Abstract
Patients with hematologic malignancies not in remission before allogeneic hematopoietic stem cell transplantation (HSCT) have a poor prognosis. To improve the antitumor activity of conditioning, we combined clofarabine with myeloablative doses of busulfan in a phase 1/2 study in nonremission hematologic malignancies. Forty-six patients were enrolled, including 31 patients with nonremission acute myelogenous leukemia (AML). Patients had a median age of 53 years, with a median comorbidity index of 3. Donors were unrelated, HLA mismatched, or both in 59% of patients. Common grade III to IV nonhematologic toxicities included transient transaminitis (50%), mucositis (24%), hand-foot syndrome (13%), transient hypoxia (13%), nausea/vomiting (9%), and diarrhea (9%). All patients engrafted. Complete remission was achieved in 80% of all patients by day +30 and in 100% of AML patients without prior hematopoietic stem cell transplantation. Two-year nonrelapse mortality for all patients was 31%, and overall survival was 28%. In AML, the overall survival was 48% at 1 year and 35% at 2 years. These data suggest that clofarabine combined with myeloablative doses of busulfan is well tolerated, secures engraftment, and possesses significant antitumor activity, particularly in nonremission AML. This study is registered at www.ClinicalTrials.gov under identifier NCT00556452.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) represents a potentially curative option for patients with advanced hematologic malignancies1 ; however, outcomes for patients not in remission at HSCT are poor. In acute myelogenous leukemia (AML), primary induction failure and relapse occur frequently and define high-risk populations needing improved treatment options.2-5 Disease-free survivals are ∼ 10%-20% in AML, highlighting remission status as a critical determinant of outcome.6-9 Relapse after HSCT consistently exceeds 50% in nonremission patients, but nonrelapse mortality (NRM) also accounts for a significant proportion of treatment failures.6-9
Reduced intensity conditioning regimens decrease early organ toxicities compared with myeloablative approaches and may limit NRM.10 Survival benefits of reduced intensity conditioning may be offset by increased relapse, particularly in the setting of nonremission disease.7,11-13 Thus, in advanced disease, conditioning that optimizes disease control but limits regimen-related toxicity is desirable. Clofarabine (Clo), a second generation purine nucleoside analog, was Food and Drug Administration–approved after demonstrating complete responses in the treatment of multiply relapsed/refractory pediatric acute lymphoblastic leukemia (ALL).14,15 This agent has since demonstrated activity in the treatment of relapsed/refractory AML,15,16 myelodysplastic syndromes (MDSs),17 and most recently non-Hodgkin lymphoma.18 In an effort to improve the antitumor effects of conditioning, we combined Clo with myeloablative doses of busulfan (Bu), CloBu4, in a phase 1/2 study in nonremission hematologic malignancies.
Patients and methods
Patient eligibility
Patients up to age 70 years with nonremission hematologic malignancies were eligible after providing informed consent in accordance with the Declaration of Helsinki to a protocol approved by the University of Michigan institutional review board (www.ClinicalTrials.gov; identifier NCT00556452). Patients with AML, chronic myelogenous leukemia, or ALL not in remission were defined as follows: (1) > 5% bone marrow blasts, (2) peripheral blasts at any degree, or (3) extramedullary disease. Patients with myelodysplastic syndrome with > 5% blasts in bone marrow were eligible. Hodgkin and non-Hodgkin lymphomas required positron-emission tomography or computed tomography scan evidence of persistent disease before HSCT. Chronic lymphocytic leukemia and multiple myeloma (MM) patients not in remission were eligible.
Patients were required to have a left ventricular ejection fraction ≥ 40%. Forced expiratory volume in 1 second, forced vital capacity, and diffusing capacity of lung for CO2 had to be ≥ 40% of predicted. Patient's total bilirubin had to be ≤ 2.0 mg/dL and hepatic transaminases (aspartate aminotransferase/alanine aminotransferase) had to be < 4 times the upper limit of normal. The minimum glomerular filtration rate was > 60 mL/min in adults and ≥ 90 mL/min in children as calculated by the Modification of Diet in Renal Disease equation or Schwartz formula, respectively. Patients with active infections or central nervous system disease were excluded.
Transplant conditioning and supportive care
Conditioning treatment consisted of intravenous Bu at 3.2 mg/kg daily based on adjusted ideal body weight infused over 3 hours on 4 consecutive days (−5 to −2). Samples for Bu pharmacokinetics were obtained after the first Bu dose on day −5 and were analyzed at the Seattle Cancer Alliance Clinical Pharmacokinetics Laboratory (Seattle, Washington). Bu dose was adjusted on days −3 and −2 if necessary to target an area under the curve (AUC) of 4800 μmol min. Patients were assigned to 1 of 3 dosing cohorts of IV Clo (20, 30, or 40 mg/m2) that was administered over 1 hour on days −6 to −2. Treatment assignment is discussed below. Clo dose was calculated based on adjusted ideal body weight and was administered immediately after Bu. Stem cells were administered on day 0.
Levetiracetam was given starting on day −6 for seizure prophylaxis. On days of Clo, dexamethasone at 8-12 mg oral or intravenous was administered as an antiemetic and to prevent possible capillary leak syndrome. GVHD prophylaxis consisted of tacrolimus from day −3 to day +180 and mycophenolate mofetil from day 0 to day +28. No antithymocyte globulin (ATG) or other T cell–depleting methods were used in this study. Patients received ursodiol for sinusoid obstruction syndrome (SOS) prophylaxis from day −6 until day +100. Fungal prophylaxis included micafungin at 50 mg intravenous daily in lieu of azole antifungals until day +5 or when liver function testing declined to grade I; then, patients were switched to either oral fluconazole (related donor recipients) or voriconazole (unrelated donor recipients).
Posttransplant assessment, treatment assignment, and statistics
All patients had peripheral blood samples and bone marrow aspiration and biopsy performed at scheduled intervals on days +30, +100, +180, and +365 and in the event of clinical suspicion for relapse. Lymphoma and MM patients had additional imaging or disease-appropriate assessments performed at similarly scheduled intervals. Remission status also was assessed by flow cytometry, cytogenetic, and molecular methods when appropriate. Peripheral blood and bone marrow chimerism was assessed on CD3+- and CD33+-enriched cells by comparative analysis of donor and recipient microsatellite markers using a PCR-based assay.19 Peripheral blood lymphocyte subsets were quantified by the University of Michigan clinical flow cytometry laboratory.
This phase 1/2 study sought to determine the maximum tolerated dose (MTD) defined as the dose of Clo combined with Bu that led to dose-limiting toxicity (DLT) in 20% of patients. Dose levels were determined using the time-to-event continual reassessment method (TITE-CRM).20 TITE-CRM assumes a simple model for the probability of a DLT as a function of dose, and it uses the occurrence of DLT in the patients enrolled in the trial to sequentially determine which dose is likely to be the MTD and allocates that dose to new patients.
Overall survival (OS) was calculated from the day of transplantation (day 0) until death. Event-free survival (EFS) was calculated from the day of transplantation until death or posttransplant detection of persistent or relapsed disease. OS and EFS were estimated using the method of Kaplan-Meier. Cumulative incidence of relapse, NRM, and GVHD were estimated using the methods of Gray, and group differences also were assessed with the methods of Gray.21 All analyses were done in the statistical package R (R Development Core Team).
Results
Patients characteristics
Forty-six consecutive patients with relapsed or refractory hematologic malignancies not in remission received unmanipulated HSCT with CloBu4 conditioning from October 2007 to November 2009 at the University of Michigan. Patient characteristics are summarized in Table 1. Patients had received a median of 3 prior regimens (range, 1-5) and had a median comorbidity index of 3 (range, 0-9).22 Eight patients (17%) received previous allogeneic HSCT (median time between HSCT, 8 months; range, 3-121 months). Two patients received fludarabine/Bu myeloablative regimens within 4 months of CloBu4 conditioning. Although all eligible diseases were initially enrolled, the later stages of accrual predominantly enrolled AML patients; thus, 68% of patients enrolled had AML. Disease characteristics for AML patients are shown in Table 2. Before HSCT, patients had an average of 30% blasts (0%-91%) in bone marrow. One AML patient with < 5% bone marrow blasts had extramedullary relapse. High-risk cytogenetics (defined as −5, −7,inv(3), t(3;3), t(6;9), t(v;11q23) excluding t(9;11q23), or ≥ 3 clonal chromosomal abnormalities)23 were present in 58% of patients. The median and average prognostic score as reported by Duval et al24 for AML was 3.0 and 2.5, respectively (range, 0-5).
Patient characteristics
| Characteristic . | Value . |
|---|---|
| Patients, n | 46 |
| Age, y | |
| Median | 53 |
| Range | 1-68 |
| Sex | |
| Female | 22 |
| Male | 24 |
| Prior regimens | |
| Median | 3 |
| Range | 1-5 |
| Previous allogeneic transplant, n (%) | 8 (17) |
| Unrelated donor, n (%) | 26 (56) |
| HLA mismatched donor, n (%) | 6 (14) |
| Comorbidity index* | |
| Median | 3 |
| Range | 0-9 |
| Diagnosis to HSCT, mo | |
| Median | 10 |
| Range | 2-123 |
| Disease, n (%) | |
| AML | 31 (68) |
| CML | 2 (4) |
| ALL | 4 (9) |
| CLL | 4 (9) |
| NHL | 3 (6) |
| MDS | 1 (2) |
| MM | 1 (2) |
| Clofarabine dose, n (%) | |
| 20 mg/m2 | 6 (13) |
| 30 mg/m2 | 21 (46) |
| 40 mg/m2 | 19 (41) |
| Characteristic . | Value . |
|---|---|
| Patients, n | 46 |
| Age, y | |
| Median | 53 |
| Range | 1-68 |
| Sex | |
| Female | 22 |
| Male | 24 |
| Prior regimens | |
| Median | 3 |
| Range | 1-5 |
| Previous allogeneic transplant, n (%) | 8 (17) |
| Unrelated donor, n (%) | 26 (56) |
| HLA mismatched donor, n (%) | 6 (14) |
| Comorbidity index* | |
| Median | 3 |
| Range | 0-9 |
| Diagnosis to HSCT, mo | |
| Median | 10 |
| Range | 2-123 |
| Disease, n (%) | |
| AML | 31 (68) |
| CML | 2 (4) |
| ALL | 4 (9) |
| CLL | 4 (9) |
| NHL | 3 (6) |
| MDS | 1 (2) |
| MM | 1 (2) |
| Clofarabine dose, n (%) | |
| 20 mg/m2 | 6 (13) |
| 30 mg/m2 | 21 (46) |
| 40 mg/m2 | 19 (41) |
CML indicates chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; and NHL, non-Hodgkin lymphoma.
Comorbidity index as reported by Sorror et al.19
Characteristics of AML patients
| Characteristic . | Value . |
|---|---|
| Patients, n | 31 |
| Age, y | |
| Median | 53 |
| Range | 1-68 |
| Cytogenetics, n (%) | |
| Standard risk | 13 (42) |
| High risk* | 18 (58) |
| Previous HSCT, n (%) | 7 (23) |
| Disease status, n (%) | |
| Primary induction failure | 14 (45) |
| < 2 relapses | 10 (32) |
| ≥ 2 relapses | 7 (23) |
| Antecedent MDS | 8 (26) |
| BM blasts, % | |
| Mean | 30 |
| Range | 0-91 |
| PB blasts, %† | |
| Mean | 16 |
| Range | 1-90 |
| Characteristic . | Value . |
|---|---|
| Patients, n | 31 |
| Age, y | |
| Median | 53 |
| Range | 1-68 |
| Cytogenetics, n (%) | |
| Standard risk | 13 (42) |
| High risk* | 18 (58) |
| Previous HSCT, n (%) | 7 (23) |
| Disease status, n (%) | |
| Primary induction failure | 14 (45) |
| < 2 relapses | 10 (32) |
| ≥ 2 relapses | 7 (23) |
| Antecedent MDS | 8 (26) |
| BM blasts, % | |
| Mean | 30 |
| Range | 0-91 |
| PB blasts, %† | |
| Mean | 16 |
| Range | 1-90 |
PB indicates peripheral blood; and BM, bone marrow.
High risk described by Dohner et al.20
PB blasts were present in 22/31 (71%) of patients at HSCT.
Clo dose and regimen-related toxicity
This phase 1/2 study used TITE-CRM for patient enrollment as described in “Methods.”20 The first enrolled patient (30-mg/m2 dose) experienced a DLT (grade IV transaminitis); thus, Clo was de-escalated to 20 mg/m2 in the next 6 patients. Four of 6 patients receiving the 20 mg/m2 Clo dose had transient grade III transaminitis. The next 6 patients enrolled at the 30-mg/m2 dose, and there were 2 cases of transient grade III transaminitis. All grade III to IV transaminitis resolved to grade I or less by 2 weeks. Thus, transient grade III to IV elevations in transaminases were excluded as a DLT for the remainder of the study so long as they returned to grade I or less in 14 days. The remaining patients were enrolled into the 30 mg/m2 and 40 mg/m2 dosing cohorts. No relationship between Clo dose and degree of transaminitis was observed (data not shown). Overall, Clo dose assignments were 20 (n = 6), 30 (n = 21), and 40 mg/m2 (n = 19).
Regimen-related toxicities are shown in Table 3. Transient transaminitis was the most common grade III to IV nonhematologic toxicity occurring in 50% of patients. Pre-HSCT ferritin levels were measured in 42 (91%) patients as a surrogate for transfusion iron overload. The median pre-HSCT ferritin level was 2011 ng/mL (range, 32-5646 ng/mL). Higher ferritin levels were observed in patients who developed grade III to IV transaminitis compared with those developing grade 0 to II transaminitis (2624 vs 1888 ng/mL; P = .04). Other frequently observed toxicities through day +30 included fever (46%), mucositis (24%), transient hypoxia (13%), and hand-foot syndrome (13%). There were a total of 3 cases of ascites and 2 cases of SOS, including one patient who developed ascites after SOS. Ascites occurred in the first posttransplant month in 2 patients receiving the 30 mg/m2 Clo dose and at 18 months in 1 patient receiving the 20 mg/m2 Clo dose. In 1 case, ascites occurred after suspected SOS, but liver biopsy findings were inconclusive for SOS. Interestingly, marked intrahepatic iron deposition was observed in liver biopsies from all patients with ascites. One patient treated at the 40 mg/m2 Clo dose developed severe SOS but recovered without complications. Excluding transient transaminitis, only 1 DLT (severe SOS) occurred at the 40 mg/m2 Clo dose; therefore, an MTD was not reached in this study.
Regimen-related toxicity
| Organ/system . | n (%) . | |||
|---|---|---|---|---|
| Grade I . | Grade II . | Grade III . | Grade IV . | |
| Liver | ||||
| Transaminitis* | 14 (30) | 6 (13) | 17 (37) | 6 (13) |
| Hyperbilirubiniemia | 5 (11) | 1 (2) | 1 (2) | 1 (2) |
| Ascites | 0 | 0 | 3 (7) | 0 |
| Veno-occlusive disease | 0 | 0 | 2 (4) | 0 |
| Gastrointestinal | ||||
| Mucositis | 5 (11) | 15 (33) | 11 (24) | 0 |
| Nausea/vomiting | 5 (11) | 15 (33) | 4 (9) | 0 |
| Diarrhea | 4 (9) | 2 (4) | 4 (9) | 0 |
| Abdominal pain | 0 | 3 (7) | 2 (4) | 0 |
| Genito-urinary | ||||
| Creatinine elevation | 2 (4) | 1 (2) | 0 | 0 |
| Hemorrhagic cystitis | 0 | 0 | 1 (2) | 0 |
| Cardiopulmonary | ||||
| Hypertension | 0 | 0 | 3 (7) | 0 |
| Hypotension | 0 | 1 (2) | 0 | 0 |
| Hypoxia | 0 | 0 | 5 (11) | 1 (2) |
| Neurologic | ||||
| Seizure | 0 | 0 | 1 (2) | 0 |
| Syncope | 0 | 0 | 2 (4) | 0 |
| Headache | 3 (7) | 1 (2) | 0 | 0 |
| Confusion | 0 | 1 (2) | 0 | 0 |
| Skin | ||||
| Hand-foot syndrome | 0 | 3 (7) | 6 (13) | 0 |
| Rash | 0 | 2 (4) | 0 | 0 |
| Constitutional | ||||
| Fever | 0 | 0 | 21 (46) | 0 |
| Musculoskeletal pain | 0 | 0 | 2 (4) | 0 |
| Other | ||||
| Hypersensitivity | 0 | 0 | 1 (2) | 0 |
| Thrombosis | 0 | 0 | 2 (4) | 0 |
| Organ/system . | n (%) . | |||
|---|---|---|---|---|
| Grade I . | Grade II . | Grade III . | Grade IV . | |
| Liver | ||||
| Transaminitis* | 14 (30) | 6 (13) | 17 (37) | 6 (13) |
| Hyperbilirubiniemia | 5 (11) | 1 (2) | 1 (2) | 1 (2) |
| Ascites | 0 | 0 | 3 (7) | 0 |
| Veno-occlusive disease | 0 | 0 | 2 (4) | 0 |
| Gastrointestinal | ||||
| Mucositis | 5 (11) | 15 (33) | 11 (24) | 0 |
| Nausea/vomiting | 5 (11) | 15 (33) | 4 (9) | 0 |
| Diarrhea | 4 (9) | 2 (4) | 4 (9) | 0 |
| Abdominal pain | 0 | 3 (7) | 2 (4) | 0 |
| Genito-urinary | ||||
| Creatinine elevation | 2 (4) | 1 (2) | 0 | 0 |
| Hemorrhagic cystitis | 0 | 0 | 1 (2) | 0 |
| Cardiopulmonary | ||||
| Hypertension | 0 | 0 | 3 (7) | 0 |
| Hypotension | 0 | 1 (2) | 0 | 0 |
| Hypoxia | 0 | 0 | 5 (11) | 1 (2) |
| Neurologic | ||||
| Seizure | 0 | 0 | 1 (2) | 0 |
| Syncope | 0 | 0 | 2 (4) | 0 |
| Headache | 3 (7) | 1 (2) | 0 | 0 |
| Confusion | 0 | 1 (2) | 0 | 0 |
| Skin | ||||
| Hand-foot syndrome | 0 | 3 (7) | 6 (13) | 0 |
| Rash | 0 | 2 (4) | 0 | 0 |
| Constitutional | ||||
| Fever | 0 | 0 | 21 (46) | 0 |
| Musculoskeletal pain | 0 | 0 | 2 (4) | 0 |
| Other | ||||
| Hypersensitivity | 0 | 0 | 1 (2) | 0 |
| Thrombosis | 0 | 0 | 2 (4) | 0 |
Recorded National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 3.0 nonhematologic toxicities from initiation of conditioning to day +30 or CTCAE 3.0 toxicities after day +30 possibly, probably, or definitely related to conditioning.
Transient resolving within 14 days.
Engraftment, chimerism, and immune reconstitution
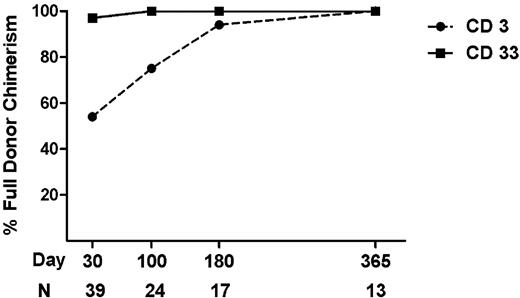
The stem cell source was peripheral blood in 93% of patients and bone marrow in 7% of patients with a median cell dose of 6.3 × 106 CD34+ cells/kg (range, 2.9-10.6 × 106 CD34+ cells/kg). All patients engrafted with neutrophils and platelets at a median of 11 days (range, 9-18) and 12 days (range, 9-19), respectively. The time to engraftment did not differ by Clo dose (data not shown). We assessed lineage specific chimerism for CD3 and CD33 compartments in bone marrow and peripheral blood at day +30, +100, +180, and +360. Of patients in remission, 54% achieved full donor CD3 chimerism by day +30 and 75% by day +100 (Figure 1). Persistent mixed chimerism has been previously associated with inferior survival and increased relapse after reduced intensity conditioning25,26 but attaining full donor chimerism for CD3 at day +30 or +100 did not correlate with relapse in this small set (data not shown). Most patients (97%) achieved full donor CD33 chimerism by day +30.
Donor chimerism after HSCT. The percentage of patients achieving full donor chimerism (> 95% donor cells) for CD3+- and CD33+-enriched cells at day +30, +100, +180, and +365 after HSCT. Patients with relapse or progressive disease were excluded.
Donor chimerism after HSCT. The percentage of patients achieving full donor chimerism (> 95% donor cells) for CD3+- and CD33+-enriched cells at day +30, +100, +180, and +365 after HSCT. Patients with relapse or progressive disease were excluded.
Pharmacokinetics
We targeted a Bu AUC of 4800 μmol min, and pharmacokinetics was obtained in 34 patients after the first Bu dose on day −5. Pharmacokinetic monitoring led to dose adjustments on days −3 and −2 in 20 patients (59%) to precisely target the AUC. Bu dose adjustment was typically made if the day −5 concentration steady state was outside the range of 700-900 ng/mL. No difference in Bu AUC was seen according to Clo dose, despite Clo being administered before Bu on day −6. Bu AUCs in 26 patients receiving fludarabine/Bu conditioning were also similar, even though the first dose of Bu was given before fludarabine.
GVHD
Acute GVHD was staged according to modified Glucksberg criteria,27 and characteristics of GVHD are listed in Table 4. Skin involvement occurred in 61%, gut involvement in 31%, and liver involvement in 17% of patients. GVHD was histologically confirmed in 85% of cases. All instances of transaminitis after CloBu4 resolved before the onset of hepatic GVHD and severity (grade III-IV vs grade 0-II) of transaminitis did not correlate with development of hepatic GVHD. Grade III to IV GVHD was observed in 30% of patients. Patients receiving the 40 mg/m2 Clo dose had a trend toward greater grade III to IV GVHD rates compared with lower doses (42% vs 19%; P = .05). Seven patients (21%) who developed GVHD had immunosuppression withdrawn or rapidly tapered before onset because of overt (n = 3) or suspected relapse (n = 4). Of patients withdrawing immunosuppression, 1 patient developed grade II GVHD and 6 patients developed grade III to IV GVHD. Twenty-six patients were in remission at day 100 and evaluable for chronic GVHD. At a median follow-up of 17 months (range, 1-38 months), 12 patients (46%) developed chronic GVHD, most being mild (n = 4) or moderate (n = 6) in severity according to National Institutes of Health criteria.28
GVHD
| Characteristic . | Value . |
|---|---|
| Acute GVHD | |
| Days to onset | |
| Median | 28 |
| Range | 13-151 |
| Maximum severity, n (%) | |
| Grade 0 | 13 (28) |
| Grade I | 10 (22) |
| Grade II | 9 (20) |
| Grade III | 6 (13) |
| Grade IV | 8 (17) |
| Target organ, n (%) | |
| Skin | 28 (61) |
| Liver | 8 (17) |
| Gut | 15 (33) |
| Multiorgan | 16 (35) |
| Chronic GVHD | |
| Evaluable patients, n (%) | 26 (57) |
| Mild | 4 (15) |
| Moderate | 6 (23) |
| Severe | 2 (8) |
| Total | 12 (46) |
| Characteristic . | Value . |
|---|---|
| Acute GVHD | |
| Days to onset | |
| Median | 28 |
| Range | 13-151 |
| Maximum severity, n (%) | |
| Grade 0 | 13 (28) |
| Grade I | 10 (22) |
| Grade II | 9 (20) |
| Grade III | 6 (13) |
| Grade IV | 8 (17) |
| Target organ, n (%) | |
| Skin | 28 (61) |
| Liver | 8 (17) |
| Gut | 15 (33) |
| Multiorgan | 16 (35) |
| Chronic GVHD | |
| Evaluable patients, n (%) | 26 (57) |
| Mild | 4 (15) |
| Moderate | 6 (23) |
| Severe | 2 (8) |
| Total | 12 (46) |
Disease response, relapse, and NRM
A complete remission (CR) at day +30 was achieved in 37 (80%) patients with nonremission hematologic malignancies. CR occurred more frequently in AML patients compared with all other diseases (94% vs 54%; P = .001). AML patients receiving CloBu4 as their first transplant had a 100% CR rate, whereas 2 of 7 (28%) AML patients receiving a prior allogeneic HSCT did not reach CR. The median time between prior HSCT and CloBu4 was 4.6 months (range, 2.6-121 months). Three patients receiving Clo as a component of prior chemotherapy (ALL = 2; AML = 1) either did not achieve CR (n = 1) or relapsed (48 and 102 days). Among the 4 patients with ALL, 3 relapsed and the fourth died from complications of acute GVHD.
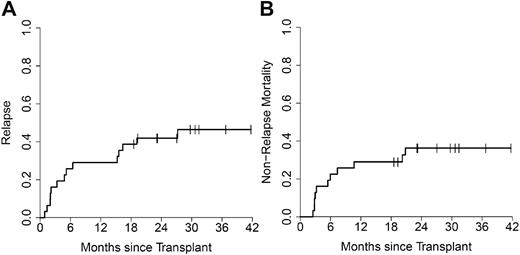
The median time to relapse was 6 months (range, 2-27 months) for AML patients achieving CR (n = 29), whereas non-AML patients achieving CR (n = 8) relapsed earlier (median, 3 months; range, 2-8 months). Including all AML patients (n = 31), the cumulative incidence of relapse was 29% (95% confidence interval [CI], 16%-48%) at 1 year and 42% (95% CI, 26%-60%) at 2 years (Figure 2A). Interestingly, 4 of 5 relapses occurring in AML after 1 year were extramedullary without simultaneous bone marrow involvement. A higher relapse rate was observed at 1 year in patients with pre-HSCT blasts greater than or equal to 10% compared with those with less AML burden (hazard ratio = 2.3; 95% CI, 1.03%-5.2%; P = .02). Other pretransplant factors such as age, cytogenetics, and presence of circulating blasts did not correlate with relapse incidence.
Relapse incidence and NRM in AML. The cumulative incidence of relapse (A) and NRM (B) for AML patients (n = 31).
Relapse incidence and NRM in AML. The cumulative incidence of relapse (A) and NRM (B) for AML patients (n = 31).
The 2 year cumulative incidence of NRM was 31% (95% CI, 19%-46%) for all patients and 36% (95% CI, 21%-55%) for AML patients receiving CloBu4 conditioning (Figure 2B). Causes of NRM were infection (n = 6), acute GVHD (n = 5), hemorrhage (n = 2), idiopathic pneumonia syndrome (n = 1), and unknown (n = 1). Supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) lists all causes of mortality according to AML and non-AML patients.
OS and EFS
At a median follow up of 29 months (range, 19-42 months) the 2 year OS for the entire study population was 28% (95% CI, 17%-45%). The 2 year OS for patients treated at the maximum 40 mg/m2 Clo dose (n = 19) was 32% (95% CI, 16%-61%) compared with 26% (95% CI, 14%-49%) for patients treated at the 20- and 30-mg/m2 doses (n = 27; P = .82).
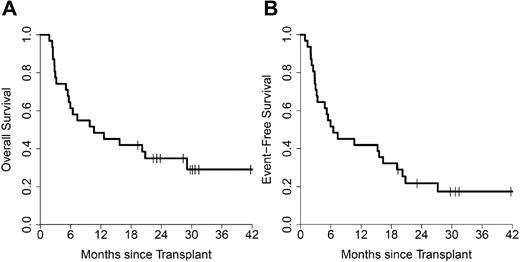
In AML (n = 31), OS at 1 and 2 years was 48% (95% CI, 34%-70%) and 35% (95% CI, 22%-57%), respectively (Figure 3A). Two-year OS was 36% (95% CI, 18%-72%) for 14 patients receiving the maximum Clo dose and 35% (95% CI, 19%-67%) for 17 patients receiving lower Clo doses (P = .88). AML patients receiving CloBu4 as their first allogeneic HSCT (n = 24) had an OS of 41% (95% CI, 25%-67%) at 2 years. EFS for all AML patients was 42% (95% CI, 28%-63%) at 1 year and 22% (95% CI, 11%-43%) at 2 years (Figure 3B).
Survival in AML. Kaplan-Meier survival plots for OS (A) and EFS (B) for AML patients (n = 31).
Survival in AML. Kaplan-Meier survival plots for OS (A) and EFS (B) for AML patients (n = 31).
Discussion
We tested the safety and efficacy of combining Clo with Bu in 46 patients with very high-risk hematologic malignancies. This trial evaluated patients with a high burden of disease at transplantation, representing a significant risk factor for both relapse and NRM.29,30 Patients enrolled had high comorbidity indices (median, 3), and AML patients had high risk scores as described by Duval et al22,24 (median, 3) reflecting the aggressive disease of this population. We hypothesized that improving the antitumor activity of conditioning without significant regimen toxicity would limit relapse and improve early survival after HSCT.
CloBu4 was frequently associated with transient elevations in liver transaminases. In addition, 4 patients developed ascites, SOS or both, possibly related to CloBu4. The correlation of elevated ferritin levels (≥ 2000 ng/mL) and grade III to IV transaminitis suggests patients with transfusion iron overload may be at higher risk for hepatic toxicity from CloBu4. Future studies may consider using chelation therapy to minimize hepatic toxicity, however, because prior chemotherapy and disease burden may have elevated ferritin levels this issue requires further study. In addition, no association between pre-HSCT ferritin and acute GVHD, NRM, or OS were observed. There were insufficient dose limiting toxicities to reach a maximum tolerated Clof dose.
Overall, we observed a 2-year NRM incidence of 31% that was not unexpected when considering the study population's age, disease burden, and comorbidity index. In younger patients with less advanced disease, Sorrer et al reported NRM rates exceeding 40% for comorbidity indices of ≥ 3.22 Grade II to IV GVHD occurred in 50% of patients, accounting for a third of NRM, which is higher than fludarabine-based conditioning but similar to nonremission patients receiving conventional myeloablative regimens.9 Importantly, overt or suspected relapse led to withdrawal of immunosuppression before GVHD in 21% of cases, possibly contributing to the high GVHD rates. These findings suggest myeloablative conditioning with CloBu4 can be used in patients with advanced hematologic malignancies.
Clo in combination with cytarabine was evaluated previously for pretransplant conditioning,31 because of considerable activity in advanced leukemia.15,16,32 This trial was stopped after enrolling 7 patients because of significant early mortality and poor engraftment despite the use of ATG. In the current study, increasing doses of Clo were administered, but combining with myeloablative doses of Bu led to stable engraftment in all patients. These findings support the secure engraftment seen when Clo was combined with Bu in other regimens,33,34 suggesting myeloablative doses of Bu are an essential component for engraftment. The M. D. Anderson group reported excellent survival outcomes with no primary or secondary graft failures when escalating doses of Clo were combined with decreasing doses of fludarabine and myeloablative doses of Bu.33 Notable differences between this report and the current study include enrollment of patients in complete remission, the use of ATG for unrelated and HLA-mismatched donors, and the combination of fludarabine and Clo. Based on our results, combining Clo with myeloablative doses of Bu possesses sufficient immunoablative properties to secure engraftment. Clo dose level did not effect Bu AUC, relapse, NRM or OS, but such analysis are limited by small patient numbers. Because no MTD was reached, Clo at 40 mg/m2 for 5 days was recommended for future studies.
CloBu4 had significant activity in the treatment of nonremission AML. Patients with AML after relapse or induction failure are unlikely to achieve durable remissions after additional chemotherapy.35,36 The lack of available donors, treatment toxicities, and poor responses to salvage chemotherapy result in less than half of such patients proceeding to HSCT.5 Earlier HSCT in refractory AML may be desirable, but conditioning must then overcome a high disease burden to facilitate sustainable remissions through the graft-versus-leukemia effect.5 AML patients in this study had a 94% CR rate (100% for AML without prior HSCT), suggesting significant activity with CloBu4. After HSCT, nonremission AML patients remain at increased risk for relapse, with rates uniformly exceeding 50% in studies of conventional myeloablative conditioning.7-9 The 2-year relapse rate of 42% for CloBu4 is encouraging but remains the predominant source of treatment failure in nonremission AML. Because half of all relapses occurred more than 6 months after HSCT, future studies might combine CloBu4 with maintenance therapies to further limit relapse.
The 1-year OS of 48% for CloBu4 compares favorably to historical 1-year OS rates of ∼15% for AML patients of similar risk reported by Duval et al in their review of the Center for International Blood and Marrow Transplant Research database.24 Notably, patients reported in this study received myeloablative conditioning with cyclophosphamide plus total body irradiation or Bu and had a median age of 38 years, an age that was significantly younger than the population studied in CloBu4 (median age, 53 years). The MD Anderson group similarly reported 1-year OS of ∼ 20% for HSCT in AML refractory to induction and 1 salvage chemotherapy.5 These findings suggest that CloBu4 conditioning has the potential to improve early survival in nonremission AML patients undergoing HSCT, but additional follow-up and confirmatory studies are needed.
In conclusion, we report that Clo and myeloablative doses of Bu conditioning are tolerable, facilitate engraftment, and have significant activity in nonremission hematologic malignancies, particularly AML. Planning is currently underway to validate these results in a larger multicenter phase 2 study of CloBu4 conditioning in nonremission AML.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Research funding and publication support in part for this study were provided by Genzyme.
Authorship
Contribution: J.M. participated in study planning, performed research, analyzed data, and wrote the manuscript; H.T. analyzed data; T.B. was the study statistician; K.E.-J. and J.S. performed chimerism analysis; A.P., E.P., P.R., C.K., S.C., G.Y., D.F., A.H., H.E., L.K., D.J., S.P., J.F., and J.L. contributed to patient accrual, quality control of the clinical endpoints, research discussion, and manuscript editing; and S.M. conceived and planned the study design, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.M. received honoraria from Otsuka Pharmaceutical and Genzyme. H.E. was a consultant for Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Shin Mineishi, Blood and Marrow Transplant Program, University of Michigan, 5303 Cancer Center, 1500 Medical Center Dr, Ann Arbor, MI 48109; e-mail: smineish@med.umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal