 33 (19) |
| Hepatomegaly, n (%) | 76 (44) |
| Male/female (ratio) | 90/82 (1.1:1) |
| Circulating blasts > 1%, n (%) | 60 (36) |
| Immature granulocytes > 5%, n (%) | 109 (64) |
| Treatment regimen, n (%) | |
| Chemotherapy | 84 (49) |
| Androgens | 33 (19) |
| Splenectomy | 28 (16) |
| Lille score, n (%) | |
| Low | 78 (45) |
| Intermediate | 77 (45) |
| High | 17 (10) |
| IWGMRT score,* n (%) | |
| Low | 18 (11) |
| Intermediate-low | 51 (31) |
| Intermediate-high | 56 (34) |
| High | 39 (24) |
| Characteristic . | Value . |
|---|---|
| Patients, no. | 172 |
| Age, y, median ± SD | 65 ± 9.69 |
| Hemoglobin, g/L, median ± SD | 104 ± 24.5 |
| White blood cell count, ×109/L, median ± SD | 11.25 ± 14.26 |
| Platelet count, ×109/L, median ± SD | 297 ± 276 |
| Abnormal cytogenetics* (%) | 30 of 89 (34) |
| Weight loss, n (%) | 33 (19) |
| Hepatomegaly, n (%) | 76 (44) |
| Male/female (ratio) | 90/82 (1.1:1) |
| Circulating blasts > 1%, n (%) | 60 (36) |
| Immature granulocytes > 5%, n (%) | 109 (64) |
| Treatment regimen, n (%) | |
| Chemotherapy | 84 (49) |
| Androgens | 33 (19) |
| Splenectomy | 28 (16) |
| Lille score, n (%) | |
| Low | 78 (45) |
| Intermediate | 77 (45) |
| High | 17 (10) |
| IWGMRT score,* n (%) | |
| Low | 18 (11) |
| Intermediate-low | 51 (31) |
| Intermediate-high | 56 (34) |
| High | 39 (24) |
SD indicates standard deviation.
Data were not available for all patients.
Prognostic analyses for the risk of death
In competing risk analysis, adverse prognostic factors at diagnosis for the risk of death were advanced age (CSHR: 1.47, P = .02), anemia (hemoglobin < 100 g/L [10 g/dL], CSHR: 2.69, P < .001), leukocytosis (WBC greater than 30 × 109/L, CSHR: 2.56, P < .001), thrombocytopenia (platelet count less than 150 × 109/L, CSHR: 1.67, P = .005), the presence of circulating blasts (CSHR: 1.57, P = .004), weight loss (CSHR: 2.28, P < .001), hepatomegaly (CSHR: 1.78, P = .001), intermediate or high Lille score (CSHR: 2.95, P < .001), intermediate-high or high IWGMRT score (CSHR: 2.81, P < .001), and the presence of an abnormal karyotype (CSHR: 1.78, P = .009). The following time-dependent characteristics retained a prognostic significance: first occurrence of anemia (CSHR: 4.51, P < .001), leukocytosis (CSHR: 3.68, P < .001), thrombocytopenia (CSHR: 3.01, P < .001), circulating blasts (CSHR: 1.97, P = .001), intermediate-high or high IWGMRT score (CSHR: 4.43, P < .001), and time to splenectomy (CSHR: 1.78, P = .01). Similar findings were observed when analyzing the total duration of follow-up with main adverse characteristics (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Evaluating the clinical consequences of these results, we found that median subsequent survival of patients with leukocytosis was invariably estimated at 19 months or less at diagnosis and between 1 and 4 years of follow-up (data not shown). Time to chemotherapy initiation and total duration of follow-up with chemotherapy had no prognostic value for the risk of death.
Prognostic analyses for the risk of blast phase
Adverse prognostic factors at diagnosis for the risk of BP were leukocytosis (CSHR: 5.85, P < .001), the presence of an abnormal karyotype (CSHR: 3.19, P = .03), unfavorable Lille and IWGMRT scores (CSHR = 3.69 [P = .003] and 2.44 [P = .04], respectively). The following time-dependent characteristics retained a prognostic significance: first occurrence of anemia (CSHR: 11.4, P < .001), leukocytosis (CSHR: 8.77, P < .001), thrombocytopenia (CSHR: 5.49, P < .001), weight loss (CSHR: 10.33, P = .02), time to chemotherapy initiation (CSHR: 2.32, P = .05), and time to splenectomy (CSHR: 2.91, P = .03).
Test for a difference of effects of covariates between the risks of death and BP was significant for time to first occurrence of leukocytosis and time to initiation of therapy (supplemental Table 1). Introducing these 2 covariates in multivariate Cox model, only time to onset of leukocytosis retained significant prognostic value for the risk of BP, suggesting a correlation between the 2 covariates.
Prognostic factors during the follow-up of low-risk patients
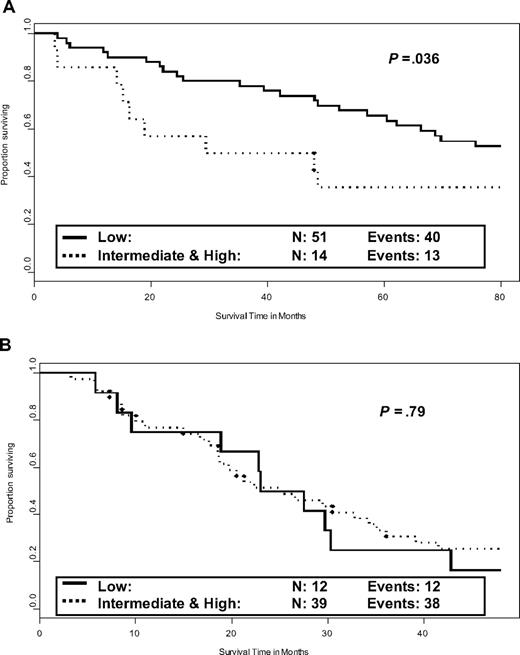
Of the 78 patients at low risk according to Lille system, 66 (85%) were alive at 3 years. The median subsequent overall survival was 82.4 months (95% CI, 47.9-101). Thrombocytopenia (median survival 26.8 vs 92.1 months if absent, P = .02), anemia (38.6 vs 85.1 months, P = .03), hepatomegaly (52.2 vs 104.7 months, P = .03), and intermediate- or high-risk Lille scoring system (38.6 vs 84.8 months, P = .036, Figure 2A) at 3 years were adverse prognostic factors for subsequent survival. Leukocytosis had no significant prognostic value because this characteristic was observed at 3 years in only 3 patients. Cytogenetic analysis had been performed at diagnosis in 47 low-risk patients (normal, 32; abnormal, 15). The previously described prognostic value for overall survival in low-risk patients was confirmed (P = .03). Four patients with abnormal karyotype had died during the first 3 years of follow-up. Thus, initial cytogenetics had no more prognostic value for subsequent survival after 3 years in the 43 patients still alive at this time (P = .16), probably because of the small number of remaining patients with an abnormal initial karyotype.
Prognostic value for subsequent survival of the Lille scoring system evaluated during the follow-up. (A) Prognostic value of the Lille scoring system evaluated at 3 years in patients with Lille low-risk score at diagnosis and still alive at 3 years. Data were missing for 1 patient. (B) Lille scoring system evaluated at 18 months in patients with Lille intermediate- or high-risk score at diagnosis and still alive at 18 months.
Prognostic value for subsequent survival of the Lille scoring system evaluated during the follow-up. (A) Prognostic value of the Lille scoring system evaluated at 3 years in patients with Lille low-risk score at diagnosis and still alive at 3 years. Data were missing for 1 patient. (B) Lille scoring system evaluated at 18 months in patients with Lille intermediate- or high-risk score at diagnosis and still alive at 18 months.
Prognostic factors during the follow-up of intermediate- or high-risk patients
Of the 94 patients at intermediate or high risk, 51 (54%) were alive at 18 months. The median subsequent survival was 24.9 months (95% CI, 18.8-32.7 months). Adverse prognostic factors for subsequent survival were initial blastosis (P = .049, median survival 21 months if present vs 29.5 months if absent), thrombocytopenia at time point (20.4 months vs 30.4 months, P = .06), and abnormal cytogenetics at diagnosis (18 months vs 32.7 months, P = .003). Only the latter characteristic was significant in multivariate Cox model for survival after 18 months, although cytogenetic abnormality at diagnosis had no prognostic value for survival in this subgroup of intermediate or high-risk patients, as previously reported.4 Lille scoring system evaluated at 18 months had no prognostic value for subsequent survival in these patients (Figure 2B).
Discussion
The natural history of PMF ranges from indolent condition lasting years to rapid progression toward acute leukemia. Thus, making decision on treatment choice and timing is frequently a tricky issue. In this study, we identified a significant prognostic value for leukocytosis, low hemoglobin concentration, and thrombocytopenia coded as time-dependent covariates in proportional hazard models for the 2 following competing risks: BP and death. The prognostic value of these characteristics at diagnosis had already been identified for the overall survival of PMF patients in several scoring systems,4 including the recently reported IWGMRT score.5
Similar prognostic factors have been reported for survival in post–polycythemia vera myelofibrosis patients,10 but longitudinal studies have not been published in PMF so far. More recently, Passamonti et al27 performed a similar analysis of the database of the IWGMRT. Their results agreed with our analyses of time-dependent prognostic factors for the risk of death. Tam et al11 aimed to identify characteristics associated with a median subsequent survival of less than 12 months and an increased risk of BP. Thus, they identified thrombocytopenia less than 50 × 109/L, marked excess of blood or bone marrow blasts (> 10%), and chromosome 17 abnormality as features of accelerated phase at diagnosis or later during the evolution. Discrepancies in the cutoffs identified in this report and the present series may be explained by differences in inclusion criteria and design of the analyses. In addition, cytogenetics was not available during follow-up in our study. The present report and the study of Tam et al11 showed that almost all covariates had a similar prognostic value for the 2 risks (death and BP). This result confirmed that BP could be considered as a part of the natural history of the disease. However, we found that leukocytosis retained some unique prognostic characteristics: First, developing leukocytosis was associated with similar median subsequent survival at several time points during the first 4 years of follow-up. Second, Cox model evaluating the difference of effect between the 2 competing risks indicated that the prognostic value of leukocytosis was more pronounced for the risk of BP than for the risk of death. These results agree with the adverse prognostic value of increased WBC and CD34+ cell count during the follow-up of PMF.28 Nevertheless, leukocytosis was not selected as a feature of accelerated phase.11 In the present series, the median subsequent survival of patients with leukocytosis at diagnosis or later during the first 4 years was greater than the criterion used for the definition of the accelerated phase (12 months or less). Finally, leukocytosis might be a useful manifestation of rapid subsequent evolution; it could be especially helpful in making the decision of whether to perform allogeneic bone marrow transplantation.
Although chemotherapy may markedly modify blood cell counts, a major influence of treatment on the outcome of patients is unlikely in the present series: First, the Cox model including chemotherapy as time-dependent covariate showed no influence on survival, Second, conventional drug therapies for PMF (including androgen preparations, corticosteroids, low-dose oral chemotherapy) are largely palliative and have never been shown to improve survival. Third, Lille scoring system had no prognostic value for subsequent survival in patients with initial high risk and still alive after 18 months, thus improvement in the value of prognostic characteristics was not associated with survival improvement. For all these reasons, it is unlikely that the effect of treatment limits the interpretation of the present analyses. Our data suggest that anemia and thrombocytopenia observed during treatment in PMF are more likely related to the disease evolution itself rather than to the toxicity of therapy. Taken together, the adverse prognostic value associated with the onset of anemia, thrombocytopenia, or leukocytosis suggested that these abnormalities are demonstration of the natural history of the disease. We found that the time to onset of chemotherapy and its total duration were prognostic factors for the risk of BP, but it remains unclear whether prolonged exposure to chemotherapy may favor the occurrence of BP in addition to the risk of this complication as part of the natural history of the disease: In our model, with times to onset of leukocytosis and chemotherapy, only the former covariate retained a significant prognostic value for the risk of BP. Regardless, chemotherapy should be closely evaluated and delivered in patients with leukocytosis. Besides chemotherapy, an increased risk of BP has been suspected to be associated with erythropoiesis-stimulating agents or danazol29 and splenectomy.30 However, the prognostic value associated with time to splenectomy for both risks in the present series should be interpreted very cautiously, given the high number of confounding factors for the choice and timing of this procedure.
Treatment decision is frequently a difficult issue during the course of chronic disorders and few studies used Cox models with time-dependent covariate for assessing the prognostic consequences of the changes observed during the follow-up for different types of risk.25,31 The pattern of distribution of events in the present series precluded any conclusion for some covariates such as the prognostic role of the acquisition of weight loss for the risk of death, an unfavorable IWGMRT score for the risk of BP, and an unfavorable Lille score for both risks (supplemental Table 1). Further studies using additional statistical analyses22 or other series are warranted. Thus the prognostic value of constitutional symptoms coded as time-dependent covariate has been recently demonstrated for the risk of death.27 Using only results of Cox models with time-dependent covariates, Passamonti et al27 stated that the combination of several time-dependent covariates allowed prognostic assessment of PMF patients at any time during the clinical course of the disease. However, such model provides only an average hazard ratio for the risk associated with the acquisition of an adverse characteristic during the follow-up. In addition, it is likely that comorbidities and delivery of previous therapy also have a prognostic influence for subsequent outcome. Furthermore, the analyses of patients with similar risk at diagnosis and still alive several years (for low-risk patients) or months (for intermediate- or high-risk patients), after diagnosis suggested that besides the values observed during follow-up, initial scoring system and initial characteristics of the disease should probably be taken into account. Besides initial blastosis, initial cytogenetics retained a significant prognostic value for subsequent survival in patients with initial high-risk score and still alive 18 months after diagnosis. By contrast, initial cytogenetics of patients at low risk at diagnosis was a prognostic factor for overall survival of these patients,4 but it had no prognostic value for subsequent survival 3 years later, because a significant percentage of patients with abnormal karyotype had already died. Cytogenetics performed in advanced phase of the disease should be useful in this case, as recently reported,6 especially in case of abnormality of chromosome 17.11 Finally, for all these reasons, results provided by Cox model with time-dependent clinical covariates should be considered only as one of the useful tools for assessing subsequent prognosis of the disease during its clinical course.
Classical treatment of PMF patients rested on conventional low-dose chemotherapy, androgen, corticosteroid therapies, splenectomy,30 or interferon32 until recent years. Improved response rates or promising results have been observed with antiangiogenic13-15 or anti-Jak217 agents, whereas intensive chemotherapy regimens with allogeneic stem cell rescue33,34 are being increasingly investigated in high-risk patients. The identification of adverse prognostic factors for subsequent survival during the clinical course will help to initiate therapy during the follow-up of patients who did not require early management. It may be useful for selecting patients eligible for new therapeutic approaches and for evaluating treatment effect. For example, the analysis conducted in low-risk patients suggested that intensive therapy could be delayed in low-risk patients until the occurrence of anemia or thrombocytopenia, especially in absence of cytogenetic abnormality.
Finally, we conclude that onset of anemia, leukocytosis, and thrombocytopenia should be considered to evaluate the severity of the ongoing disease and to initiate therapy during the clinical course of PMF. However, the prognostic importance of these characteristics may depend on the scoring system evaluated at diagnosis. Patients with leukocytosis should be closely monitored and delivery of conventional chemotherapy in this case carefully evaluated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the paper; A.D. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the paper; B.H. and L.S. performed research, collected and interpreted data, and wrote the paper; and J.-L.D. and B.D. designed and performed research, collected, analyzed, and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Morel, Service Hematologie Clinique, Centre Hospitalier Schaffner, 99 route de la Bassée, 62300 Lens, France; e-mail: pmorel@ch-lens.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal